Translate this page into:
Safety of autologous intramuscular platelet lysate injections in patients with critical limb ischaemia: A pilot, exploratory study
For correspondence: Prof Awidi Abdalla, Cell Therapy Center, Jordan University, Queen Rania Street, 11942 Amman, Jordan e-mail: abdalla.awidi@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Platelet concentrate contains a rich cocktail of growth factors that support growth and proliferation of cells. The primary goal of this study was to investigate the safety of platelet lysate (PL) in patients with critical limb ischaemia (CLI) not suitable for standard revascularization. Data on the preliminary efficacy are also presented.
Methods:
Seven patients (18-70 yr) with CLI classified in the Rutherford grades 3-5, with history of intermittent claudication for more than eight weeks and were not suitable for standard revascularization, underwent autologous intramuscular injections of PL. These patients were examined physically alongside other parameters such as TcPO2, toe pressure, and ankle brachial index, at baseline and were followed up for 12 months.
Results:
The procedure was well tolerated with no serious adverse or any adverse events reported during follow up. Although not the primary aim of this report, preliminary data showed significant clinical improvement in Rutherford stage, ankle-brachial index and toe pressure which persisted for a year.
Interpretation & conclusions:
Intramuscular injection of PL was well tolerated with no major adverse events reported in our study participants. With the observed satisfactory safety data, preliminary efficacy data of PL should be further validated.
Keywords
Critical
ischaemia
limb
lysate
platelet
Peripheral arterial disease (PAD) broadly refers to the disease that mostly affects extra-cardiac arteries, most commonly those of the lower limbs. It is derived from any disease that causes arteriostenosis, usually a familiar sign of systemic atherosclerosis, and is associated with an increased risk of death and ischaemic events12. Critical limb ischaemia (CLI) is the most advanced clinical stage of PAD3. The incidence of PAD increases with age and its prevalence is relatively high in developed countries, nearly 12 per cent of the adult population. Limited reports exist about the prevalence of the disease in the Middle East and the Arab world; only two studies reported the prevalence of PAD in Gulf countries to be between two and three per cent45. In Jordan, one study aimed in part, to study the prevalence of PAD in 2120 patients undergoing coronary angiography through the year 2014 and found it to be 12.8 per cent6.
Conventional treatments of CLI aim to achieve a better outcome of the patient, by improving the function of the limb and reducing the risks of morbidity and mortality from cardiovascular diseases7. Revascularization is indicated for intermittent claudication impacting the quality of life and for cases when amputation is necessary after the tissue has been extensively deteriorated and cannot be salvaged, however, is not applicable in many cases8.
Consequently, novel alternative cellular therapies have been introduced to regenerate collateral vessels. Stem cell-based therapies, such as autologous bone marrow-derived mesenchymal stem cells (BM-MSCs), were utilized in the premise that these have pro-angiogenic properties9. Nonetheless, the neovascularization capacity of administered autologous BM-MSCs from old patients is impaired due to decreased angiogenic efficiency of the old stem cells, along with noticed poor cell retention and survival after delivery which would limit the use of these autologous cells in patients diagnosed with CLI1011.
Platelets are well recognized for their haemostatic functions, in addition to their diverse content of biologically active metabolites, such as pro-angiogenic factors12.
Platelet lysate (PL) is a platelet derivative with potential clinical applications. It contains a combination of immediately available growth factors (GFs) and cytokines that can assist in tissue regeneration, either by direct impact on the injured tissue or by providing the necessary chemokines to attract MSCs to the site of injury13.
In this study, the aim was to evaluate the safety of using PL in patients with CLI and to make a preliminary assessment of the efficacy of PL in these patients.
Material & Methods
Patients: This was a pilot, exploratory study, in which seven patients with CLI were recruited in this study. Written informed consent was obtained from all participants in accordance with the Helsinki Declaration. The study was approved by the cell therapy Institutional Review Board (39/2013) and prospectively registered on ClinicalTrials.gov (NCT02941419).
Inclusion criteria: (i) Male or female patients between 18 and 70 yr; (ii) Rutherford stage 3, 4 and 5; (iii) history of intermittent claudication for more than eight weeks; (iv) patients meeting any of the following criteria: resting ankle-brachial index (ABI) <0.8, toe pressure ≤60 mmHg, toe brachial index (TBI) <0.6 or transcutaneous oxygen pressure (TcPO2) ≤60 mmHg in the foot; (v) those not eligible for surgical or radiological revascularization; (vi) in the case of diabetic patients, he/she should be on medication and fairly controlled (HbA1c <10%); (vii) normal values for the following blood tests: liver enzymes including alanine aminotransferase, aspartate transaminase and bilirubin and serum creatinine; (viii) normal haemoglobin and platelet count; (ix) controlled blood pressure and on regular medication for hypertension if any; and (x) competent and willing to give informed consent and to be available for all baseline treatment and follow up examinations required by the protocol.
Exclusion criteria: (i) Pregnant and lactating women; (ii) systemic autoimmune disease such as rheumatoid arthritis and/or receiving immunosuppressant medications; (iii) history of neoplasm or malignancy in the past 10 yr; (iv) reported unstable cardiovascular disease or heart failure; (v) leg oedema; (vi) inflammatory or progressive fibrotic disorder; (vii) renal insufficiency or failure; (viii) history of infectious disorder diseases such as hepatitis B and C and HIV; (ix) chronic inflammatory disease; (x) history of stroke or myocardial infarction (less than three months); (xi) bleeding or clotting disorder and use of oral anticoagulant therapy (heparin and warfarin); and (xii) poorly controlled diabetes (HbA1c ≥10).
Baseline evaluation: Complete history and thorough physical examination were performed for all patients, in addition to ABI test, toe pressure test, TcPO2 test and computed tomography angiography.
Complete laboratory tests and serologic profile were obtained for all patients and they were also screened for HIV and hepatitis viruses.
Follow up evaluations: Patients were followed up and evaluated for 12 months at the following intervals: baseline, four, eight and 12 months respectively. Follow ups included ABI, toe pressure and TcPO2 tests in addition to thorough physical examination and detailed history for each patient for baseline and recovery evaluations in each follow up visit.
Patients’ assessment: The primary outcome measures were safety and tolerability and the secondary end point was preliminary efficacy.
Safety assessment: The safety was assessed by monitoring any adverse event at the time of the injection, after one hour, 24 h and a week post-injection. This was done through an adverse event questionnaire developed by our team. Moreover, safety and tolerability were also evaluated by observing the injection site for bleeding, bruising, pain, swelling, hypo- and hyperthermia, erythema and urticarial and by measuring the vital signs. In addition to history and physical examination after two weeks of the injection, haematology and blood chemistry three months post-injection were performed. These panels of blood tests included complete blood count and differential, erythrocyte sedimentation rate, kidney function test and electrolytes, liver function test, C-reactive protein, glucose, HbA1c, lipid profile, urinalysis and coagulation tests.
Efficacy assessment: The efficacy of our treatment was evaluated by an increase in patients’ haemodynamics: ABI, toe pressure and TcPO2. Physical examination was also performed at each follow up visit.
Platelet lysate preparation: 40-45 ml citrated whole blood was collected to prepare four doses of 5 ml autologous PL for each patient. Platelet-rich plasma (PRP) was obtained by low-speed centrifugation (110 × g for 10 min). Then, pooled PRP was centrifuged at high speed (3300 × g for 10 min). Platelets were activated by three cycles of freezing at −80°C and rapid thawing at 37°C. After the last freeze-thaw cycle, PL was centrifuged at 3300 × g for 10 min and the supernatant was filtered through 0.22-um syringe filter. This was stored as 5 ml aliquots, in which four tubes were prepared for each patient.
Injection: Five millilitres of prepared autologous PL was given intramuscularly in the affected gastrocnemius as multiple point injections in a circular fashion. This procedure was done for all patients in all four injections.
Assessment of outcomes: The primary outcome measures were safety and tolerability, which were assessed by monitoring any local inflammatory reaction, complication or any adverse event at the time points mentioned above. The secondary end point was efficacy, which was evaluated by ABI, TcPO2 and toe pressure.
Statistical analysis: Wilcoxon signed-rank test was performed to determine the differences between before and after the interference. Statistical significance was assumed at a value of P<0.05. All statistical analyses were performed using SPSS for Windows version 22 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics: Seven consecutive patients with CLI who were nonsuitable for revascularization were screened. The median age was 57 yr. All seven patients had atheromatous PAD in origin, all of whom also had CLI since more than two years ago. All patients showed severe stages of CLI: five patients with Rutherford stage 5 and two patients with stage 3. The medians for their ABI, toe pressure and TcPO2 were 0.7, 48 mmHg and 33 per cent, respectively.
Follow up evaluations:
Safety of the injection: The primary outcome measures of this study were safety and tolerability. The PL injection was well tolerated. There was no bruising, swelling, erythema, urticarial or hypo- and hyperthermia resulting from the intramuscular injection of the PL, suggesting that the PL prepared was safe. No major adverse events or complications related to the injected PL were detected in any patient immediately nor after one year of injection. No significant changes were detected in patients’ CBC (complete blood count) and biochemistry tests. Only minor side effects occurred such as mild pain and local bleeding at the site of the injection during the procedure.
Clinical efficacy of the injection: All patients were followed up for one year. Over the study period, patients showed an overall significant improvement demonstrated in ABI and toe pressure. Moreover, pain-free walking distance was significantly extended in limbs treated with PL from baseline to one year after injection (P<0.05) (Table I). The Rutherford stage significantly improved for these patients after eight months of the injections and persisted for a year (Table II). ABI (Fig. 1), toe pressure (Fig. 2) and pain-free walking distance of the limbs injected with PL were significantly increased from baseline to the level four months after injection (P<0.05). Furthermore, this increase persisted until one year after the injections. However, there was no significant change in the TcPO2 readings over the study period (Fig. 3).
| Pain-free walking distance | ||||
|---|---|---|---|---|
| Rest pain | ≤50 m | ≥100 m- <200 m | ≥200 m | |
| Baseline | ||||
| Number of patients | 5 | 2 | 0 | 0 |
| Four months | ||||
| Number of patients | 2 | 3 | 2 | 0 |
| Eight months | ||||
| Number of patients | 1 | 0 | 4 | 2 |
| 12 months | ||||
| Number of patients | 1 | 0 | 4 | 2 |
| Rutherford stage | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Baseline | ||||||
| Number of patients | 0 | 0 | 0 | 2 | 0 | 5 |
| Four months | ||||||
| Number of patients | 0 | 1 | 1 | 3 | 2 | 0 |
| Eight months | ||||||
| Number of patients | 2 | 0 | 4 | 0 | 1 | 0 |
| 12 months | ||||||
| Number of patients | 2 | 4 | 0 | 0 | 1 | 0 |
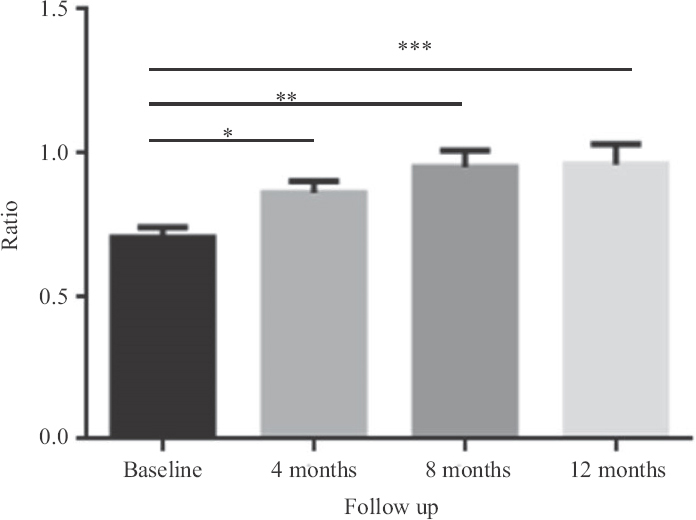
- ABI changes over one-year period for seven patients. *P=0.005 (after four months),**P=0.005 (after eight months), ***P=0.007 (after one year). Data represented as mean ±SEM (n=7). ABI, ankle-brachial index; SEM, standard error of mean.
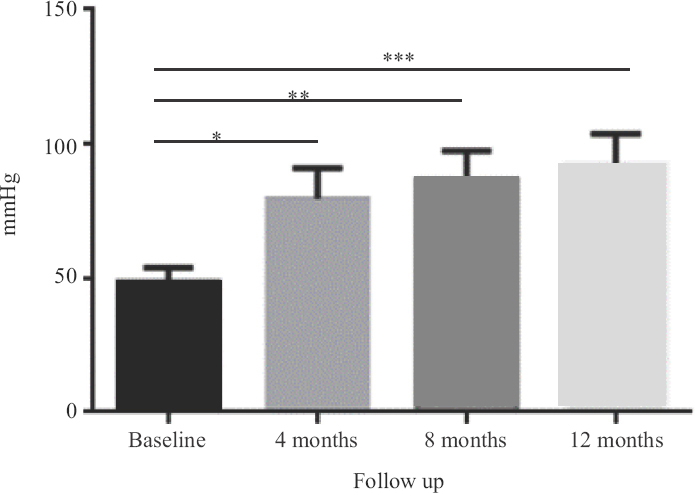
- Toe pressure changes over one-year period for seven patients. *P=0.005 (after four months), **P=0.005 (after eight months), ***P=0.005 (after one year).
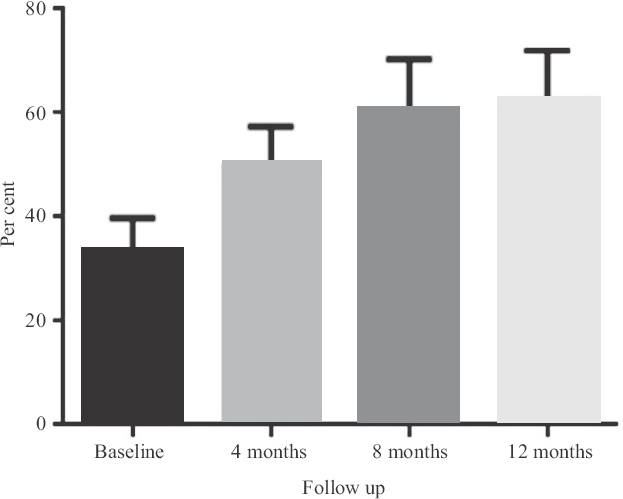
- TcPO2 changes over one-year period for seven patients. Data represented as mean±SEM (n=7). TcPO2, transcutaneous oxygen pressure; SEM, standard error of mean.
Discussion
In the past decade, gene and cellular therapies have shown promising safety and efficacy trial data for CLI patients14. One study conducted by Matoba et al15 showed significant improvements in leg pain, ulcer size and pain-free walking distance that were maintained for two years in patients injected with bone marrow mononuclear cells, while another study assessed the safety of intramuscular injection of hepatocyte GF plasmid in patients with CLI, where their injections were safe and well tolerated. However, there was no difference between groups in their ABI, TBI, pain relief or wound healing16.
Despite the early optimistic reports, there should be further consideration towards the limitations that stand in the way of the success of cellular therapies in the prevention of amputations. The impaired regenerative function of stem cells and progenitor cells is apparent in diabetes, hypertension, atherosclerosis and ageing, which are all associated with PAD17. Not to forget the existing retention problem of stem cells at the site of damage after administration. It has been reported that cells stay at the site of administration for two hours at best before circulating back to the heart11, which is a short period of time for a significant effect to take place. Moreover, it has not been elucidated by which mechanisms MSCs benefit flow recovery in PAD18, some justify the angiogenic potential of MSCs to a paracrine effect that leads to modulation of angiogenic molecules and may even involve crosstalk between several pathways9.
Autologous PL has been greatly explored in many clinical settings to promote tissue regeneration19. This significance stems from the fact that platelets release a wide range of powerful mitogenic and chemotactic GFs such as PDGF, TGF-β, EGF, VEGF, IGF-1, FGF, HGF and other molecules that are involved in regenerative processes20. These bioactive GFs could influence tissue repairing through angiogenesis and promote cellular chemotaxis and restitution of extracellular matrix2122.
Platelet lysate, obtained by mechanical activation of platelets, has been demonstrated to deliver more GFs compared with PRP and to assess the efficacy of platelets’ growth elements on the compromised tissue without any additional substances1923. Furthermore, traditional PRP constitutes an increased concentration of white blood cells, which could cause pro-inflammatory effects24. This freeze-thaw method for platelet activation; following a high-speed centrifugation and supernatant abstraction, a leucocyte-free PL was obtained. Numerous studies have demonstrated the positive effects of PL products in tissue culture and clinical settings252627282930. These clinical trials have illustrated favourable PL clinical outcomes in different diseases such as lateral epicondylitis, knee osteoarthritis and lumbar radicular pain282930. These promising outcomes can be as a result of the cocktail of bioactive substances present in the activated PL and signalling molecules which have significant roles in cell behaviour, tissue repair and induce the migration of reparative cells such as MSCs2122.
As a primary goal, the safety of autologous intramuscular PL injection was investigated. In this group of patients, no major adverse events related to the treatment were observed. The clinical condition improved for all patients except for two, as per in their TcPO2, toe pressure and Rutherford stage. The overall improvement in the ABI measurements was statistically evident through the course of PL administration on follow up. However, the significant differences in ABI readings were between baseline and four months, between baseline and eight months and between baseline and 12 months. The overall toe pressure readings showed a significant improvement as well. As demonstrated in the data, the substantial progress in toe pressure readings was explicit between baseline and the follow up points of four, eight and 12 months, respectively as well as between four and eight months. These preliminary data suggest a cumulative and sustainable amelioration of ABI and toe pressure values following PL injections through the time plan. In addition, the Rutherford stage and the distance rest pain showed an overall consequential difference on follow up.
Despite those findings, this pilot study is associated with several limitations, such as the sample size and the lack of a control group.
In order to validate the efficacy data, the use of PL as a treatment for CLI still needs optimization and guidelines to be set, despite the initial optimistic data retrieved. In fact, platelet profiles and outcomes can be correlated to the original blood samples’ characteristics and methods of fabrication31. Furthermore, since platelet function can be affected by the metabolic status of the patient32, this should be considered when assessing the efficacy of PL injections. Furthermore, activation, aggregation and stress of platelet might occur during PL preparation. For example, on in vitro activation, there are an early alpha-granule release and a possible loss of GFs during the collection procedure3133. Consequently, it is crucial to define and unify the degree of platelet activation by setting a consensus in the preparation method. For instance, injecting platelets with a narrow range of concentration should be performed and platelet stress should be avoided during PL preparation. Not to mention that the efficacy of the treatment can be statistically evaluated if proper sample size is determined. Since the sample size of this study was too small and there remains a need to have a conclusive end result. Therefore, a larger study with a larger group of patients is being conducted to investigate the efficacy of using pooled allogeneic PL in the treatment of CLI.
Financial support & sponsorship: The study was funded by the University of Jordan.
Conflicts of Interest: None.
References
- Peripheral arterial disease in the Middle East:Underestimated predictor of worse outcome. Glob Cardiol Sci Pract. 2013;2013:98-113.
- [Google Scholar]
- Peripheral arterial disease in patients presenting with acute coronary syndrome in six middle eastern countries. Int J Vasc Med. 2011;2011:815902.
- [Google Scholar]
- Polyvascular disease in patients presenting with acute coronary syndrome:Its predictors and outcomes. ScientificWorld Journal. 2012;2012:284851.
- [Google Scholar]
- Prevalence of previously unrecognized peripheral arterial disease in patients undergoing coronary angiography. Medicine (Baltimore). 2018;97:e11519.
- [Google Scholar]
- Peripheral arterial disease:Epidemiology, natural history, diagnosis and treatment. Int J Angiol. 2007;16:36-44.
- [Google Scholar]
- Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25:903-10.
- [Google Scholar]
- Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol Ther. 2014;22:1960-70.
- [Google Scholar]
- A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization. Acta Biomater. 2016;36:86-98.
- [Google Scholar]
- Overview of platelet physiology:Its hemostatic and nonhemostatic role in disease pathogenesis. ScientificWorldJournal. 2014;2014:781857.
- [Google Scholar]
- Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg. 2012;56:264-6.
- [Google Scholar]
- Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010-8.
- [Google Scholar]
- Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58-65.
- [Google Scholar]
- Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem Cell Rev Rep. 2018;14:337-45.
- [Google Scholar]
- Mesenchymal stem cells as a treatment for peripheral arterial disease:Current status and potential impact of type II diabetes on their therapeutic efficacy. Stem Cell Rev Rep. 2013;9:360-72.
- [Google Scholar]
- Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol. 2013;13:47.
- [Google Scholar]
- Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma:An in vitro study. PLoS One. 2014;9:e107813.
- [Google Scholar]
- The effect of Platelet Lysate on osteoblast proliferation associated with a transient increase of the inflammatory response in bone regeneration. Biomaterials. 2013;34:9318-30.
- [Google Scholar]
- Platelet lysate coating on scaffolds directly and indirectly enhances cell migration, improving bone and blood vessel formation. Acta Biomater. 2013;9:6630-40.
- [Google Scholar]
- Platelet lysates promote mesenchymal stem cell expansion:A safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228-36.
- [Google Scholar]
- Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS One. 2015;10:e0121713.
- [Google Scholar]
- Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797-811.
- [Google Scholar]
- The effect of platelet lysate in culture of PDLSCs:An in vitro comparative study. PeerJ. 2019;7:e7465.
- [Google Scholar]
- Human platelet lysate in mesenchymal stromal cell expansion according to a GMP grade protocol:A cell factory experience. Stem Cell Res Ther. 2018;9:124.
- [Google Scholar]
- The use of lumbar epidural injection of platelet lysate for treatment of radicular pain. J Exp Orthop. 2017;4:38.
- [Google Scholar]
- Safety and efficacy of autologous intra-articular platelet lysates in early and intermediate knee osteoarthrosis in humans:A prospective open-label study. Clin J Sport Med. 2015;25:524-8.
- [Google Scholar]
- Autologous platelet lysate local injections for the treatment of refractory lateral epicondylitis. J Orthop Surg Res. 2016;11:17.
- [Google Scholar]
- Tissue regeneration and in loco administration of platelet derivatives:Clinical outcome, heterogeneous products, and heterogeneity of the effector mechanisms. Transfusion. 2005;45:1759-67.
- [Google Scholar]
- Determining the effect of preparation and storage:An effort to streamline platelet components as a source of growth factors for clinical application. Transfus Med Hemother. 2015;42:174-80.
- [Google Scholar]






