Translate this page into:
Safety evaluation of mercury based Ayurvedic formulation (Sidh Makardhwaj) on brain cerebrum, liver & kidney in rats
Reprint requests: Dr Y.K. Gupta, Professor & Head, Department of Pharmacology, All India Institute of Medical Sciences New Delhi 110 029, India e-mail: yk.ykgupta@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Sidh Makardhwaj (SM) is a mercury based Ayurvedic formulation used in rheumatoid arthritis and neurological disorders. However, toxicity concerns due to mercury content are often raised. Therefore, the present study was carried out to evaluate the effect of SM on brain cerebrum, liver and kidney in rats.
Methods:
Graded doses of SM (10, 50, 100 mg/kg), mercuric chloride (1 mg/kg) and normal saline were administered orally to male Wistar rats for 28 days. Behavioural parameters were assessed on days 1, 7, 14 and 28 using Morris water maze, passive avoidance, elevated plus maze and rota rod. Liver and kidney function tests were done on day 28. Animals were sacrificed and brain cerebrum acetylcholinesterase activity, levels of malondialdehyde (MDA), reduced glutathione (GSH) in brain cerebrum, liver, kidney were estimated. The levels of mercury in brain cerebrum, liver and kidney were estimated and histopathology of these tissues was also performed.
Results:
SM in the doses used did not cause significant change in neurobehavioural parameters, brain cerebrum AChE activity, liver (ALT, AST, ALP bilirubin) and kidney (serum urea and creatinine) function tests as compared to control. The levels of mercury in brain cerebrum, liver, and kidney were found to be raised in dose dependent manner. However, the levels of MDA and GSH in these tissues did not show significant changes at doses of 10 and 50 mg/kg. Also, there was no histopathological change in cytoarchitecture of brain cerebrum, liver, and kidney tissues at doses of 10 and 50 mg/kg.
Interpretation & conclusions:
The findings of the present study suggest that Sidh Makardhwaj upto five times the equivalent human dose administered for 28 days did not show any toxicological effects on rat brain cerebrum, liver and kidney.
Keywords
Brain cerebrum
kidney
liver
mercury
oxidative stress
Sidh Makardhwaj
Sidh Makardhwaj is a popular Kupipakwa rasayan, prepared with swarna (gold), parada (mercury), gandhaka (sulphur) in a specific ratio (1:8:24) mentioned in Ayurvedic Formulary of India1. It has been used in the Indian Systems of Medicine for centuries with claimed efficacy and safety for the treatment of rheumatoid arthritis, neurological disorders, as a rasayana for vigour and longevity of life2. It is prepared by a specific process of constant heating for more than 24 h converting it in a stable compound (mercury sulphide)1.
Recent reports on the presence of heavy metals in Ayurvedic or herbal preparations have raised concern and controversy. Saper et al3 have reported that one out of five Ayurvedic herbal medicine products (HMPs), produced in South Asia contains potentially harmful levels of lead, mercury, and arsenic. However, mercury along with sulphur (mercury sulphide) is one of the ingredients used in many traditional Ayurvedic medicines. Ayurvedic experts have estimated that approximately 20 per cent of the Ayurvedic formulations contain mercury sulphide as an ingredient14. Therefore, heavy metals content in bhasma can be thousand folds higher2. As per the classic Ayurvedic text, the processed mercury along with sulphur is converted to mercury sulphide and in low dose shows good therapeutic activities without producing toxic effects in the human subjects5. However, safety issues have been raised about mercury content present in herbo-mineral and bhasma preparations6. We, therefore, undertook this experimental study to evaluate safety profile of Sidh Makardhwaj mercury based Ayurvedic formulation.
Material & Methods
This study was conducted in the department of Phamacology, All India Institute of Medical Sciences (AIIMS), New Delhi, India. Male Wistar adult rats (150-200 g) were obtained from the Central Animal Facility of AIIMS, and stock bred in the departmental animal house. The rats were group housed in polyacrylic cages (38×23×10 cm) with not more than four animals per cage and maintained under standard laboratory conditions with natural dark and light cycle. They were allowed free access to standard dry rat diet (Ashirwad, Punjab, India) and tap water ad libitum. However, 12 h before the behavioural testing, the rats were deprived of food. The study protocol was approved by the Institutional Animal Ethics Committee, All India Institute of Medical Sciences, New Delhi, India.
Drugs preparation and duration of treatment: Sidh Makardhwaj (Batch number, MDR 022; date of manufacturing, September 2009; Maharshi Ayurveda Pharmaceutical Limited, New Delhi, India) was suspended in honey (Dabur Pharmaceuticals Pvt. Ltd, Gaziabad, India) and mercuric chloride (Sigma, USA) solution made in distilled water. Rats were randomly divided into five groups consisting of six rats each i.e. normal control, mercuric chloride (1 mg/kg/day) and Sidh Makardhwaj (10, 50 and 100 mg/kg) treated. The doses of Sidh Makardhwaj (10, 50 and 100 mg/kg/day) for rat were calculated by extrapolating the equivalent human dose (1, 5 and 10 times)7 and were administered orally between 10.00 and 11.00 h every day for 28 days, in a volume not exceeding 1 ml/100 g rat weight. On 28th day, the rats were subjected to the behavioural tests and then sacrificed for biochemical and histopathological studies.
Neurobehavioural activity (cognition and motor coordination)
One trial passive avoidance task: Passive avoidance (Ugo Basile, USA) was used to evaluate the memory retention deficit and was evaluated according to the method described by Nakahara et al8. In acquisition trial, the rat was placed in a lighted chamber and guillotine door separating the light and dark chambers was opened. Initial latency (IL) to enter the dark chamber was recorded. Immediately after the rat enters the dark chamber, the guillotine door was closed and an electric foot shock (75 V, 0.2mA, 50 Hz) was delivered to the floor grids for 3 sec. The rat was removed from the dark chamber 5 sec later and returned to its home cage. After 24 h, retention latency (RL) time was noted in the same way as in the acquisition trial.
Morris water maze: Morris water Maze (Ugo Basile, USA) was use to evaluate the learning and memory. The Morris water maze consisted of a large circular pool filled with water (1.8 m in diameter, 0.6 m in height) and a platform (10 cm in diameter) submerged 1 cm below the water's surface. An automated tracking system (Video tracking system, Stoelting, USA) analyzed the total path lengths. Rats were given four acquisition sessions with an inter-trial interval of 10 min. Once a rat located the platform, it was allowed to remain there for 10 sec before being removed from the tank. If a rat failed to locate the platform within 120 sec, it was manually guided to it9.
Elevated plus maze: Elevated plus maze was used to evaluate the memory retention deficit10. Rats were placed at one end of an open arm, facing away from the central square. Time taken by the rat to move from open arm to closed arms was recorded and marked as “initial transfer latency” (ITL). Animal was allowed to explore the maze for 30 sec after recording initial transfer latency. Retention transfer latency (RTL) was recorded by placing the rats similarly on the open arm at specified intervals.
Rota rod: Rota rod was use to evaluate the muscle coordination of rats. Rats were conditioned to the accelerating rod (Ugo Basile). Each animal received a training session on the rota rod at constant speed of 8 rpm and was tested until learned to remain on the rotating spindle for 60 sec. Each rat received single base line trial on the accelerating rota rod in which the spindle speed increased from 4 to 40 rpm over a period of 5 min. After administration of selected drugs for 28 days, each rat received a test trial11.
Biochemical estimation and histopathology: At the end of behavioural experiments, blood was withdrawn by puncturing retro-orbital sinus for biochemical estimations. Serum was separated to measure liver and kidney functions test parameters. Animals were then sacrificed under ether anaesthesia and the brain cerebrum, liver, kidney were quickly removed. tissues (n=50%) were cleaned with ice cold saline and stored at -80°C to determine the level of melondialdehyde (MDA), glutathione (GSH), acetyl cholinesterase (AChE) activity, mercury level and the remaining tissues were kept in 10 per cent formalin for histopathological study.
AChE activity was measured in the brain according to the method of Ellman et al12, and was expressed as change in optical density/min/mg protein. Protein estimation was carried out by the method of Lowry et al13. MDH was determined by the method of Ohkawa et al14, and its concentration was expressed in nmol/g wet tissue. Glutathione was measured according to the method of Ellman15 and concentration was expressed as mg/g wet tissue.
Mercury level was estimated in tissue by inductively coupled plasma – atomic emission spectrophotometer (ICP-AES, JY 2000-2, France). Brain cerebrum, liver and kidney tissues were digested by cold vapors digestion procedure according to the method of Jacob et al16. Mercury levels were expressed in µg/g wet tissue.
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, urea and craetinine levels were estimated separately using individual kit by semi auto analyzer (Mini techno, USA). The enzyme activities were reported as the instructions of the manufacturer of assay kits (Logitech India Pvt. Ltd, Delhi, India).
Histopathological study: Tissue specimens from brain cerebrum, liver, and kidney fixed in 10 per cent formalin were processed by conventional method, embedded in paraffin, sectioned at 4-5 µm and stained by haematoxylin and eosin17. Tissues were examined under a light microscope (Nikon, Japan). Histopathological study was carried out in the department of Pathology, AIIMS, blinded to the groups.
Statistical analysis: Data were expressed as mean ± SEM. A one-way analysis of variance (ANOVA), followed by Post-hoc multiple comparisons of Tukey test was used for statistical analysis. SPSS (version 16) statistical software USA was used for the analysis of data and P< 0.05 was taken as the level of significance.
Results
Effect of Sidh Makardhwaj on behavioural parameters
Passive avoidance task - There was no significant change in the initial latencies and retention latencies of Sidh Makardhwaj (10, 50 and 100 mg/kg) treated groups as compared to normal control. However, there was significant decrease in mean retention latencies of mercuric chloride treated group as compared to normal control group (P< 0.001) (Table I).
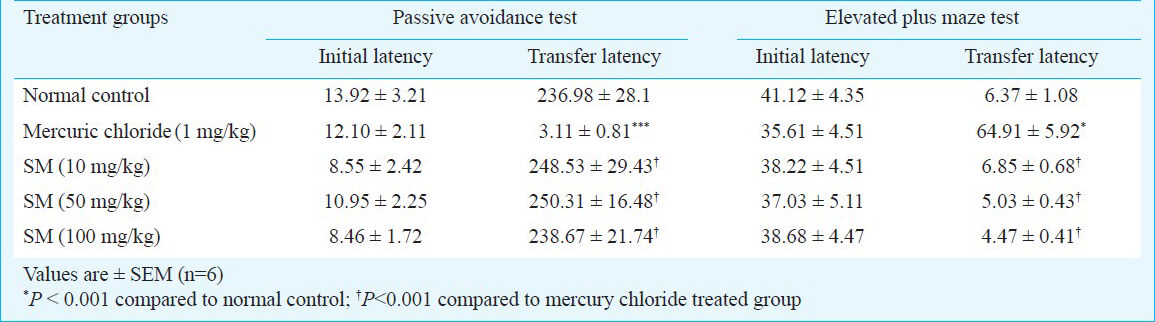
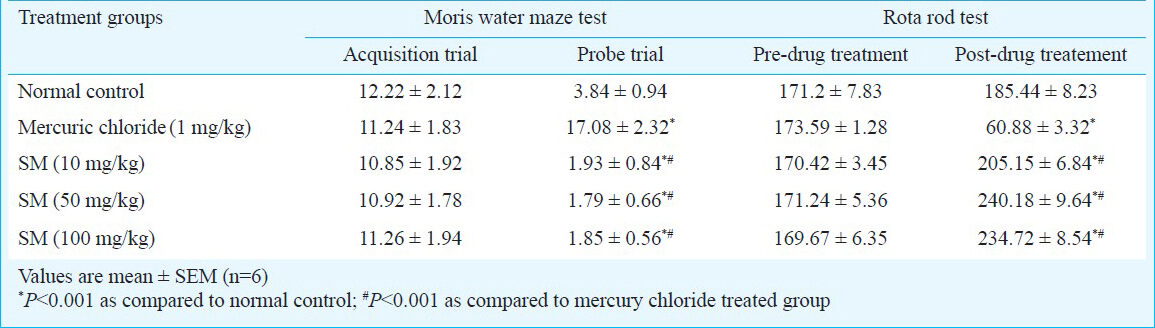
Morris water maze test - Sidh Makardhwaj (10, 50 and 100 mg/kg) treated groups did not show significant change in total distance travelled during the acquisition trials and probe trial to reach the platform as compared to normal control group. However, there was increase in distance travelled during probe trial to reach the platform of mercuric chloride treated group as compared to normal control (P< 0.001) (Table II).
Elevated plus maze test - There was no significant change in initial transfer latencies and retention transfer latencies of Sidh Makardhwaj (10, 50 and 100 mg/kg) treated groups as compared to normal control group. However, there was significant increase in mean retention transfer latencies as compared to normal control group (P< 0.001) (Table I).
Rota rod test - There was no significant change in the time spent on the spindle of the rota rod before drug treatment and post drug treatment as compared to normal control group. However, there was significant decrease in time spent on spindle of mercuric chloride treated group as compared to control group (P<0.001) (Table II).
Effect of Sidh Makardhwaj on biochemical parameters
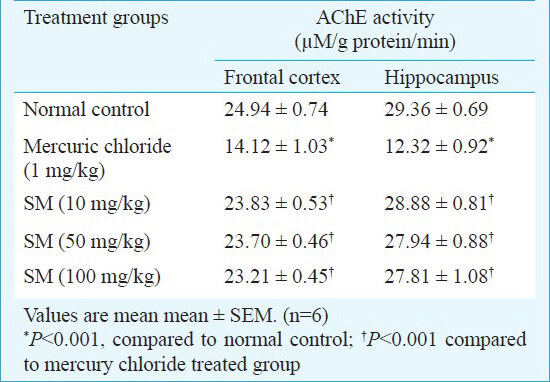
AChE activity of frontal cortex and hippocampus - There were no significant difference in AChE activity of Sidh Makardhwaj (10, 50 and 100 mg/kg) groups as compared to normal control group in frontal cortex as well as in hippocampus. However, there was significant (P<0.001) decrease in AChE activity in frontal cortex as well as in hippocampus of mercuric chloride group as compared to normal control group (Table III).
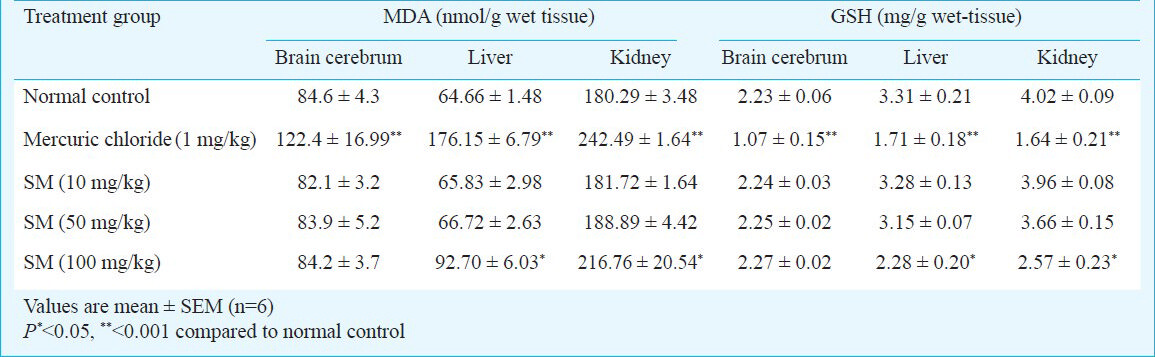
MDA and GSH levels in brain cerebrum, liver and kidney - There were no significant difference in brain cerebrum's MDA and GSH levels at studied doses of Sidh Makardhwaj while increased levels were observed in mercuric chloride group as compared to normal control group (P<0.001). Significantly increased MDA and decreased GSH levels in liver and kidney of mercuric chloride treated group (P< 0.001) and Sidh Makardhwaj (100 mg/kg) treated group (P< 0.05) were observed while no significant change was observed at lower doses of Sidh Makardhwaj (10 and 50 mg/kg) as compared to the control group (Table IV).
Mercury level in brain cerebrum, liver and kidney - There was significant increase in rat's brain, liver and kidney mercury levels of Sidh Makardhwaj (10, 50 and 100 mg/kg) groups (P< 0.05, P< 0.001) as well as in mercuric chloride treated group (P< 0.001) as compared to normal group (Table V).
Liver and kidney function test parameters - There was no significant change in the serum ALT, AST, ALP, bilirubin, urea and creatinine levels of Sidh Makardhwaj treated groups at doses of 10, 50 and 100 mg/kg as compared the normal control group, while significant change was observed in mercuric chloride treated group (Table VI).
Effect of Sidh Makardhwaj on brain cerebrum, liver and kidney histology: The brain cerebrum, liver and kidney of normal control and those treated with lower doses of Sidh Makardhwaj (10, 50 mg/kg) showed no abnormal histopathological changes but mild histopathological change was observed with higher dose of Sidh Makardhwaj (100 mg/kg). Microscopically, necrosis of neurons in cerebrum (Fig. 1), inflammed periportal zone in liver (Fig. 2) and disruption of epithelium in proximal convoluted tubules in kidney (Fig. 3) were observed at higher dose (100 mg/kg). However, mercuric chloride treated group showed expected toxicities i.e. necrosis of neurons in cerebrum, inflammed periportal zone in liver and disruption of epithelium in proximal convoluted tubules in kidney.
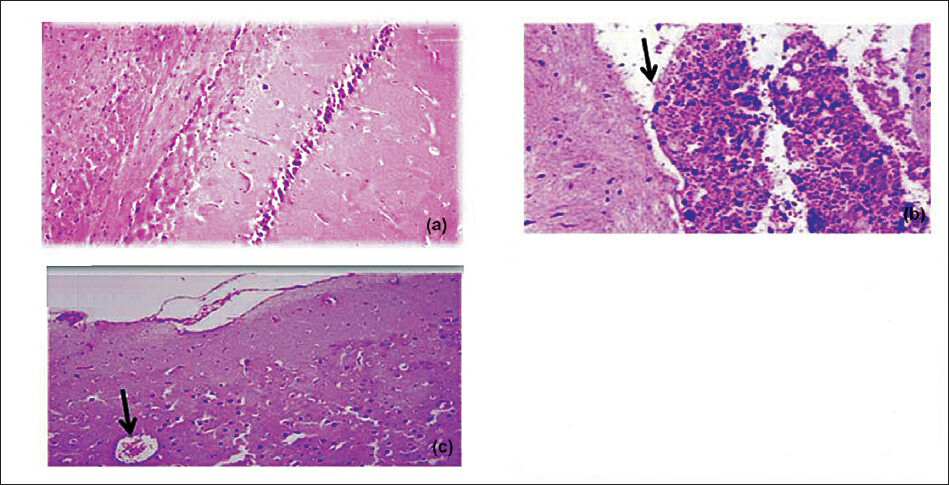
- Effect of Sidh Makardhwaj on cerebrum histology. Lingh micrograph of (a) control brain cerebrum showing normal architecture (b) 1 mg/kg/day, HgCl2 treated rat showing pyknosis, congestion of blood vessels and necrosis of Purkinje cells (arrow) (c) 100 mg/kg/day, Sidh Makardhwaj treated showing pyknosis of neurons and congestion of blood vessels (arrow). Original magnification 20X.
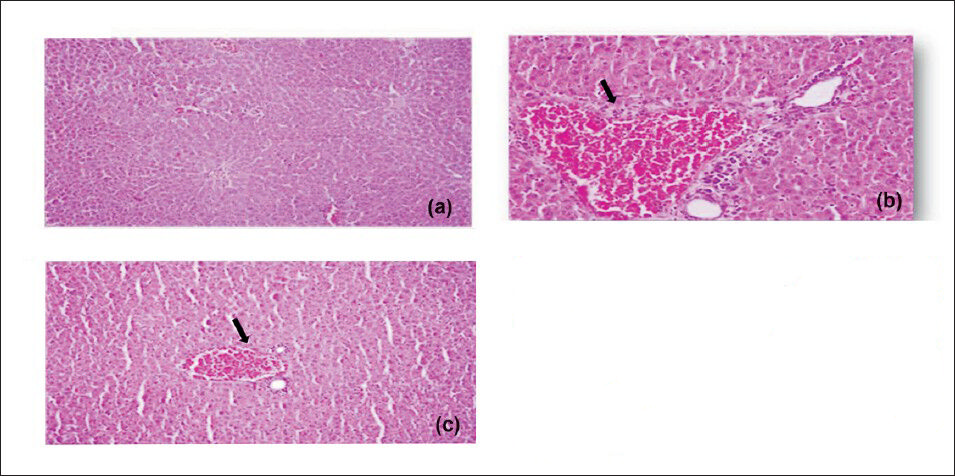
- Effect of Sidh Makardhwaj on rat's liver histology. Light micrograph of (a) control liver showing normal architecture (b) 1 mg/kg/day, HgCl2 treated showing pyknosis, vascular, degenerative and necrotic changes in the liver (arrow) (c) 100 mg/kg/day, Sidh Makardhwaj treated showing pyknosis and congestion of blood vessels (arrow). Original magnification 20X.
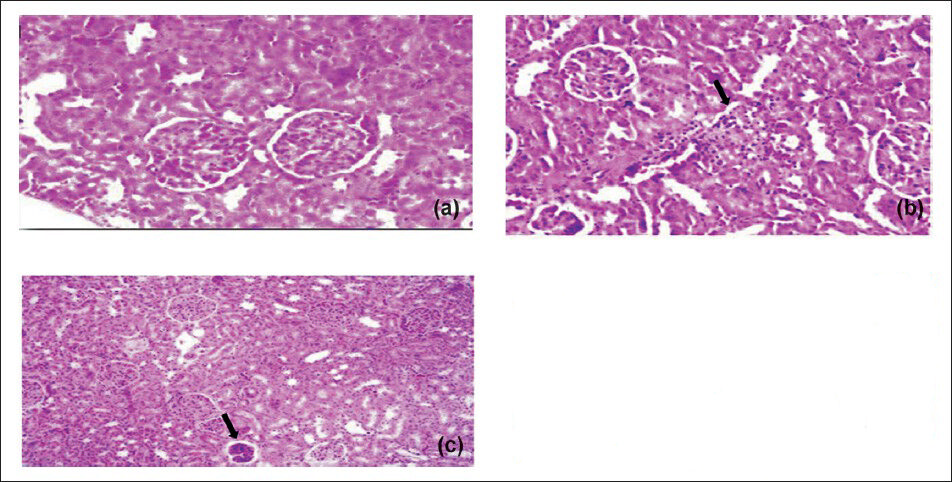
- Effect of Sidh Makardhwaj on rat's kidney histology. Light micrograph of (a) control liver showing normal architecture (b) 1 mg/kg/ day, HgCl2 treated showing pyknosis, vascular, degenerative and necrotic changes in the liver (arrow) (c) 100 mg/kg/day, Sidh Makardhwaj treated showing pyknosis and congestion of blood vessels (arrow). Original magnification 20X.
Discussion
Neurotoxicity, hepatotoxicity and nephrotoxicity due to mercury exposure are well known1819. However, mercury based Ayurvedic formulations (Sidh Makardhwaj) have been widely used in India for centuries. The US Environmental Protection Agency (EPA) has adopted a reference dose (RfD) for methyl mercury of 0.1 µg/kg body weight/day20. However, the total mercury content of Sidh Makardhwaj formulation used in the present study was 35454.2 µg/g. The calculated total ingested mercury at doses of 10, 50 and 100 mg/kg were 354.5, 1772.5 and 3545.4 µg/kg. Thus, in therapeutic dose of Sidh Makardhwaj (10 mg/kg), the per day ingested mercury was many fold higher than the reference dose. It was interesting to note that even this high concentration of mercury in Sidh Makardhwaj for 28 days did not cause significant toxicity in liver, kidney and brain cerebrum. The absence of toxicity could be due to the fact that Ayurvedic detoxification process (Sodhana) might have contributed in some modification of metal property resulting in abolition of toxicity, yet retaining its pharmacological property. Another reason could be due to mercuric sulphide content in Sidh Makardhwaj because Son et al21 have reported that mercuric sulphide at the dose of 2 g/kg did not cause hepatotoxicity in the biochemical and histological examination in mice.
Previous studies have shown behavioural and spatial learning deficits in animals due to mercury exposure22. Baraldi et al23 showed cognitive impairment in chronically mercury exposed rats and there was decreased ability to learning in water maze model. Mercuric chloride (1 mg/kg/day, p.o) caused impairment of memory and motor activity in our study and also reported in literature24. However, Sidh Makardhwaj (10, 50 and 100 mg/kg), admininistered orally for 28 days did not affect cognitive and motor function in rats.
Acetylcholine, acetyl cholinesterase (AChE) and choline acetyltransferase (ChAT) are involved in cognition function and motor activity. several studies have shown decreased ChAT and AChE activity after mercury exposure242526 which could be the reason for impairment in cognition function and motor control. In our study, decreased acetylcholinesterase activity was found in hippocampus and frontal cortex in mercuric chloride treated group while Sidh Makardhwaj treatment for 28 days did not cause significant change in rat frontal cortex and hippocampus AChE activity as compared normal control group.
Mercuric chloride administration reduces renal and hepatic GSH content and increases lipid peroxide formation2728. The results of the present study were in conformity with the earlier studies which showed that mercuric chloride caused oxidative stress in brain cerebrum, liver and kidney. However, Sidh Makardhwaj (10, 50 and 100 mg/kg) did not affect brain cerebrum's MDA and GSH levels indicating that Sidh Makardhwaj in therapeutic doses does not cause oxidative stress.
There is evidence that chronic exposure to low concentration of mercury causes tissue or organ damage29. In the present study, there was significant increase in brain cerebrum, liver and kidney mercury levels at all doses of Sidh Makardhwaj (10, 50 and 100 mg/kg) as compared to normal control group. However, levels of mercury in brain cerebrum, liver and kidney were significantly lower as compared to mercuric chloride treated group.
Several studies have shown significant elevations in serum ALT, AST, ALP, bilirubin due to mercury exposure19282930. In the present study also rats exposed to mercuric chloride showed elevated levels. However, no significant change in serum ALT, AST, ALP, and bilirubin was observed in Sidh Makardhwaj treated groups.
Kidney damage is indicated by elevated serum urea and creatinine levels. Mercuric chloride treatment has been shown to cause a significant increase in serum creatinine and serum urea nitrogen indicating an impaired renal function31. In our study, Sidh Makardhwaj administered orally for 28 days did not cause nephrotoxicity in rats.
Inorganic mercury (mercuric chloride) has been shown to accumulate in the renal cortex and affect the morphology and function of the proximal tubules32. Jadhav et al33 have observed dose-dependent vascular, degenerative and necrotic changes in the brain cerebrum and liver of male rats exposed to mercury via drinking water. In the present study, congestion of blood vessels, neuronal degeneration, necrosis of hepatic cells, cellular necrosis involving primarily the pars recta of proximal tubules was observed at higher dose of Sidh Makardhwaj (100 mg/kg) and mercuric chloride treated group. normal architecture of brain cerebrum, liver and kidney was seen at lower dose of Sidh Makardhwaj (10 and 50 mg/kg).
Mercury sulphide is one of the ingredients of many traditional Ayurvedic medicines. Liu et al34 have reported that cinnabar is chemically inert with a relatively low toxic potential when taken orally. In risk assessment, cinnabar is less toxic than many other forms of mercury. There are many studies showing the safety of cinnabar and mercury sulphide which could be the reason for non toxic nature of Sidh Makardhwaj213435.
In conclusion, the findings of the present study suggest that Sidh Makardhwaj in the doses equivalent to human dose given for 28 days does not have any adverse effects on brain cerebrum, liver and kidney. Importantly, there were no changes in biochemical parameters at therapeutic dose. The histopathological examination also showed normal cytoarchitecture of brain cerebrum, liver and kidney ruling out its toxic potential at the therapeutic dose levels. However, mild histopathological changes were observed at higher dose (10 times of therapeutic dose) of Sidh Makardhwaj.
Acknowledgment
Author thank Dr. A. K. Dinda, Professor, Department of Pathology, All India Institute of Medical Sciences, New Delhi for his suggestions and expertise with histopathology. The financial support by Central Council for Research in Ayurveda and Sidha (CCRAS), Department of AYUSH, Ministry of Health and Family Welfare, Government of India, New Delhi for this research work is duly acknowledged (F. No. Z31014/04/2009/EMR-CCRAS).
Conflict of Interest: The authors declare no conflicts of interest.
References
- Ayurvedic Formulary of India, Part I & II. New Delhi: Department of AYUSH, Ministry of Health & Family Welfare, Government of India; 2005.
- [Google Scholar]
- Some observations on the metal-based preparations in the Indian Systems of Medicine. Indian J Traditional Knowledge. 2010;9:562-75.
- [Google Scholar]
- Ayurvediya rasashastra. Varanasi, India: Chaukhabha, Surbharati Prakashan; 2005. p. :84.
- [Google Scholar]
- Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci. 2002;23:136-9.
- [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), July. 2005. Available from: http://www.fda.gov/downloads /Drugs/Guidances/UCM078932.pdf
- [Google Scholar]
- Effects of intra cerebru ventricular injection of AF64A on learning behaviors in rats. Jpn J Pharmacol. 1988;48:121-30.
- [Google Scholar]
- Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47-60.
- [Google Scholar]
- Evaluation of learning and memory mechanisms employing elevated plus-maze in rate and mice. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:117-25.
- [Google Scholar]
- Correlation between motor impairment and infract volume after permanent and transient middle cerebral artey occlusion in the rat. Stroke. 1997;28:2060-6.
- [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88-95.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-8.
- [Google Scholar]
- Theory and practice of histological techniques. (4th ed). New York: Churchill Livingstone; 1996. p. :111.
- [Google Scholar]
- Mercury: Metal and their compound in environment. New York: VCH Publisher Inc; 1991. p. :1045-8.
- [Google Scholar]
- Comparative evaluation of the protective effect of selenium and garlic against liver and kidney damage induced by mercury chloride in the rats. Pharmacol Rep. 2008;60:199-208.
- [Google Scholar]
- U.S. Environmental Protection. Water quality criterion for the protection of human health: methylmercury. Washington: U.S. Environmental Protection Agency, Office of Science and Technology, Office of Water; EPA-823-R-01-001 January; 2001.
- [Google Scholar]
- Toxic effects of mercuric sulfide on immune organs in mice. Immunopharmacol Immunotoxicol. 2010;32:277-83.
- [Google Scholar]
- Effects of low dose methylmercury administration during the postnatal brain growth spurt in rats. Neurotoxicol Teratol. 2007;29:282-7.
- [Google Scholar]
- Cognitive deficits and changes in gene expression of NMDA receptors after prenatal methylmercury exposure. Environ Health Perspect. 2002;110(Suppl 5):855-8.
- [Google Scholar]
- ATSDR. Toxicological Profile for Mercury (update) USA: Agency for Toxic Substances and Disease Registry (ATSDR) Atlanta; 1999. p. :1-485.
- [Google Scholar]
- Effect of mercury compounds on cholineacetyl transferase. Res Commun Chem Pathol Pharmacol. 1980;30:381-4.
- [Google Scholar]
- Do metals inhibit acetylcholinesterase (AChE). Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity? Biomarkers. 2005;10:360-75.
- [Google Scholar]
- Renal oxidant injury and oxidant response induced by mercury. Kidney Int. 1996;50:1032-43.
- [Google Scholar]
- Hepatoprotective effects of taurine against mercury induced toxicity in rats. J Environ Biol. 2007;28:753-6.
- [Google Scholar]
- Mercuric compounds inhibit human monocyte function by inducing apoptosis: evidence for formation of reactive oxygen species, development of mitochondrial membrane permeability transition and loss of reductive reserve. Toxicology. 1997;124:211-24.
- [Google Scholar]
- Spirulina fusiformis: A food supplement against mercury induced hepatic toxicity. J Health Sci. 2005;51:424-30.
- [Google Scholar]
- Effect of mercuric chloride various hydroxyproline fractions in rat serum. Mol Cell Biochem. 2005;271:159-65.
- [Google Scholar]
- Histopathology of preclinical toxicity studies: interpretation and relevance in drug safety evaluation. (2nd ed). Amsterdam: Elsevier Science; 2000.
- [Google Scholar]
- Induction of oxidative stress in erythrocytes of male rats subchronically exposed to a mixture of eight metals found as groundwater contaminants in different parts of India. Arch Environ Contam Toxicol. 2007;52:145-51.
- [Google Scholar]
- Mercury in traditional medicines: is cinnabar toxicologically similar to common mercurials? Exp Biol Med (Maywood). 2008;233:810-7.
- [Google Scholar]
- Realgar, cinnabar and An-Gong-Niu-Huang Wan are much less chronically nephrotoxic than common arsenicals and mercurials. Exp Biol Med (Maywood). 2011;236:233-9.
- [Google Scholar]






