Translate this page into:
Role of secretory phospholipase A2 in women with metabolic syndrome
Reprint requests: Dr Pop Dana, Bihorului 5 Str, Cluj-Napoca, Romania e-mail: pop7dana@yahoo.com, liana_stanca@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Secretory phospholipase A2 (sPLA2), a member of the phospholipase A2 superfamily of enzymes that hydrolyses phospholipids, is a potentially useful plasma biomarker for atherosclerotic cardiovascular disease. Cardiovascular diseases are the leading cause of mortality in women. The purpose of this study was to investigate the correlation between cardiovascular risk factors and the sPLA2 levels in women with metabolic syndrome as compared to women without metabolic syndrome and men with metabolic syndrome.
Methods:
Patients (n=100) with various cardiovascular risk factors consecutively evaluated at the Rehabilitation Hospital-Cardiology Department, Cluj-Napoca, Romania were enrolled during 2011, of whom 10 were excluded. The patients were divided in three groups: group 1 (37 women with metabolic syndrome), group 2 (27 men with metabolic syndrome), and group 3 (26 women without metabolic syndrome). Body weight, smoking habits, glycaemia, hypertension, and serum lipids fractions were analysed as cardiovascular factors. Serum sPLA2 activity was measured using the chromogenic method.
Results:
There were no statistically significant correlations between sPLA2 levels and the investigated risk factors, irrespective of patient groups. However, there were significant positive correlations between sPLA2 and hsCRP in all three groups (P<0.05). In women with no metabolic syndrome an negative correlation was found between sPLA2 levels and HDL-C- r=-0.419, P=0.03. In men with metabolic syndrome there was a direct correlation between sPLA2 levels and HOMA, r=0.43, P<0.05, 95% CI (-0.098; 1.15).
Interpretation & conclusions:
Women with metabolic syndrome did not display different sPLA2 levels as compared to men with metabolic syndrome and women without metabolic syndrome. However, women with metabolic syndrome demonstrated a low but positive correlation between sPLA2 and hsCRP levels.
Keywords
Cardiovascular risk factors
metabolic syndrome
sPLA2
Cardiovascular diseases are the leading cause of death in women1; in Europe almost 55 per cent of mortality in women is caused by cardiovascular diseases, chiefly coronary disease and stroke2. In this context, the present research takes into special consideration the novel markers of cardiovascular risk3, under the circumstances in which cardiovascular risk factors and charts downplay cardiovascular risk in women. Moreover, endothelial dysfunction and inflammation play a major role in the pathogenesis of microvascular angina, common among women4. The respective markers may be divided into three categories: inflammation, haemostasis, and other factors. It is well known that lipoprotein (a) is highly atherothrombotic, and its elevated levels increase the risk of cardiovascular disease5 and represent an independent risk factor for coronary disease6. The lipoprotein (a) is shown to be correlated to the progression, extension, and severity of the coronary disease, as well as to a negative prognostic after myocardial infarction6. There is evidence that its levels increase with age in women6. Elevated levels in women indicate an independent atherogenic risk factor both in pre- and postmenopausal women7. It has been shown that high lipoprotein levels in women are associated with an increasing risk of cerebrovascular accident (CVA)8. There are studies which show that secretory phospholipase A2 (sPLA2) levels are more elevated in women than in men, along with an increase in C-reactive protein (CRP) levels9.
There is evidence that the sPLA2 hydrolyses phospholipids, generating free fatty acids and lisophospholipides, which lead to an increase of proinflammatory factors in the arterial wall10. These have a chemotactic and oxidative effect on the smooth muscular arterial cells and on the monocytes/macophages. sPLA2 also determines the generation of platelet-activating factor (PAF), with proatherogenic and procoagulant role10.
The present study was undertaken to find out the relationship between sPLA2 levels and cardiovascular risk factors in women with metabolic syndrome (MS) and compare with women without MS and men with MS.
Material & Methods
A total of 100 patients with various cardiovascular risk factors consecutively evaluated at the Rehabilitation Hospital-Cardiology Department, Cluj-Napoca, Romania, were in retrospectively included in the study, of whom 10 were excluded because some of the laboratory parameters were not determined accurately. Patients with ischaemic heart disease were also excluded. They were divided in three groups: group 1 (37 women with metabolic syndrome), group 2 (27 men with metabolic syndrome), and group 3 (26 women without metabolic syndrome). The study was carried out in 2011. As cardiovascular risk factors body weight, smoking habits, glycaemia, hypertension, serum lipids fractions and high sensitivity C-reactive protein (hsCRP) were analysed. Blood pressure was measured according to standard protocol as the mean of two readings after the participant was at rest for 5 min in a sitting position. The metabolic syndrome was defined according to the criteria of the International Diabetes Federation11. Body mass index (BMI) (kg/m2) was derived from height and weight measured in the clinic with participants wearing light clothing and no shoes. Blood samples (10 ml) were obtained by venipuncture according to the standard Lipid Research Clinics Protocol12. Low-density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald formula13. The homeostasis model assessment (HOMA-IR) was used to estimate insulin resistance [(HOMA-IR (Insulin resistance) (mmol/LxμU/ml) = fasting glucose (mmol/l) X fasting insulin (μU/ml)/405]. Plasma glucose levels were measured by the glucose oxidase method14. The hsCRP was analyzed by chemiluminescent immunometric assay (IMMULITE 2000)15. sPLA2 levels were measured by Cayman's secretory PLA2 (sPLA2) assay kit (Cayman Chemical Company, Ann Arbor MI, USA). The detection range of the assay was from 0.02 to 0.2 μU/min/ml of sPLA activity, which was equivalent to an absorbance increase of 0.01 to 0.1/min.
Arterial stiffness (pulse wave velocity) was measured using TensioMedTMArteriograph (KFT, Hungary). The study protocol was approved by the ethics committee of University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Statistical analysis: Microsoft Excel 2007 (v.6.0: Microsoft Corporation, Redmond, WA), Epiinfo 2000 (v.6.0, EpiInfo Development team CDC, GA, Atlanta) and SPSS 13 (v.13.0; IBM Corporation, Armonk, NY, USA) for Windows were used for data analysis. The data were presented as mean ± 1 SD or percentages when appropriate. First, the data normality was tested by applying the Kolmogorov-Smirnov test. Then, in agreement with the results, either Levene's test for equality of variances or Bartlett's chi square test was applied. In the former case, the results led to the application of the independent samples t test- equal variances. In Bartlett's chi square test, Mann-Whitney (Wilcoxon Rank Sum) or the Student t-test was applied. In the case of dichotomous variables, depending on the context, either Fisher or chi-square test was applied.
Results
Male patients with metabolic syndrome displayed a significantly elevated glycaemia than women with MS (P<0.01). On the contrary, women without MS had higher levels of total cholesterol and LDL-C (P=0.01, P<0.05). HDL-C was also more elevated in women with MS, (P<0.01) (Table).
In the remaining cardiovascular risk factors, there were no significant differences between the sexes. There were no significant differences in sPLA2 levels among between the three groups of patients (Table).
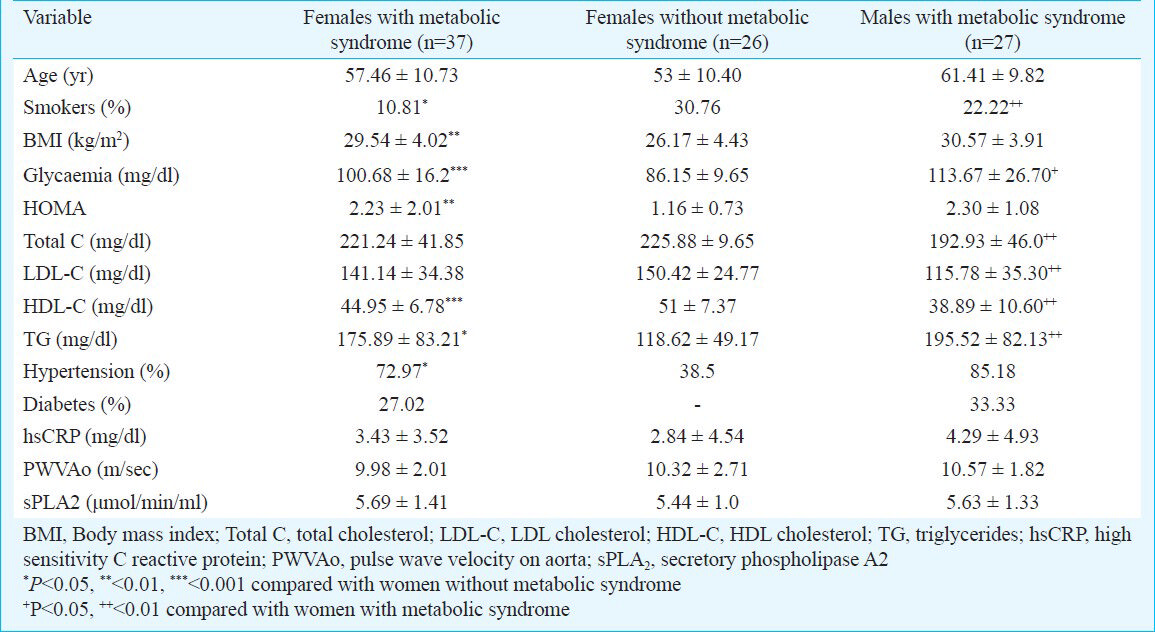
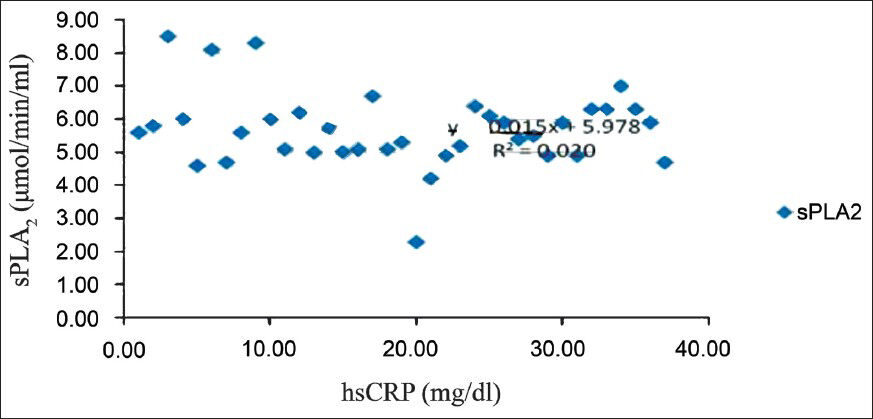
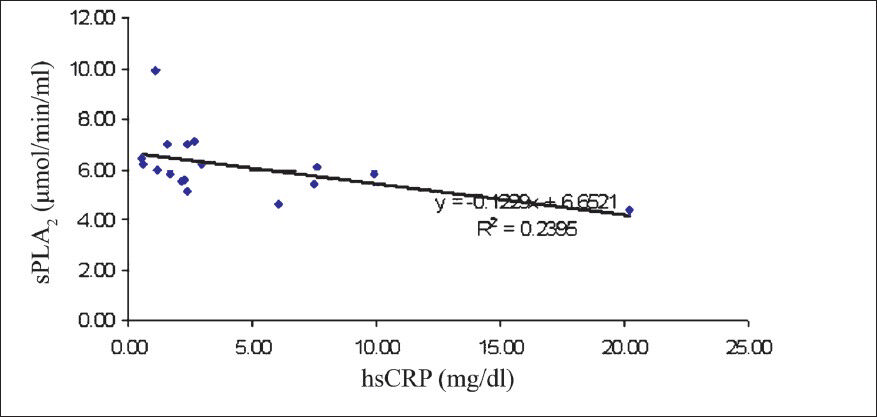
To assess whether there were correlations between cardiovascular risk factors, and sPLA2 the analyses were done. In general, there were no significant correlations between the sPLA2 levels and either of the studied risk factors, irrespective of the group of patients under examination. However, in women with metabolic syndrome there was a low positive correlation (r=0.141, P<0.05) between sPLA2 levels and hsCRP (Fig. 1). But the same correlation appeared to be present in women with no metabolic syndrome also (Spearman coefficient rho=-0.243, P<0.05). A significant correlation between sPLA2 and hsCRP was also found in men with metabolic syndrome (-r=0.39, (P<0.05) (Fig. 2). At the same time, the female patients without metabolic syndrome displayed a significantly inverse correlation between sPLA2 and HDL-C- r=-0.419, P<0.05 (Fig. 3). In men with metabolic syndrome there was a direct relationship between sPLA2 and HOMA, r=0.43, P<0.05) (Fig. 4).
- Correlation between sPLA2 (μmol/min/ml) and hsCRP (mg/dl) in women with metabolic syndrome.
- Correlation between sPLA2 (μmol/min/ml) and hsCRP (mg/dl) in men with metabolic syndrome.
- Correlation between sPLA2 (μmol/min/ml) and HDL-C (mg/dl) in women without metabolic syndrome.
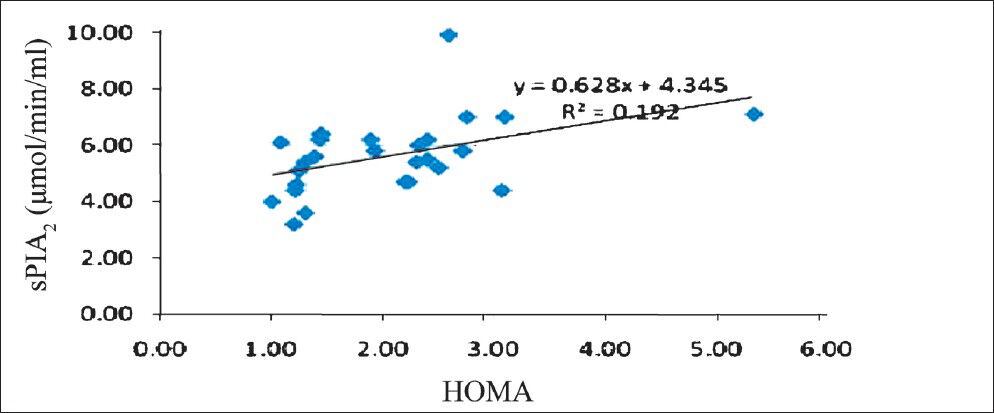
- Correlation between sPLA2 (μmol/min/ml) and HOMA in men with metabolic syndrome.
Discussion
Traditional risk factors can predict the risk of cardiovascular disease in many, but not in all, patients. About 10 to 20 per cent patients with coronary heart disease display no identifiable risk factor16. Many studies have established that lipoprotein associated phospholipase A2 (Lp-PLA2) is a cardiovascular marker independent of but correlated and augmentative to established risk factors17. Lp-PLA2 is produced by the inflammatory cells involved in atherogenesis and is accumulated in atherosclerotic lesions. It is well known that about 70 - 80 per cent of the Lp-PLA2 circulates in blood bound to LDL, and the rest to Lp(a), HDL cholesterol, and a very small quantity to VLDL18.
Secretory phospholipase A2 (sPLA2), a member of the phospholipase A2 superfamily of enzymes, is a potentially useful plasma biomarker for atherosclerotic cardiovascular disease. The 2010 ACCF/AHA (American College of Cardiology Foundation / American Heart Association) Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults19 recommends that Lp-PLA2 might be reasonable for cardiovascular risk assessment in intermediate-risk asymptomatic adults (IIbB). Gong et al20 found increased plasma levels of Lp-PLA2 in patients with metabolic syndrome.
There are five major classes of PLA2: the secreted and cytosolic forms, the calcium-independent PLA2s, the lysosomal PLA2s and the platelet-activating factor acetylhydrolases (PAF-AH, which include Lp-PLA2 and are also calcium independent)10. sPLA2 is also involved in atherogenesis in several ways: it changes the affinity of LDL particles for extracellular matrix proteins212223, with their accumulation in the arterial walls, favours lipid peroxidation24 and mediates the hydrolysis of certain lysophospholipids and free fatty acids25. Circulating levels of sPLA2 are higher in patients with documented CAD than in controls26.
We found an inverse correlation between sPLA2 levels and HDL cholesterol levels in women without metabolic syndrome. An increase in HDL-C levels has been shown to be accompanied by a decline in the levels of inflammatory factors27. An increase in the sPLA2 activity determines the hydrolysis of HDL-C particles both within and without the context of inflammatory processes2829. sPLA2 may determine changes in the HDL-C levels that can eventually upset the beneficial mechanisms involved in the macrophage cholesterol efflux29.
An association was seen between sPLA2 and CRP levels, which represents both acute-phase proteins and markers of vascular inflammation, in patients with and without metabolic syndrome. Koenig et al30 also found a significant direct correlation between the levels of sPLA2 type IIA and CRP. Another study conducted in patients with insulin resistance, documented correlations between sPLA2 and CRP, as well as interleukin (IL)-6 and soluble adhesion molecules31. The question arises whether sPLA2 also plays a part in the pathogenesis of metabolic syndrome. Ravaux et al32 have shown that the ligands of all three peroxisome proliferator activated receptors (PPARs) in the vascular smooth muscle may determine a decrease in the sPLA2 expression. Results from UDACS study demonstrated that in patients with type 2 diabetes mellitus, changes in the PLA2G2A genes might alter sPLA2 levels33.
Pulse wave velocity on aorta (PWVAo) is deemed to be an investigation that may contribute in assessing individual cardiovascular risk, but is still not used on a large scale. No significant correlation was recorded between sPLA2 and PWVAo in the present study. Men with metabolic syndrome showed higher values of both sPLA2 and PWVAo than women with MS.
In conclusion, women with metabolic syndrome did not display different sPLA2 values as compared to men with metabolic syndrome and women without metabolic syndrome, irrespective of traditional cardiovascular risk factors taken into account. However, sPLA2 levels increased with the increase in hsCRP levels in women with MS.
Acknowledgment
This study was conducted within the framework of Research Project No. 947, ID_2246/ 2009 Code, part of PN II Program funded by the Romanian Ministry of Education, Research and Innovation-The National University Research Council.
References
- Effectiveness-Based guidelines for the prevention of cardiovascular disease in women - 2011 update. Circulation. 2011;123:1243-62.
- [Google Scholar]
- Cardiovascular diseases in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J. 2006;27:994-1005.
- [Google Scholar]
- Multimarker Prediction of Coronary Heart Disease Risk: The Women's Health Initiative. J Am Coll Cardiol. 2010;55:2080-91.
- [Google Scholar]
- Cardiovascular disease in women. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald's heart disease - A textbook of cardiovascular medicine (9th ed). Philadelphia: Elsevier; 2011. p. :1757-69.
- [Google Scholar]
- A comprehensive view of sex-specific issues related to cardiovascular disease. CMAJ. 2007;176:S1-41.
- [Google Scholar]
- Changes in traditional risk factors no longer explain time trends in cardiovascular mortality and its socioeconomic differences. J Epidemiol Community Health. 2008;62:251-7.
- [Google Scholar]
- Lipoprotein-associated phospholipase A 2 , hormone use, and the risk of ischemic stroke in postmenopausal women. Hypertension. 2008;51:1115-22.
- [Google Scholar]
- Inflammatory biomarkers and the prediction of coronary events among people at intermediate risk: the EPIC-Norfolk Prospective Population Study. Heart. 2009;95:1682.
- [Google Scholar]
- Phospholipase A2s: Developing drug targets for atherosclerosis. Atherosclerosis. 2010;2:357-66.
- [Google Scholar]
- The usefulness of the International Diabetes Federation and the National Cholesterol Education Program's Adult Treatment Panel III definitions of the metabolic syndrome in predicting coronary heart disease in subjects with type 2 diabetes. Diabetes Care. 2007;30:1206-11.
- [Google Scholar]
- The Lipid Research Clinics Coronary Primary Prevention Trial Results I. Reduction in Incidence of Coronary Heart Disease. JAMA. 1984;251:351-64.
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative centrifuge. Clin Chem. 1972;18:499-500.
- [Google Scholar]
- Evaluation of Trinder's glucose oxidase method for measuring glucose in serum and urine. Clin Chem. 1975;21:1754-60.
- [Google Scholar]
- Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898-904.
- [Google Scholar]
- Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82:159-65.
- [Google Scholar]
- Lipoprotein-associated phospholipase A2: pathogenic mechanism and clinical utility for predicting cardiovascular events. Curr Atheroscler Rep. 2006;8:374-81.
- [Google Scholar]
- 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-103.
- [Google Scholar]
- Plasma lipoprotein-associated phospholipase A2 in patients with metabolic syndrome and carotid atherosclerosis. Lipids Health Dis. 2011;19:10:13.
- [Google Scholar]
- Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 1998;139:205-22.
- [Google Scholar]
- Phospholipase A(2) modification of low density lipoproteins forms small high density particles with increased affinity for proteoglycans and glycosaminoglycans. J Biol Chem. 1999;274:25913-20.
- [Google Scholar]
- Lipolysis of LDL by human secretory phospholipase A(2) induces particle fusion and enhances the retention of LDL to human aortic proteoglycans. Arterioscler Thromb Vasc Biol. 2001;21:1053-8.
- [Google Scholar]
- Secretory phospholipase A2 and lipoprotein lipase enhance 15-lipoxygenase-induced enzymic and nonenzymic lipid peroxidation in low-density lipoproteins. Biochemistry. 1998;37:9203-10.
- [Google Scholar]
- Modification of LDL with human secretory phospholipase A2 or sphingomyelinase promotes its arachidonic acid-releasing propensity. J Lipid Res. 2004;45:831-8.
- [Google Scholar]
- Prognostic value and the changes of plasma levels of secretory type II phospholipase A2 in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur Heart J. 2003;24:1824-32.
- [Google Scholar]
- High-density lipoproteins neutralize C-reactive protein proinflammatory activity. Circulation. 2004;109:2116-22.
- [Google Scholar]
- Lipoproteins are substrates for human secretory group IIA phospholipase A2: preferential hydrolysis of acute phase HDL. J Lipid Res. 1998;39:2150-60.
- [Google Scholar]
- Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J Biol Chem. 2000;275:10077-84.
- [Google Scholar]
- Association between type II secretory phospholipase A2 plasma concentrations and activity and cardiovascular events in patients with coronary heart disease. Eur Heart J. 2009;30:2742-8.
- [Google Scholar]
- The proinflammatory mediator platelet activating factor is an effective substrate for human group X secreted phospholipase A2. Biomed Biochem Acta. 2006;1761:1093-9.
- [Google Scholar]
- Inhibition of interleukin-1beta-induced group IIA secretory phospholipase A2 expression by peroxisome proliferator-activated receptors (PPARs) in rat vascular smooth muscle cells: cooperation between PPARbeta and the proto-oncogene BCL-6. Mol Cell Biol. 2007;27:8374-87.
- [Google Scholar]
- Tagging-SNP haplotype analysis of the secretory PLA2IIa gene PLA2G2A shows strong association with serum levels of sPLA2-IIa:results from the UDACS study. Hum Mol Genet. 2006;15:355-61.
- [Google Scholar]






