Translate this page into:
Role of homocysteine & MTHFR C677T gene polymorphism as risk factors for coronary artery disease in young Indians
Reprint requests: Dr Santosh Kumar Gupta, C-1/29, 1st Floor, Besides SL Sharma Open University, Sector 2, Devendra Nagar, Raipur (CG) 492 001, India, e-mail: maj_sgupta@yahoo.co.in
-
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Hyperhomocysteinaemia (HCA) either due to mutation of MTHFR gene or deficiency of vitamin B12 and folic acid, has been reported as a risk factor for coronary artery disease (CAD). The present study was aimed to determine plasma homocysteine (Hcy) levels and to evaluate MTHFR C677T gene polymorphism as risk factors for CAD, and to study the role of Hcy in conjunction with a few other risk factors of CAD in young Indians. The effect of vitamin B12 and folic acid supplements on the raised plasma Hcy levels in patients of CAD was also assessed.
Methods:
The present study included 199 consecutive angiography confirmed CAD patients, <45 yr of age, without any other known pro- coagulant state and 200 age- and sex-matched healthy controls. Fasting blood samples were collected in EDTA and plasma Hcy was estimated by ELISA test and the MTHFR C677T polymorphism detection was carried out by PCR-RFLP method.
Results:
Significant difference (P<0.001) was found between mean fasting levels of plasma Hcy in cases (22.14 ± 10.62 μmol/l) and controls (17.38 ± 8.46 μmol/l) with an Odds ratio as 1.93 (95% CI, 1.27-2.94). Levels of cholesterol, LDL, and triglycerides were significantly (P<0.001) higher in cases compared with controls.
Interpretation & conclusions:
Our study showed significant correlation between hyperhomocysteinaemia and coronary artery disease. Multivariate analysis by logistic regression of the various risk factors of CAD, found high levels of Hcy, cholesterol, LDL and low levels of HDL and smoking as independent predictors of CAD when all other factors were controlled. Significant post-treatment decrease found in HCA was easily modifiable by vitamin intervention irrespective to their CT or TT genotype of C677T MTHFR gene. Further studies to look at the plasma levels of folate and cobalamines and their association with Hcy are required to be done.
Keywords
CAD - case-control
gene polymorphism
homocysteine
MTHFR
risk factors
Coronary artery disease (CAD) due to atherosclerosis is associated with increased mortality and morbidity. Various risk factors have been found to be associated with the development of CAD. The role of diabetes mellitus, smoking and hyperlipidaemia as risk factors for CAD is well established.
Hyperhomocysteinemia (HCA) known to be associated with increased thrombotic tendency has been considered as a risk factor for coronary artery disease and atherosclerosis1 whereas others2 have found no association between acute myocardial infarction (MI) and HCA. Mild to moderate HCA is known to be due to genetic factors like mutation in methylene tetrahydrofolate reductase (MTHFR) genes or due to environmental factors like deficiency of vitamin B12 or folic acid. The presence of the C677T mutation is known to correlate with increased MTHFR thermolability and homozygotes for the C677T allele are predisposed to HCA in context to suboptimal folate status3. Vitamin intervention is known to bring down levels of homocysteine (Hcy), an endothelial toxin, which has been implicated in pathogenesis of CAD1. A meta-analysis showed a significant association between plasma Hcy (normal range 5-15 μmol/l) and MTHFR gene polymorphism with ischaemic heart disease, stroke, deep vein thrombosis and pulmonary embolism4. The odds ratios for a 5 μmol/l increase in Hcy were 1.42 in 72 case-control and 1.32 in 20 prospective studies of CAD4.
Folate levels are reported to be lower in patients with MI and CAD than in control subjects1. There are some reports on prevention of arterial disease recurrence or progression by prophylactic doses of folic acid or cobalamin. In one study, combined folic acid, cobalamin and pyridoxine daily for 1 year was associated with decreased rate of restenosis of coronary arteries compared with placebo in patients who had undergone angioplasty and stenting5. A meta analysis of 80 studies6 found a 14 per cent greater risk of CAD in association with HCA in individuals, irrespective of their genotype. In Europe and North America, no association of Hcy with the MTHFR C677T mutation was observed in contrast to the regions of Middle East and Japan6. However, a recent meta analysis by Clarke et al7 studied 19 unpublished data sets and found only 2 per cent increase risk of CAD in TT homozygotes as compared to CC Homozygotes. In contrast the meta- analysis of 86 published studies showed of the association of the polymorphism with CAD, the excess CAD risk in TT Homozygotes as compared to CC Homozygotes was 15 per cent. Authors suggested the reason of discrepancy between published and unpublished studies could be due to publication bias. They also found that there is no significant effect of folate supplementation on 5 year incidence of CAD7.
The frequency of the MTHFR C677T mutation is shown to vary amongst different populations8. The few Indian studies available provide conflicting results. Four studies from south India found no association between Hcy and CAD9–12 whereas a study from UK13 and some from India1415 found HCA to be correlated to CAD in Indians. A study from Mumbai16 found no difference in Hcy levels in cases of CAD and controls but heterozygosity of the MTHFR mutation in 54.5 per cent patients of CAD with HCA, thereby implying that genetic predisposition to HCA could be a risk factor for CAD in the presence of folate deficiency.
There is paucity of data on prevalence of these deficiencies in Indians with one study finding 33 per cent of the population in India to have low folate levels17 and another, carried out in Pune, found evidence of cobalamine deficiency in 47 per cent and HCA in 77 per cent of the urban population18. Thus, the present study was carried out to evaluate plasma Hcy levels and MTHFR C677T polymorphism as risk factors for CAD and to study the role of Hcy in conjunction with a few other risk factors of CAD. The secondary objective was to assess the effect of vitamin B12 and folic acid supplements on the raised plasma Hcy levels in patients of CAD.
Material & Methods
This study was conducted at the Army Hospital (Research and Referral), New Delhi from September 2005 to September 2008. The cases (n=199) were consecutively selected from Cardiology OPD and Ward/CCU and all of them were angiography confirmed CAD patients, <45 yr of age, without any known pro-coagulant state. Controls (n=200) were age- and sex-matched healthy serving soldiers of Indian Army who came to hospital for annual medical check up. Those who successfully completed basic physical efficiency test (BPET) stress test during their physical training were included in study. Clinical details of cases were obtained after examination in consultation with attending cardiologist. Thrombophilia workup was carried out in all patients so as to rule out the other causes of CAD. Thrombophilia workup included complete blood count (CBC) with platelets, prothrombin time (PT), activated partial thromboplastin time (APPT), Protein C and S, Factor V leiden mutation, fibrinogen assay and d-dimer. Plasma Hcy and C677T MTHFR gene polymorphism investigations were performed on the blood samples as per protocol. Details of type of cardiac problems along with angiography findings, blood pressure, lipid profile, history of smoking, hypertension, diabetes, etc. were also recorded. In addition, the cases were administered vitamin B12 (1000 μg/day) and folic acid (5 mg/day) therapy for 3 months in consultation with attending physician and investigations repeated after 3 months to assess their effect on Hcy levels. Informed consent was obtained from each individual. The protocol of present study was approved by ethics committee of Army Hospital (R&R), New Delhi.
Plasma Hcy was estimated by enzyme immunoassay using AXIS shield AS kits (Scotland). Fasting blood samples were collected in EDTA tubes and plasma was separated immediately within one hour and stored at -20°C till analysis. HCA refers to increase in plasma Hcy level above the normal range (5-15 μmol/l).
MTHFR assay by PCR: The point mutation at 677 position picked up by PCR by relevant probes was followed by restriction fragments length polymorphism (RFLP) using Hinf 1 enzyme19. Post digestion documentation was done on gel and TT, CT, CC genotypes were seen.
Sample size calculation and statistical analysis: By taking the proportion of cases having HCA to be around 60 per cent as compared to 25 per cent of controls. In order to estimate a difference of 25 per cent, at α = 0.05, a two tailed test of proportions required a sample size of 85 in each group for a power of 90 per cent. This sample size was also adequate to capture a difference of 0.3 μmoles/l of Hcy levels between the cases and controls (STATA ver 8, USA). The data were analyzed using SPSS ver 13.0.1. Measures of central tendency and dispersion were estimated and compared. Multivariate analysis with logistic regression was carried out and for statistical significance t test and Chi square were used.
Results
The mean age of cases (185 males, 14 females) was 37.4 ± 5.8 yr (range 22-45 yr) and that for controls (184 males, 16 females) was 36.35 ± 6.58 yr (range 28 to45 yr). The maximum number of cases n=97 (48.7%) was seen in age group 40-45 yr and least (n= 3.6%) in <25 yr. Majority of the cases (121 of 199, 61.1%) had single vessel disease, almost a quarter (n=54, 26.8%) double vessel and 24 (12.1%) had triple vessel disease.
The mean fasting levels of plasma Hcy in cases (22.14 ± 10.62 μmol/l) and controls (17.38 ± 8.46 μmol/l) showed a statistically significant difference (P<0.001). Using 18 μ mol/l as cut-off for Hcy levels, 111 controls and 78 cases had less than 18 μ mol/l and 89 controls and 121 cases had more than 18 μ mol/l Hcy levels (P<0.001). The odds ratio was 1.93 (95% CI, 1.27-2.94). Statistical analysis of comparison between males and females was not attempted due to inadequate sample of females (14 cases and 16 controls).
Table I shows the distribution of study subjects as per their C677T MTHFR genotype. Statistically significant difference in distribution of MTHFR gene in cases and controls was observed (P<0.05). Statistically significant difference (P<0.001) in Hcy levels was seen between cases and controls having CC and CT genotype. The numbers of cases with TT mutation were very inadequate to be analyzed statistically. Irrespective of genotype, the Hcy levels were higher in cases as compared to controls (P<0.001) (Table I).
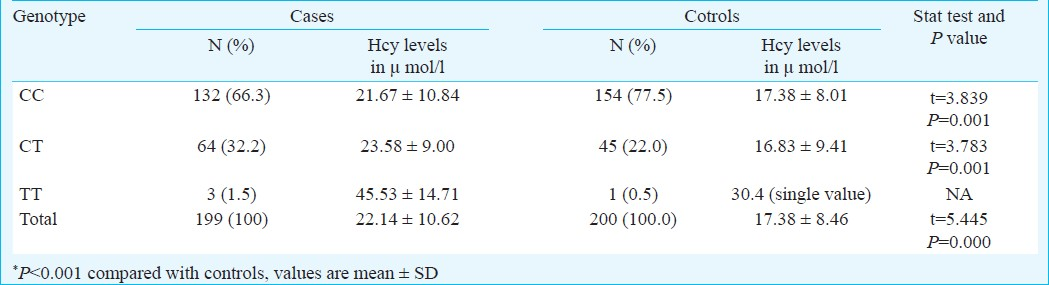
Association of smoking with Hcy was seen in controls (P<0.05) but not in cases of CAD where Hcy levels were high irrespective of smoking status (Table II). Table III shows the mean levels of lipid profile in the cases of CAD and controls. The difference was significant (P<0.001) for cholesterol, LDL and triglycerides and HDL levels when cases of CAD were compared with controls.
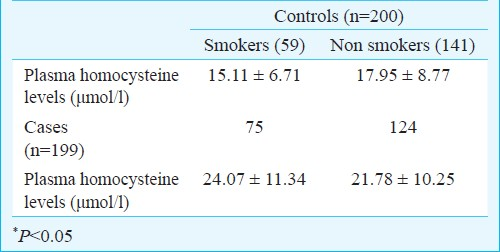
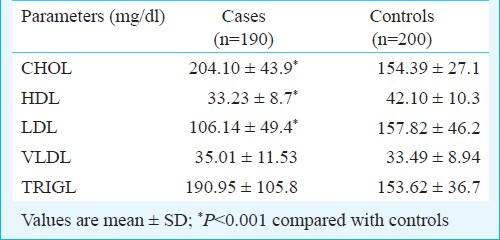
Taking cut-offs for lipid profile parameters as per the ATPIII guidelines20, univariate analysis was done. The cut-off for cholesterol, LDL showed a significant positive association and HDL a negative association with cases of CAD. The cut-off of triglycerides >150 mg/dl gave a non significant association. Thus analysis using the high value of >199 mg/dl was carried out and showed a significant association (Table IV).
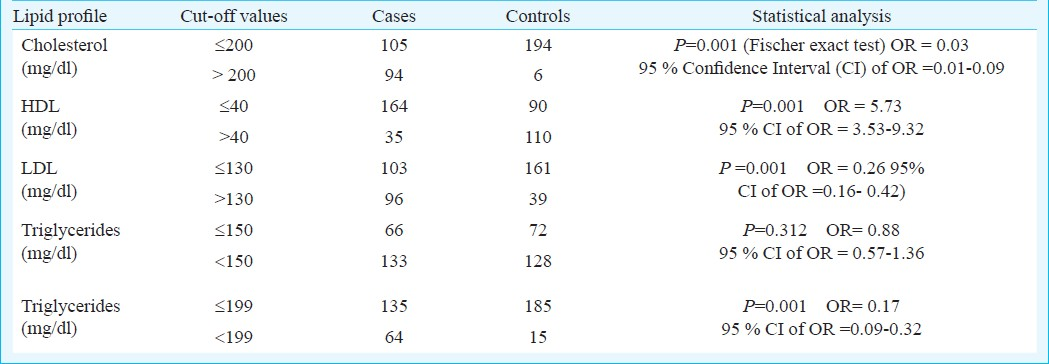
Table V shows logistic regression carried out for various risk factors found to be associated with CAD in univariate analysis. The variables included in the model were Hcy levels, cholesterol, HDL, LDL, triglycerides and smoking status. High levels of Hcy, cholesterol, LDL and smoking were found to be independent predictors of CAD when all other factors were controlled. Triglyceride levels with the cut-off of 150 mg/dl were not found to be significant independent predictor of CAD on multivariate analyses. HDL >40 mg/dl provided a significant protection to CAD. Age between 35 to 45 yr was an independent risk factor for CAD in young.

The mean post-treatment plasma Hcy levels decreased significantly (P<0.001) to 11.64 ± 5.52 μmol/l from pre-treatment levels of 22.14 ± 10.62 μmol/l after 3 months of treatment with 5 mg folic acid and 1000 μg of vitamin B12 per day. The regression analysis of pre- and post-treatment Hcy showed a high Pearson correlation coefficient (r= 0.618) and a high R2 (0.381) (P<0.001) (Fig.).
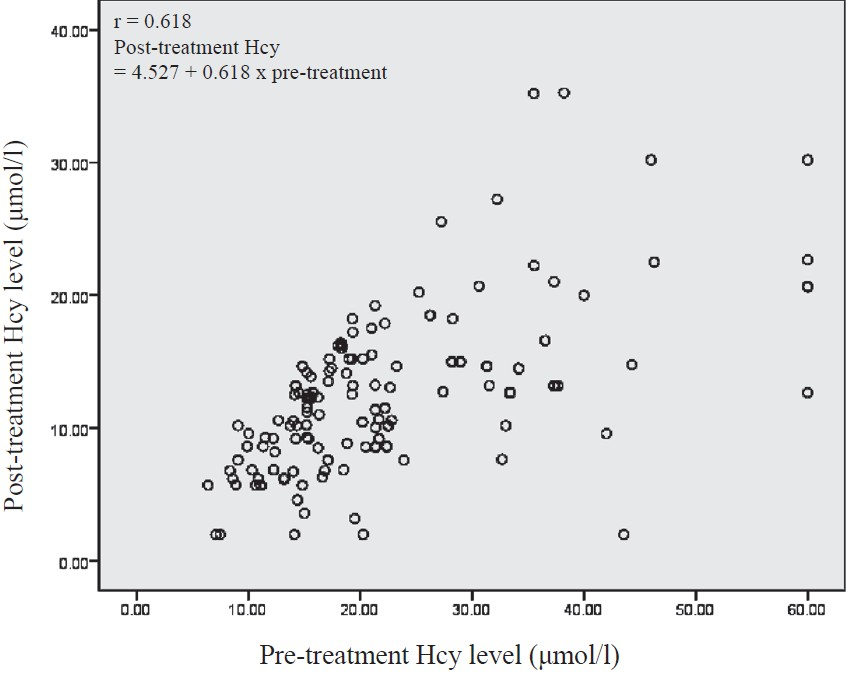
- Scatter diagram of homocysteine pre- and post-treatment with vitamin B12 and folic acid.
Discussion
Increase in Hcy concentrations with age has been reported in Spanish children age 2 months to 18 yr21 but was not seen in a group of healthy Canadian adults having mean age of 36 yr with range of 23-59 yr22. In our study, Hcy levels varied as per age with maximum being in 40 to 45 yr age group, amongst cases of CAD, however, no significant difference was seen amongst controls.
Our study showed an association of high Hcy level with CAD as supported by a few cross-sectional studies4. A large number of case-control studies have examined the association between HCA and CAD and two2324 reported significantly higher Hcy levels, either fasting or after methionine load, in persons with CAD as compared with persons without CAD.
Hcy levels depend upon the presence of homozygosity of the C677T mutation that is present in 5 to 20 per cent subjects of Caucasian descent. In these cases, with (TT) mutation higher Hcy has been observed1. However, studies from India found the prevalence of the gene mutation to be low in Indians1525. We also found very few homozygous individuals in the present study. Thus association with homozygocity (TT) could not be analysed. In our heterozygote (CT) cases and controls as well as wild homozygous (CC), no significant association was found between Hcy levels and the gene polymorphism. Thus our findings are at variance from some Indian studies9–12 which did not find association between Hcy levels and CAD but similar to other studies which found HCA correlated with CAD in Indians13–15. Our study is also at variance with a study from Mumbai which found MTHFR polymorphism to be an important risk factor for HCA and CAD16.
Smoking has been found to compound the modest effect of hypertension on plasma Hcy26. The strong relationship between blood pressure and Hcy that exists only in smokers suggests that smoking induced Hcy elevations may raise systolic blood pressure. In our study, we found significant association between smoking and Hcy levels in controls but not in cases of CAD implying that other factors may also affect Hcy levels in CAD.
Hsu et al27 found no association between the C677T MTHFR gene polymorphism and risk of CAD in Taiwan. A study on 24968 initially healthy American women found higher Hcy levels had significant association with incidents of CAD but no association with MTHFR genotype as found in our study28. A recent case-control study conducted on 121 patients having at least 50 per cent stenosis of one coronary artery compared with 155 healthy control found that MTHFR genotype had no association with the risk of CAD29. However, plasma Hcy (>12.5 μmol/l, OR-3.49; 95% CI, 1.23-9.88) had a significant association with increased risk of CAD similar to our study. A meta-analysis of MTHFR C677T polymorphism and CAD found no strong evidence to support the association of C677T gene polymorphism and CAD in Europe, North America or Australia6. This geographical variability may be attributed to higher folate intake in these countries.
A study showed that serum LDL cholesterol and triglyceride concentration were elevated and serum HDL levels were significantly lower in CAD patients than in controls and Hcy levels were significantly higher in CAD patients30. In our study, individuals with cholesterol >200 mg/dl, LDL >130 mg/dl and HDL <40 mg/dl had higher odds ratio for association with CAD, similar to other studies3132. Also, in Indians, triglyceride level >199 mg/dl may be a risk factor for CAD. Multivariate analysis by logistic regression of various risk factors of CAD found high levels of Hcy, cholesterol, high LDL and low levels of HDL and smoking as independent predictors of CAD when all other factors were controlled.
Dietary supplementation with folic acid and vitamin B12 can reduce elevated Hcy levels in most patients. Regardless of the causes of elevation, supplementation with one or more of these vitamins can lower plasma levels of Hcy4. Mean Hcy levels in our controls were much higher than the normal in the West and in comparison to other studies3334. Probably the dietary deficiency of vitamin B6,B12 and folic acid as seen in Indian population1718 plays a significant role. A significant post-treatment decrease in Hcy levels, in our study subjects, supports that supplementation of multivitamin has a definitive role in bringing down the level of Hcy in CAD cases as was evident in a meta-analysis4. Another meta-analysis6 found a strong evidence to support an association of the MTHFR C677T polymorphism and Hcy levels with CAD in Asia but not in Europe, North America and Australia. Thus, vitamin supplementation has a major role in reducing Hcy levels in patients of CAD irrespective of the cause. Further studies to assess the folate and cobalamine levels in Indian patients with CAD and its association with Hcy need to be carried out.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for the financial support.
References
- Hyperhomocysteinemia, atherosclerosis and thrombosis. Thromb Haemost. 1999;81:165-76.
- [Google Scholar]
- Relation of serum homocysteine and lipoprotein (a) concentrations to atherosclerotic disease in a prospective Finnish population based study. Atherosclerosis. 1994;106:9-19.
- [Google Scholar]
- Hyper homocysteinemia: A million ways to lose control. Arterioscler Thromb Basic Biol. 2003;23:371-3.
- [Google Scholar]
- Homocysteine and cardiovascular disease: evidence of causality from meta-analysis. BMJ. 2002;325:1202-6.
- [Google Scholar]
- Decrease rate of Coronary re-stenosis after lowering plasma homocysteine levels. N Engl J Med. 2001;345:1593-600.
- [Google Scholar]
- Meta-analysis of MTHFR 677 C→T polymorphism and coronary heart disease. Does totality of evidence support casual role for homocysteine and preventive potential of folate. BMJ. 2005;331:1053-70.
- [Google Scholar]
- Homocysteine and coronary heart disease: Meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9(2):e1001177.
- [Google Scholar]
- MTHFR association with arteriosclerotic vascular disease? Hum Genet. 1999;103:11-21.
- [Google Scholar]
- Methylene tetra hydrofolatate reductase gene polymorphism and hyperhomocysteinemia as risk factors for coronary heart disease in Indian population. J Assoc Physicians India. 2002;24:509-15.
- [Google Scholar]
- Absence of association between serum homocysteine levels and coronary artery disease in South Indian males. Indian Heart J. 2001;53:44-7.
- [Google Scholar]
- Fibrinogen and homocysteine levels in coronary heart disease. Indian Heart J. 1999;51:499-502.
- [Google Scholar]
- Plasma homocysteine levels in patients with coronary heart disease. Indian Heart J. 1998;50:295-9.
- [Google Scholar]
- Plasma homocysteine concentrations and risk of coronary heart disease in UK, Indian Asians and Europeans. Lancet. 2000;355:523-7.
- [Google Scholar]
- Homocysteine and lipid levels in young patients with coronary artery disease. J Assoc Physicians India. 2003;51:681-5.
- [Google Scholar]
- Combination of Thrombophilia markers in acute myocardial infarction. Indian J Med Sci. 2004;58:381-8.
- [Google Scholar]
- MTHFR gene mutation and hyperhomocysteinemia as risk factors in coronary heart disease in Indian population. J Assoc Physicians India. 2002;50:9-15.
- [Google Scholar]
- Homocysteine and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indian. Am J Clin Nutr. 2001;74:233-41.
- [Google Scholar]
- MTHFR gene C677T and A 1298C polymorphisms and homocysteine levels in primary open angle and primary closed angle glaucoma. Mol Vis. 2009;15:2268-78.
- [Google Scholar]
- NCEP ATP III guidelines incorporates global risk assessment. Am J Manag Care (Suppl):1. 3
- [Google Scholar]
- Plasma total homocysteine in healthy subjects; sex specific relation with biological traits. Am J Clin Nutr. 1996;64:587-93.
- [Google Scholar]
- Homozygocity for the C677→T mutation of 5, 10-methylene tetrahydrofolate reductase and total plasma homocysteine are not associated with greater than normal risk of a first myocardial infarction in Northern Sweden. Coron Artery Dis. 2001;12:85-90.
- [Google Scholar]
- Homocysteine and the methylene tetrahydrafolate reductase C677T allele in premature coronary artery disease.Case control and family studies. Eur J Clin Invest. 2001;31:24-30.
- [Google Scholar]
- Prevalence of methylene tetrahydrofolate polymorphism in South Indian population. Curr Sci. 2004;86:440-3.
- [Google Scholar]
- Relationship of systolic blood pressure with plasma homocysteine: importance smoking status. J Hypertens. 2003;21:1307-12.
- [Google Scholar]
- The C677T mutation of the methylene tetrahydrofolate reductase gene is not associated with the risk of coronary artery disease or venous thrombosis among Chinese in Taiwan. Hum Hered. 2001;51:41-5.
- [Google Scholar]
- Homocysteine, 5,10-methylene tetrehydrofolate reductase 677 C>T polymorphism, nutrient intake and incident cardiovascular disease in 24968 initially healthy women. Clin Chem. 2007;53:845-51.
- [Google Scholar]
- High plasma homocysteine is associated with the risk of coronary artery disease independent of methylene tetrahydrofolate reductase 677 C→T genes. Asia Pac J Clin Nutr. 2008;17:330-8.
- [Google Scholar]
- Plasma homocysteine levels and 677 C→T methylene tetrahydrofolate reductase gene polymorphism in patients with coronary artery disease of different severity. Indian J Med Res. 2008;127:154-8.
- [Google Scholar]
- MTHFR polymorphism (C667→ T) hyperhomocystenemia, and risk of ischaemic cardiovascular disease and venous thromboembolism: prospective and case control studies from Copenhagen City Heart Study. Blood. 2004;104:3028-37.
- [Google Scholar]
- Differences in risk factors, atherosclerosis and cardiovascular disease between ethnic groups in Canada, the study of Health Assessment and Risk in Ethnic Groups (SHARE) Indian Heart J. 2000;5(Suppl):S35-43.
- [Google Scholar]
- Homocysteinemia in daily practice: levels in coronary artery disease. Circulation. 1996;94:2154-8.
- [Google Scholar]
- Homocysteine and risk of premature coronary heart disease. Circulation. 1996;94:2154-8.
- [Google Scholar]






