Translate this page into:
Role of calcium &/or vitamin D supplementation in preventing osteoporotic fracture in the elderly: A systematic review & meta-analysis
For correspondence: Dr Manmeet Kaur, Department of Pathology, All India Institute of Medical Sciences, Mandi Dabwali Road, Bathinda 151 001, Punjab, India e-mail: puniamanmeet@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Calcium and vitamin D, separately or in combination are usually prescribed to prevent fragility fractures in elderly population. However, there are conflicting results regarding the ideal dosage and overall efficacy obtained from randomized controlled trials (RCTs) conducted in the past. The objective of this study was to assess the fracture risk with the administration of calcium or vitamin D alone or in combination in elderly population (>60 yr).
Methods:
PubMed, Cochrane and Embase databases were searched to identify the studies from inception to February 2021 with keywords, ‘vitamin D’, ‘calcium’ and ‘fracture’ to identify RCTs. The trials with comparing vitamin D, calcium or combination with either no medication or placebo were included for final analyses. The data were extracted and the study quality was assessed by two reviewers. The principal outcome measure was fractures around hip joint and secondary outcomes assessed were vertebral and any other fracture.
Results:
Eighteen RCTs were considered for the final analysis. Neither calcium nor vitamin D supplementation was associated with risk of fractures around hip joint [risk ratio (RR) 1.56; 95% confidence interval (CI), 0.91 to 2.69, I2=28%; P=0.11]. In addition, the combined administration of calcium and vitamin D was also not associated with fractures around the hip joint in comparison to either no treatment or placebo. The incidence of vertebral (RR 0.95; 95% CI, 0.82 to 1.10, I2=0%; P=0.49) or any other fracture (RR 0.83; 95% CI 0.65 to 1.06, I2=0%; P=0.14) was not significantly associated with the administration of calcium and vitamin D either individually or in combination. Further subgroup analysis of the results did not vary with the dosage of calcium or vitamin D, dietary calcium intake sex, or serum 25-hydroxyvitamin D levels.
Interpretation & conclusions:
The present meta-analysis of RCTs on calcium, vitamin D or a combination of the two in comparison to no treatment or placebo did not support the routine administration protocol of calcium and vitamin D either alone or in combination to lower the risk of fractures in elderly population.
Keywords
25-Hydroxyvitamin D
calcium
hip fracture
osteoporosis
risk
randomized controlled trials
vitamin D
With the rise in the elderly population worldwide, the incidence of osteoporotic fractures is increasing proportionately1. Reportedly, there is a probability that approximately 50 per cent women and 20 per cent men aged 50 yr suffer an osteoporotic fracture in their remaining lifetime2,3. Hip fracture is one among the most serious types of fragility fractures with approximately one third risk of death in the subsequent year4. The survivors require good nursing and social care which translates into a major social and economic burden5. Hence, the thrust should be on prevention of these fractures. Common practice is to recommend combined calcium and vitamin D supplements to patients after an osteoporotic fracture to prevent chances of the same in the future. Vitamin D is required for the maintenance of good musculoskeletal health as it promotes the absorption of calcium, osteoid mineralization and maintenance of muscle functions.
Previous studies on the effect of calcium supplementation on bone density have demonstrated that there is no substantial increase in bone density beyond one year of calcium administration6,7. Moreover, the influx of calcium ions in the blood leads to suppression in the parathyroid hormone levels, thus affecting the cycle of bone resorption and formation8. Few observational studies in the past have also shown that there is no relationship between calcium intake and risk of fracture9. The regions with low calcium intake touching Asia and Africa have a lower incidence of fracture in comparison to Europe and North America. This phenomenon has been referred to as the ‘Calcium Paradox’. In published literature, there is no consensus to support the use of either calcium alone or in combination with vitamin D to reduce the risk of subsequent fractures. Hence, this meta-analysis was planned to assess calcium and, vitamin D individually or in combination administration of calcium and vitamin D with placebo for fracture incidence in the elderly population.
Material & Methods
This systematic review and meta-analysis was performed as per the recommendations of the PRISMA statement10 and was registered in PROSPERO (CRD 42021218539).
Search strategy: PubMed, EMBASE and COCHRANE databases were searched since inception to February 2021, to collect information on published trials for evaluating the association between calcium and, vitamin D (individually or in combination) supplementation on the incidence of fractures in elderly individuals with a prior history of a fracture. In addition, clinicaltrials.gov was also searched for any undergoing trials. The keywords searched were ‘calcium’, ‘vitamin D’, and ‘fracture’. No restriction of language, date or publication status was applied on the search. The bibliographic details of all the included studies were searched manually for any additional citations. In case of duplication of publication, the study with the entire data set was excluded. The complete search strategy has been listed in the Supplementary Material.
Inclusion criteria: Randomized controlled trials (RCTs) or meta-analyses comparing calcium, and vitamin D, individually or in or combination administered either with placebo or no treatment were included in this study. Furthermore, this studies included adults older than 50 yr with a previous history of fracture.
Exclusion criteria: Studies without a treatment or placebo group, with individuals having glucocorticoid-associated osteoporosis, employed co-administration of calcium and, vitamin D individually or in combination with other treatment modalities like antiresorptive medication or included of dietary supplementation of either calcium or vitamin D were excluded from this study.
Selection of study data and data extraction: All the studies were independently screened for meeting the study criteria using Rayyan web application11. In case of any disagreement regarding the inclusion of the study, the matter was resolved by a third author. The reviewers independently extracted the characteristics of the studies and outcome measures. The extraction form was developed as per Cochrane recommendations12. Any discrepancy between the data extracted twice was resolved by analysis of the full text by all the reviewers. The patient characteristics, calcium and, vitamin D administered individually or in combination, dicalcium intake, serum vitamin D levels, cases with a history of hip, vertebral or non-vertebral fracture along with the duration of the trial were recorded.
Assessment of risk of bias: Cochrane Collaboration’s tool was used to check for the quality of studies included for the meta-analysis13. Each study was checked for random generation of sequence; reporting of selective outcome; concealment of allocation; blinding of participants; incomplete outcome data; selective outcome reporting and potential sources of bias like conflicts of interest. The performance of each study was checked for risk of bias and tabulated. The risk of bias was categorized as low, medium and high risk. When either randomization or allocation concealment was assessed as a high risk of bias regardless of other items, the trials were considered as low quality. Similarly, when both randomization and allocation concealment was assessed as a low risk of bias and other items as low or unclear risk of bias, the trial was considered as high quality. The trials which did not meet high or low quality criteria were considered as moderate quality14,15. A study was labelled with low risk of bias if six out of the seven chosen domains were found to be low risk12.
Statistical analysis: The incidence of fracture was assessed for association with calcium and, vitamin D, administration individually or in combination. Each component was also compared with a placebo or treatment given in the studies. The meta-analysis was performed to obtain relative risk ratios, absolute risk difference and 95% confidence interval. In cases where relative risk and absolute risk difference lead to similar end result, the results of relative risk were taken into consideration, especially when an intervention was targeted to prevent an unwanted event16.
The random-effect model of derSimonian and Laird approach17 was used to estimate pooled RRs and mean differences with the inverse variance approach. In case of no event in a group, the RR was estimated by adding 0.5 to each cell. The testing of heterogeneity was carried out using Chi-squared test and quantified using I2. In the case of I2>50 per cent, substantial heterogeneity was considered. P<0.10 was considered significant.
To evaluate the association of fracture with the variables under interest, the subgroups were specified based on dosage with the frequency of calcium supplementation, sex, dietary intake and baseline serum vitamin D levels. The subgroups were analyzed to look for significant results (P<0.05). Funnel plots were used to assess publication bias when the number of trials reporting the primary outcomes was ≥10. The statistical analyses were performed using Review Manager Software (Cochrane Collaboration, UK). The criteria mentioned in Cochrane Handbook were used to convert medians, standard errors and 95 per cent CI to means and standard deviations.
Results
Literature search: A total of 1273 articles were obtained in the initial search for published RCTs on the study topic. Of these, 32 duplicate articles were removed, leaving 1241 for screening. After screening titles and abstracts, 46 full text articles remained for review and 18 articles were included as per the criteria. Hence, a total of 18 RCTs which involved 39759 participants were selected in this meta-analysis (Fig. 1). The characteristics of the included RCTs are given in Table I and the list of excluded trials along with the reasons have been provided in the Supplementary Table.
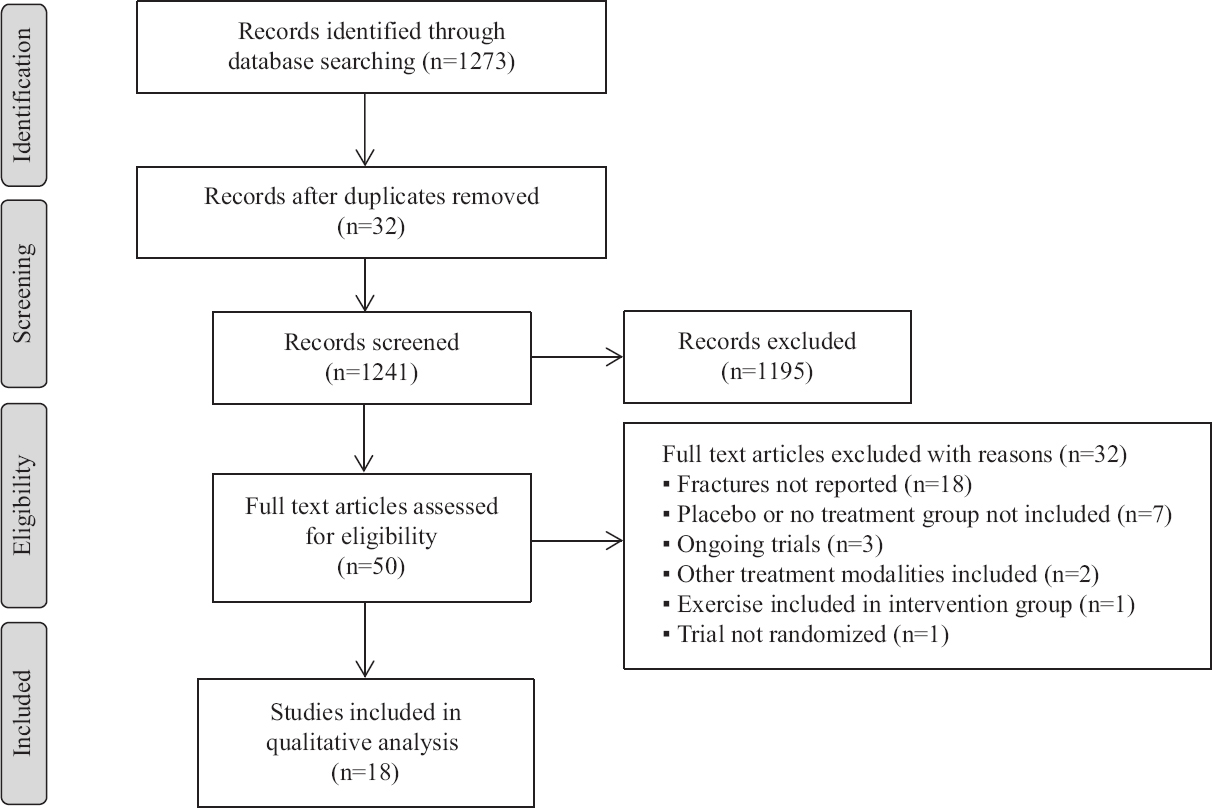
- PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for the study inclusion and exclusion procedure.
| Study | Country | Total number of participants | Male:female ratio | Intervention | Comparator | Mean age (yr) | Calcium intake (mg/day) | Serum vitamin D |
|---|---|---|---|---|---|---|---|---|
| Hansson and Roos et al18, 1987 | Sweden | 50 | 0:50 | 1 g/d calcium (n=25) | Placebo (n=25) | 65.9 | NA | NA |
| Reid et al19, 1993 | New Zealand | 135 | 0:135 | 1 g/d calcium (n=25) | Placebo (n=67) | 58 | 750 | 37.5 |
| Recker et al20, 1996 | United States | 197 | 0:197 | 1.2 g/d calcium (n=135) | Placebo (n=102) | 73.5 | 434 | 25.5 |
| Peacock et al21, 2000 | United States | 261 | 74:187 | 0.75 g/d (n=126) | Placebo (n=102) | 73.8 | 597 | 25 |
| Avenell et al22, 2004 | United States | 105 | 53:11 | 1 g/d calcium (n=35); 800 IU/day | No treatment (n=35) | 78 | NA | NA |
| Harwood et al23, 2004 | United Kingdom | 75 | 0:75 | 3,000,000 IU single dose (n=38) | No treatment (n=37) | 80.5 | NA | 11.6 |
| Porthouse et al24, 2005 | United Kingdom | 3314 | 0:3314 | 1 g/d calcium (n=1321); 800 IU/day | No treatment (n=1993) | 76.8 | 1080 | NA |
| Grant et al25, 2005 | United Kingdom | 2643 | 402:2241 | 1 g/d calcium (n=1311); 800 IU/day | Placebo (n=1332) | 77 | NA | 15.2 |
| Prince et al26, 2006 | Australia | 1460 | 0:1460 | 0.48 g/d calcium (n=730) | Placebo (n=730) | 75.2 | 915 | 31 |
| Reid et al27, 2006 | New Zealand | 1471 | 0:1471 | 1 g/d calcium (n=732) | Placebo (n=739) | 74.3 | 857 | 20.7 |
| Jackson et al28, 2006 | United States | 7972 | 0:7972 | 1 g/d calcium (n=4015); 400 IU/day | Placebo (n=3957) | 62.4 | 1151 | 18.9 |
| Smith et al29, 2007 | United Kingdom | 9440 | 4354:5086 | 3,000,000 IU single dose (n=4727) | Placebo (n=4713) | 79.1 | 625 | 22.6 |
| Sander’s et al30, 2010 | Australia | 2258 | 0:2258 | 5,000,000 IU every year (n=1131) | Placebo (n=1127) | 76.1 | 976 | 19.8 |
| Salvovaara et al31, 2010 | Finland | 3432 | 0:3432 | 1 g/d calcium (n=1718); 800 IU/day | No treatment (n=1714) | 67.3 | 957 | 19.8 |
| Punthakee et al32, 2012 | Canada | 1221 | 722:499 | 1000 IU/day (n=607) | Placebo (n=614) | 66.6 | NA | NA |
| Hin et al33, 2017 | United Kingdom | 305 | 155:150 | 4000 IU/day (n=102); 2000 IU/day (n=102) | Placebo (n=101) | 71.7 | 710 | 20.1 |
| Khaw et al34, 2017 | New Zealand | 5108 | 2969:2139 | 2,000,000 IU single dose followed by 1,00,000 monthly (n=2558) | Placebo (n=2550) | 65.9 | 810 | 25.2 |
| Xue et al35, 2017 | China | 312 | 0:312 | 0.6 g/d calcium (n=139); 800 IU/day | Placebo (n=173) | 63.6 | NA | 30.8 |
NA, not available
| Trail excluded | Year of publication | Reason for exclusion |
|---|---|---|
| Inkovaara et al1 | 1983 | Fracture data not reported |
| Lips et al2 | 1996 | Fracture data not reported |
| Dawson-Hughes et al3 | 1997 | Fracture data not reported |
| Riggs et al4 | 1998 | Fracture data not reported |
| Ruml et al5 | 1998 | Fracture data not reported |
| Baron et al6 | 1999 | Fracture data not reported |
| Trivedi et al7 | 2003 | Fracture data not reported |
| Pfeifer et al8 | 2009 | Trial did not include no treatment or placebo group |
| Bischoff-Ferrari et al9 | 2010 | Trial did not include no treatment or placebo group |
| Witham et al10 | 2010 | Trial did not include no treatment or placebo group |
| Mitri et al11 | 2011 | Fracture data not reported |
| Sambrook et al12 | 2012 | Trial included mandatory sunlight exposure in one of the groups |
| Schaller et al13 | 2012 | Fracture data not reported |
| Rossini et al14 | 2012 | Trial is non-randomized |
| Gendenneing et al15 | 2012 | Fracture data not reported |
| Aloia et al16 | 2013 | Fracture data not reported |
| Witham et al17 | 2013 | Fracture data not reported |
| Tella et al18 | 2014 | Placebo group not included |
| Takano et al19 | 2014 | Placebo or no treatment group not included |
| REVITAHIP trail et al20 | 2014 | Placebo or no treatment group not included |
| Massart et al21 | 2014 | Fracture data not reported |
| Martineau et al22 | 2015 | Fracture data not reported |
| Wang et al23 | 2015 | Multivitamin tablets were administered in the experimental group |
| Rolighed et al24 | 2015 | Fracture data not reported |
| Uusi-Rasi et al25 | 2015 | Fracture data not reported |
| Liu et al26 | 2015 | Fracture data not reported |
| Schwetz et al27 | 2017 | Fracture data not reported |
| Laiz et al28 | 2017 | Exercise was included in the intervention group |
| Pop et al29 | 2017 | Placebo or no treatment group not included |
| Leblanc et al30 | 2018 | Ongoing trial |
| Joseph et al31 | 2018 | Ongoing trial |
| DO-HEALTH32 | 2019 | Ongoing trial |
The risk of bias of the studies included was assessed (Supplementary Figs
Calcium intake and risk of fracture: Calcium was administered in the form of calcium carbonate in ten trials, calcium citrate malate in two trials, calcium citrate in two trials; combination of bicarbonate, lactate and, gluconate in one trial; lactate, gluconate, carbonate in two trials and unclear form in one trial. The association between calcium administration and hip fracture [risk ratio (RR) 1.56; 95% confidence interval (CI), 0.91 to 2.69, I2=28%; P=0.11], vertebral fracture (RR 0.95; 95% CI, 0.82 to 1.10, I2=0%; P=0.49) or other fractures (RR 0.83; 95% CI 0.65 to 1.06, I2=0%; P=0.14) was not significant (Fig. 2) in comparison to either no treatment or placebo administration. The subgroup analysis was carried out for the assessment of fracture risk in the hip, vertebra and other parts of the body, but there was no significant association based on calcium dosage, sex and serum 25-hydroxy vitamin D [25OH)D] levels (Table II).
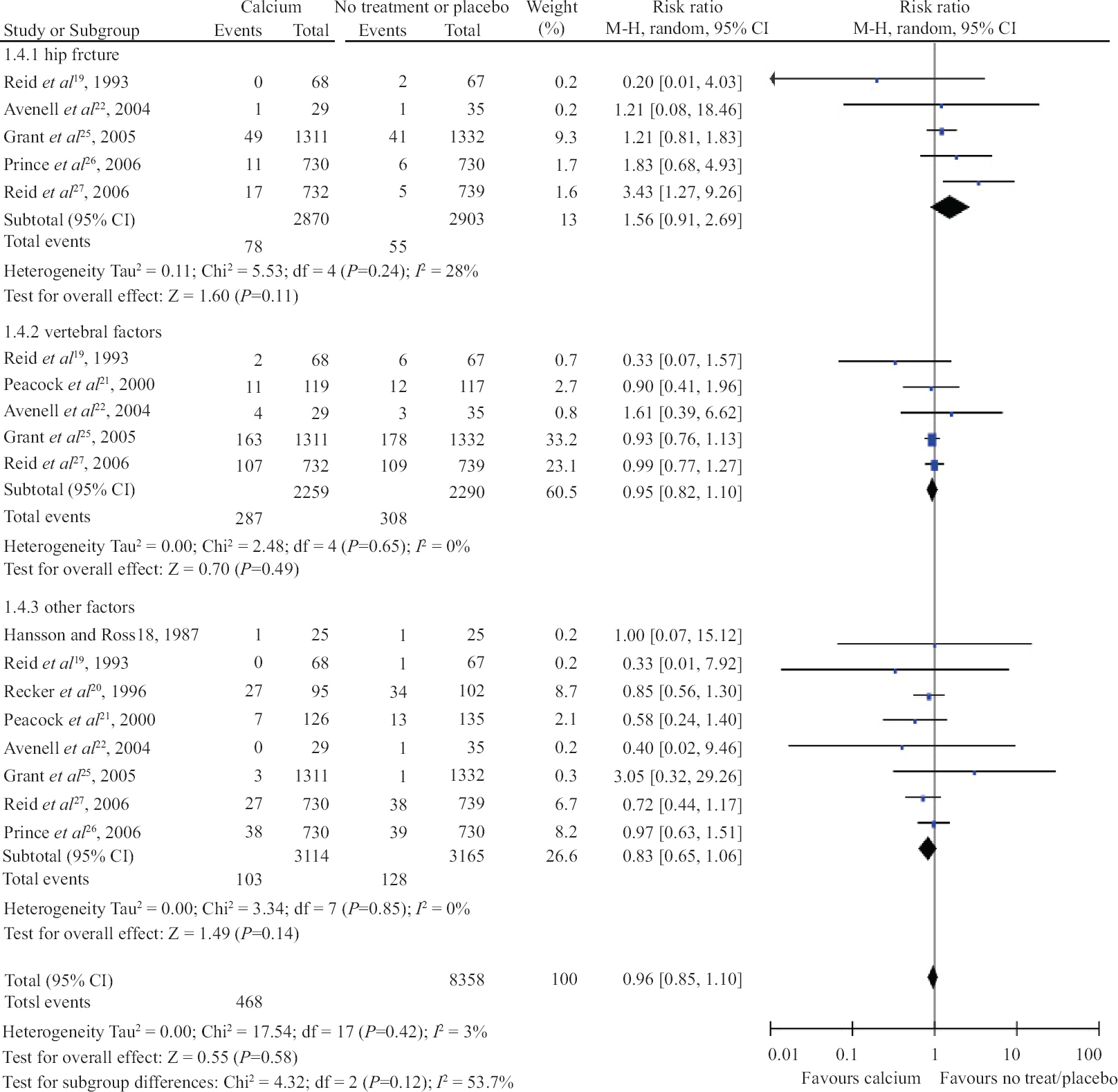
- Forrest plot of trials with administration of calcium for prevention of fracture in hip, vertebrae and other parts of the body.
| Factor | Participants with fracture | Total number of participants | RR (95% CI) | P |
|---|---|---|---|---|
| Hip fracture | ||||
| Dose of calcium | ||||
| <1 g | 17 | 1460 | 1.83 (0.68-4.93) | 0.76 |
| >1 g | 116 | 4313 | 1.51 (0.67-3.37) | |
| Sex | ||||
| Women only | 41 | 3066 | 1.97 (0.74-5.28) | 0.31 |
| Both sex | 92 | 2707 | 1.21 (0.81-1.82) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 41 | 3066 | 1.97 (0.74-5.28) | 0.16 |
| <20 ng/ml | 92 | 2707 | 1.21 (0.81-1.82) | |
| Vertebral fracture | ||||
| Dose of calcium | ||||
| >1 g | 572 | 4313 | 0.95 (0.82-1.11) | 0.63 |
| <1 g | 23 | 236 | 0.90 (0.41-1.96) | |
| Sex | ||||
| Women only | 224 | 1606 | 0.76 (0.30-1.92) | 0.94 |
| Both sex | 371 | 2943 | 0.94 (0.78-1.13) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 247 | 1842 | 0.96 (0.76-1.21) | 0.76 |
| <20 ng/ml | 341 | 2643 | 0.93 (0.76-1.13) | |
| Other fracture | ||||
| Dose of calcium | ||||
| >1 g | 158 | 1918 | 0.87 (0.65-1.15) | 0.56 |
| <1 g | 73 | 4361 | 0.75 (0.47-1.18) | |
| Sex | ||||
| Women only | 204 | 3261 | 0.85 (0.66-1.09) | 0.97 |
| Both sex | 28 | 3153 | 0.68 (0.32-1.44) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 143 | 3064 | 0.84 (0.61-1.16) | 0.84 |
| <20 ng/ml | 85 | 3101 | 0.82 (0.57-1.20) | |
25(OH)D, 25-hydroxy vitamin D; CI, confidence interval; RR, relative risk
Vitamin D intake and risk of fracture: Vitamin D supplementation with a placebo or no treatment was compared in six trials. The association between vertebral fracture (RR, 1.28; 95% CI, 0.80 to 2.05, I2=0%; P=0.31), hip fracture (RR, 1.18; 95% CI, 0.91 to 1.53, I2=0%; P=0.21), or other fracture (RR, 1.09; 95% CI, 0.94 to 1.20, I2=0%; P=0.11; Fig. 3) was not found to be significant. The subgroup analysis for different dosage and frequency of assessment of fracture risk was not found to be significantly associated (Table III).
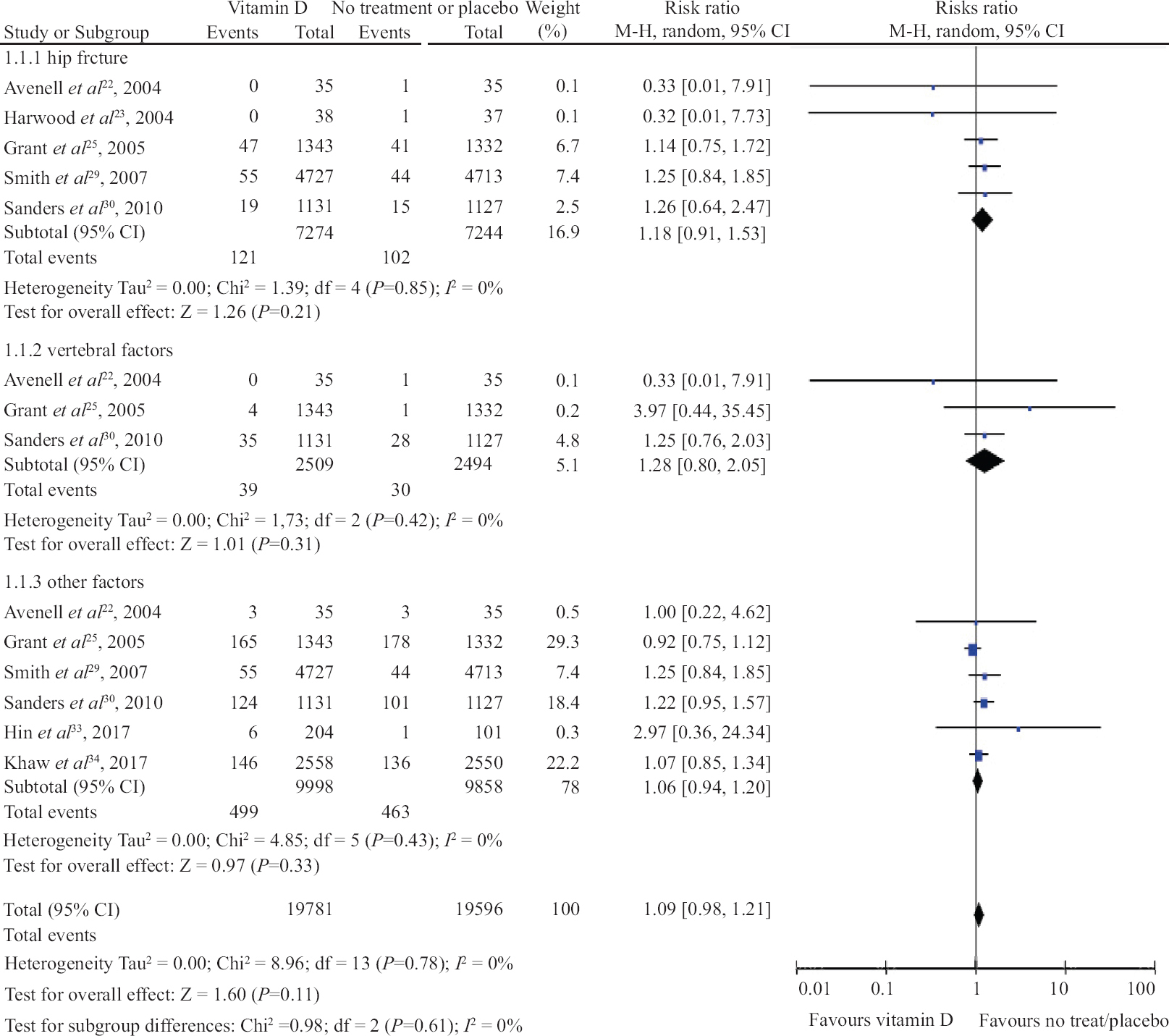
- Forrest plot of trials with the administration of vitamin D for prevention of fracture in hip, vertebrae and other parts of the body.
| Factor | Participants with fracture | Total number of participants | RR (95% CI) | P |
|---|---|---|---|---|
| Hip fracture | ||||
| Frequency of vitamin D supplementation | ||||
| Low daily dose | 89 | 2745 | 1.11 (0.74-1.68) | 0.17 |
| High dose once yearly | 35 | 2333 | 1.19 (0.62-2.3) | |
| High dose intermittently | 99 | 9440 | 1.25 (0.84-1.85) | |
| Sex | ||||
| Women only | 34 | 2258 | 1.26 (0.64-2.47) | 0.52 |
| Both sex | 89 | 2745 | 1.11 (0.74-1.68) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 222 | 14,448 | 1.19 (0.92-1.55) | NA |
| <20 ng/ml | 0 | 0 | NA | |
| Vertebral fracture | ||||
| Frequency of vitamin D supplementation | ||||
| Low daily dose | 6 | 2745 | 1.51 (0.14-16.14) | 0.21 |
| Intermittent high dose | 63 | 2258 | 1.25 (0.76-2.03) | |
| Sex | ||||
| Women only | 63 | 2258 | 1.25 (0.76-2.03) | 0.49 |
| Both sex | 6 | 2745 | 1.51 (0.14-16.14) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 0 | 0 | NA | NA |
| <20 ng/ml | 69 | 5003 | 1.28 (0.80-2.05) | |
| Other fracture | ||||
| Frequency of vitamin D supplementation | ||||
| Low daily dose | 349 | 2745 | 0.92 (0.76-1.12) | 0.23 |
| High dose once yearly | 225 | 2258 | 1.22 (0.95-1.57) | |
| High dose intermittently | 282 | 5108 | 1.07 (0.85-1.34) | |
| Sex | ||||
| Women only | 225 | 2258 | 1.22 (0.95-1.57) | 0.56 |
| Both sex | 730 | 17,293 | 1.01 (0.88-1.16) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 955 | 19551 | 1.09 (0.98-1.21) | NA |
| <20 ng/ml | 0 | 0 | NA | |
CI, confidence interval; NA, not available; RR, relative risk
Combined vitamin D and Calcium administration and fracture risk: Supplementation of calcium and vitamin D combined versus placebo or no treatment was compared in seven trials. The association between vertebral fracture (RR, 0.63; 95% CI, 0.29 to 1.40, I2=0%; P=0.26), hip fracture (RR, 1.10; 95% CI, 0.86 to 1.40, I2=0%; P=0.47) and other fractures (RR, 0.921; 95% CI, 0.78 to 1.08, I2=0%; P=0.29; Fig. 4) was not found to be significant. There was no significant difference in the subgroup analysis based on intake of calcium and vitamin D, sex, baseline 25(OH)D levels and dietary intake of calcium (Table IV).
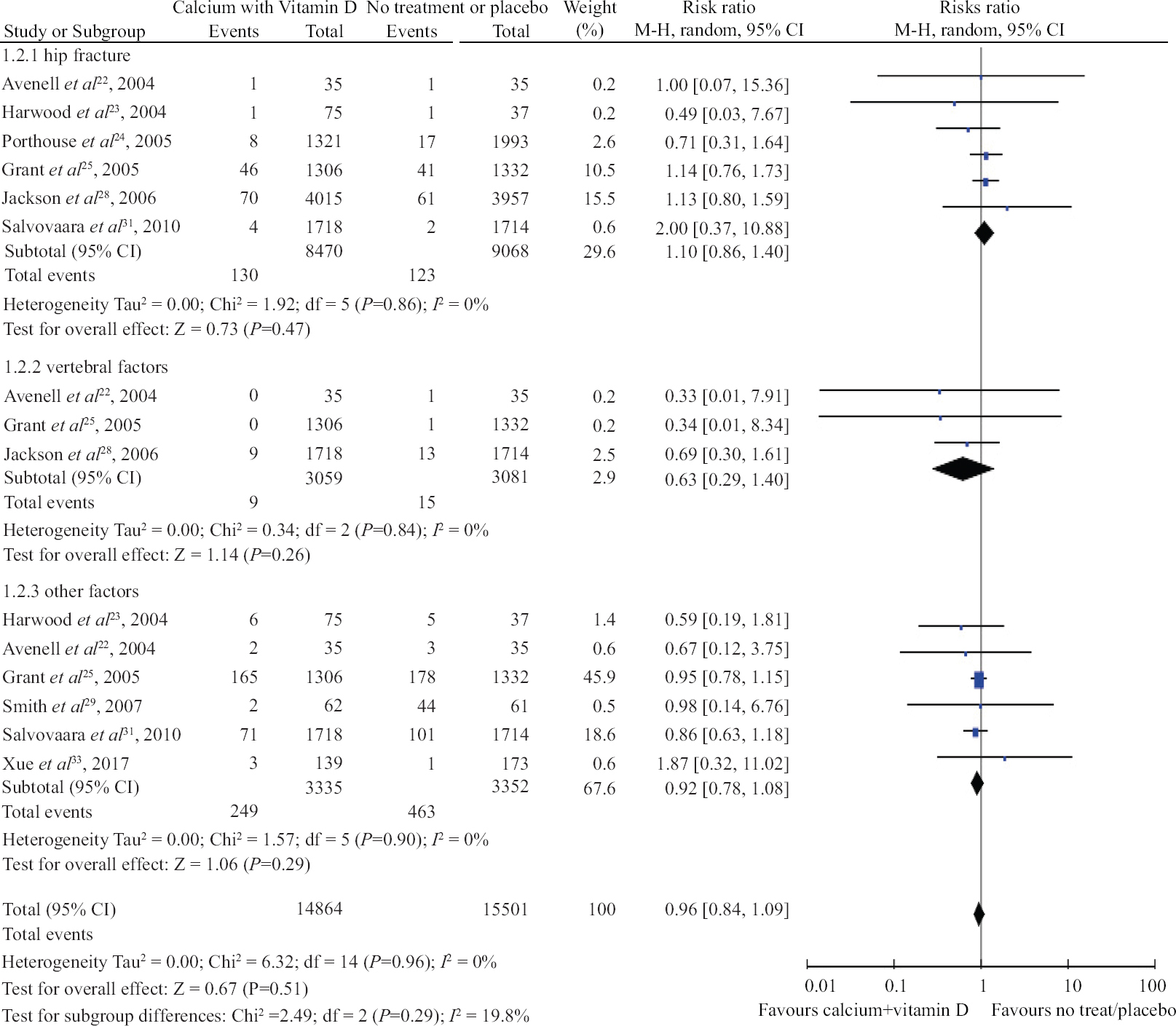
- Forrest plot of trials with the combined administration of calcium and vitamin D for prevention of fracture in hip, vertebrae and other parts of the body.
| Factor | Participants with fracture | Total number of participants | RR (95% CI) | P |
|---|---|---|---|---|
| Hip fracture | ||||
| Combined calcium and vitamin D supplementation | ||||
| Calcium with >1 g with low daily vitamin D | 120 | 9454 | 1.07 (0.75-1.54) | 0.83 |
| Other | 133 | 8084 | 1.12 (0.80-1.57) | |
| Sex | ||||
| Women only | 164 | 14,830 | 1.07 (0.79-1.46) | 0.81 |
| Both sex | 89 | 2708 | 1.14 (0.76-1.72) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 0 | 0 | NA | NA |
| <20 ng/ml | 251 | 17,468 | 1.10 (0.86-1.4) | |
| Vertebral fracture | ||||
| Combined calcium and vitamin D supplementation | ||||
| Calcium with >1 g with low daily vitamin D | 2 | 2708 | 0.34 (0.04-3.2) | 0.67 |
| Other | 22 | 3432 | 0.69 (0.30-1.61) | |
| Sex | ||||
| Women only | 22 | 3432 | 0.69 (0.30-1.61) | 0.45 |
| Both sex | 2 | 2708 | 0.34 (0.04-3.2) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 0 | 0 | NA | NA |
| <20 ng/ml | 24 | 6140 | 0.63 (0.29-1.4) | |
| Other fracture | ||||
| Combined calcium and vitamin D supplementation | ||||
| Calcium with >1 g with low daily vitamin D | 512 | 6252 | 0.91 (0.77-1.07) | 0.53 |
| Other | 4 | 123 | 0.98 (0.14-6.76) | |
| Sex | ||||
| Women only | 15 | 235 | 0.67 (0.26-1.77) | 0.51 |
| Both sex | 501 | 14,988 | 0.92 (0.78-1.08) | |
| Serum 25(OH)D levels at presentation | ||||
| >20 ng/ml | 0 | 0 | NA | NA |
| <20 ng/ml | 516 | 6375 | 0.91 (0.77-1.07) | |
CI, confidence interval; NA, not available; RR, relative risk
Discussion
The meta-analysis revealed that calcium and, vitamin D individually or in combination did not lower the chances of hip, vertebral or any other fragility fractures in the elderly population. The exclusion of low-quality trials and trials with patients using specific medication did not affect the results. The outcome was independent of calcium, vitamin D dosage or the combination of two, sex and serum 25(OH)D levels.
Prior meta-analysis carried out by Tang et al36 had reported decrease in fragility fractures with calcium supplementation. However, they had included two cluster trials15,16 with a large sample size and did not adjust for the cases which could have increased the chance of achieving low P value and narrow confidence intervals in the comparison groups. In another meta-analysis by Bolland et al9, the dietary intake of calcium did not decrease the risk of fracture, and role of calcium supplementation in the prevention of fracture was also doubtful. In the present study, no association between calcium supplementation and the risk of fracture was observed. Hence, calcium supplementation need not be a routine recommendation for lowering risk of fracture.
A meta-analysis by Bischoff-Ferrari et al37 had reported lower chances of hip fracture and other fragility fractures with the use of a high dose of vitamin D (≥800 IU) per day. The inclusion of institutional patients by Chapuy et al38 in the meta-analysis could have affected the finding of the meta-analysis. Other meta-analyses by Bergman et al39 supported the use of a high dose of vitamin D to prevent the non-vertebral and non-hip fragility fractures. However, they reported no significant association between high dose vitamin D and hip fractures. In the present study, the reason for the difference in the result could be due to reporting of neutral or negative association between vitamin D administration and risk of fracture.
In a Cochrane review by Avenell et al40, the chance of hip fracture or combined fragility fracture was suggestively reduced with the combined administration of calcium and vitamin D. In contrast, Bolland et al41 reported no beneficial effect with the administration of calcium and vitamin D in combination in osteoporotic fractures. A meta-analysis by Zhao et al14 reported inconsistent results with a combined supplementation of calcium and vitamin D due to different inclusion criteria like the restriction of RCTs to community dwellers or residents of nursing homes.
In a study by Jackson et al42, positive interaction was reported between hormonal therapy and calcium and vitamin D supplementation. They concluded that a lower risk of fragility fractures with this combination was found in individuals on hormonal therapy in contrast to the study group not on hormonal therapy which did not report any reduction in fracture risk. In the present meta-analysis, all the cases on hormonal therapy were excluded from the analysis.
The data from VITAL trial43 reported that vitamin D3 supplementation did not reduced risk of fractures.
There is a requirement of a large RCTs, especially in elderly high risk individuals involving combined administration of calcium and vitamin D to obtain a reliable evidence.
The present study did have a few limitations. First, some studies did not include the baseline values of 25(OH)D levels which could have altered the results of the subgroup analysis. Second, few RCTs were of poor quality with allocation bias. Third, there are chances of publication bias in the results reported by individual RCTs. Fourth, there could have been variations in the classification of quality of the studies.
Overall, the present meta-analysis involving RCTs that included calcium and, vitamin D administered individually or in combination compared with either no treatment or placebo do not support their routine supplementation in the elderly population. However, the results of undergoing RCTs involving high doses of vitamin D may have bearing on the future meta-analyses.
Financial support and sponsorship
None.
Conflicts of interest
None.
Supplementary Fig. 1
Supplementary Fig. 1 Risk of bias table for included trials.Supplementary Fig. 2
Supplementary Fig. 2 Risk of bias summary for included trials.Supplementary Fig. 3
Supplementary Fig. 3 Funnel plot for publication Bias assessment.References
- Osteoporosis and fracture risk evaluation and management: Shared decision making in clinical practice. JAMA. 2017;317:253-4.
- [Google Scholar]
- Organizational factors and long-term mortality after hip fracture surgery. A cohort study of 6143 consecutive patients undergoing hip fracture surgery. PLoS One. 2014;9:e99308.
- [Google Scholar]
- A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16:158.
- [Google Scholar]
- Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ. 2015;351:h4183.
- [Google Scholar]
- Calcium and/or vitamin D supplementation for the prevention of fragility fractures: who needs it? Nutrients. 2020;12:1011.
- [Google Scholar]
- Physiology, Parathyroid Hormone. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- [Google Scholar]
- Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097.
- [Google Scholar]
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, eds. Cochrane handbook for systematic reviews of interventions (2nd ed). Chichester (UK): John Wiley & Sons; 2019.
- The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- [Google Scholar]
- Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: A systematic review and meta-analysis. JAMA. 2017;318:2466-82.
- [Google Scholar]
- Endoscopic transgastric versus surgical approach for infected necrotizing pancreatitis: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2019;29:141-9.
- [Google Scholar]
- Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21:1575-600.
- [Google Scholar]
- The effect of fluoride and calcium on spinal bone mineral content: a controlled, prospective (3 years) study. Calcif Tissue Int. 1987;40:315-7.
- [Google Scholar]
- Effect of calcium supplementation on bone loss in postmenopausal women. N Engl J Med. 1993;328:460-4.
- [Google Scholar]
- Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res. 1996;11:1961-6.
- [Google Scholar]
- Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab. 2000;85:3011-9.
- [Google Scholar]
- The effects of an open design on trial participant recruitment, compliance and retention—a randomized controlled trial comparison with a blinded, placebo-controlled design. Clin Trials. 2004;1:490-8.
- [Google Scholar]
- A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: the Nottingham Neck of Femur (NONOF) study. Age Ageing. 2004;33:45-51.
- [Google Scholar]
- Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330:1003.
- [Google Scholar]
- Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365:1621-8.
- [Google Scholar]
- Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166:869-75.
- [Google Scholar]
- Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119:777-85.
- [Google Scholar]
- Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-83.
- [Google Scholar]
- Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2007;46:1852-7.
- [Google Scholar]
- Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815-22.
- [Google Scholar]
- Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: a population-based 3-year randomized, controlled trial—the OSTPRE-FPS. J Bone Miner Res. 2010;25:1487-95.
- [Google Scholar]
- Design, history and results of the Thiazolidinedione Intervention with vitamin D Evaluation (TIDE) randomised controlled trial. Diabetologia. 2012;55:36-45.
- [Google Scholar]
- Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int. 2017;28:841-51.
- [Google Scholar]
- Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017;5:438-47.
- [Google Scholar]
- Effects of enhanced exercise and combined vitamin D and calcium supplementation on muscle strength and fracture risk in postmenopausal Chinese women. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39:345-51.
- [Google Scholar]
- Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet. 2007;370:657-66.
- [Google Scholar]
- A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40-9.
- [Google Scholar]
- Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327:1637-42.
- [Google Scholar]
- Efficacy of vitamin D3 supplementation in preventing fractures in elderly women: A meta-analysis. Curr Med Res Opin. 2010;26:1193-201.
- [Google Scholar]
- Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2005;3:CD000227.
- [Google Scholar]
- The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:307-20.
- [Google Scholar]
- The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: A meta-analysis. QJM. 2007;100:185-92.
- [Google Scholar]
- Supplmental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387:299-309.
- [Google Scholar]
- Calcium, vitamin D and anabolic steroid in treatment of aged bones:double-blind placebo-controlled long-term clinical trial. Age Ageing. 1983;12:124-130.
- [Google Scholar]
- Vitamin D supplementation and fracture incidence in elderly persons:a randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400-6.
- [Google Scholar]
- Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670-6.
- [Google Scholar]
- Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res. 1998;13:168-74.
- [Google Scholar]
- The effect of calcium citrate on bone density in the early and mid-postmenopausal period:a randomized placebo-controlled study. Am J Ther. 1999;6:303-11.
- [Google Scholar]
- Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101-7.
- [Google Scholar]
- Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community:randomised double blind controlled trial. BMJ. 2003;326:469.
- [Google Scholar]
- Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20:315-22.
- [Google Scholar]
- Effect of high-dosage cholecalciferol and extended physiotherapy on complications after hip fracture:a randomized controlled trial. Arch Intern Med. 2010;170:813-20.
- [Google Scholar]
- The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure:a randomized controlled trial. Circ Heart Fail. 2010;3:195-201.
- [Google Scholar]
- Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes:the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. American J Clin Nutr. 2011;94:486-94.
- [Google Scholar]
- Does increased sunlight exposure work as a strategy to improve vitamin D status in the elderly:a cluster randomised controlled trial. Osteoporos Int. 2012;23:615-24.
- [Google Scholar]
- Mild to moderate cognitive impairment is a major risk factor for mortality and nursing home admission in the first year after hip fracture. Bone. 2012;51:347-52.
- [Google Scholar]
- Short-term effects on bone turnover markers of a single high dose of oral vitamin D? Clin Endocrinol Metab. 2012;97:E622-6.
- [Google Scholar]
- Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women:A randomized controlled trial. J Bone Miner Res. 2012;27:170-6.
- [Google Scholar]
- Calcium and vitamin D supplementation in postmenopausal women. J Clin Endocrinol Metab. 2013;98:E1702-9.
- [Google Scholar]
- Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension:the VitDISH randomized controlled trial. JAMA Intern Med. 2013;173:1672-9.
- [Google Scholar]
- Effect of vitamin D supplementation on BMD in young and elderly caucasian and African American women:two randomized, placebo controlled trials. Endocrine reviews. In: Conference:96th annual meeting and expo of the endocrine society, ENDO. Vol 2014. 2014. 35 (no pagination)
- [Google Scholar]
- Eldecalcitol Study Group. Relationship between the effect of eldecalcitol and serum 25(OH)D level. J Steroid Biochem Mol Biol. 2014;144:124-7. Pt A
- [Google Scholar]
- An initial loading-dose vitamin D versus placebo after hip fracture surgery:baseline characteristics of a randomized controlled trial (REVITAHIP) BMC Geriatrics. 2014;14:101.
- [Google Scholar]
- Biochemical parameters after Cholecalciferol repletion in hemodialysis:Results from the vitadial randomized trial. Am J Kidney Dis. 2014;64:696-705.
- [Google Scholar]
- Increased risk of upper respiratory infection with addition of intermittent bolus-dose vitamin D supplementation to a daily low-dose regimen [Abstract. Thorax. 2013;68(Suppl 3) A64 [s123]
- [Google Scholar]
- Multivitamin and mineral supplementation is associated with the reduction of fracture risk and hospitalization rate in Chinese adult males:a randomized controlled study. J Bone Miner Metab. 2015;33:294-302.
- [Google Scholar]
- No beneficial effects of vitamin D supplementation on muscle function or quality of life in primary hyperparathyroidism:Results from a randomized controlled trial. Eur J Endocrin. 2015;172:609-17.
- [Google Scholar]
- Exercise and vitamin D in fall prevention among older women:a randomized clinical trial. JAMA Intern Med. 2015;175:703-11.
- [Google Scholar]
- The effect of the modified eighth section of eight-section brocade on osteoporosis in postmenopausal women:A prospective randomized trial. Medicine (Baltimore). 2015;94:991.
- [Google Scholar]
- Impact of 3-monthly vitamin D supplementation plus exercise on survival after surgery for osteoporotic hip fracture in adult patients over 50 years:A pragmatic randomized, partially blinded, controlled trial. J Nutr Health Aging. 2017;21:413-20.
- [Google Scholar]
- Effects of vitamin D supplementation on bone turnover markers:A randomized controlled trial. Nutrients. 2017;9:432.
- [Google Scholar]
- Three doses of vitamin D, bone mineral density, and geometry in older women during modest weight control in a 1-year randomized controlled trial. Osteoporos Int. 2016;28:377-88.
- [Google Scholar]
- Screening for Vitamin D Deficiency:Systematic Review for the U. S. In: Preventive Services Task Force Recommendation [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. Report No.:13-05183-EF-1
- [Google Scholar]
- The International Polycap Study-3 (TIPS-3):Design, baseline characteristics and challenges in conduct. Am Heart J. 2018;206:72-9.
- [Google Scholar]
- DO-HEALTH:Vitamin D3 - Omega-3 - Home exercise - Healthy aging and longevity trial - Design of a multinational clinical trial on healthy aging among European seniors. Contemp Clin Trials. 2021;100:106124.
- [Google Scholar]
Supplementary Material
Search strategy in database
PUBMED:
#1 “calcium”[MeSH Terms] OR “calcium”[All Fields]
#2 “vitamin d”[MeSH Terms] OR “vitamin d”[All Fields] OR “ergocalciferols”[MeSH Terms] OR “ergocalciferols”[All Fields]
#3 “fractures, bone”[MeSH Terms] OR (“fractures”[All Fields] AND “bone”[All Fields]) OR “bone fractures”[All Fields] OR “fracture”[All Fields]
#4 trial”[Title/Abstract] OR “randomised trial”[Title/Abstract] OR “randomised controlled trial” [Title/Abstract]
#5 #1 or #2
#6 #3 and #5
#7 #4 and #6
EMBASE:
#1 ‘calcium’/exp OR calcium
#2 ‘vitamin d’/exp OR ‘vitamin d’
#3 ‘fracture’/exp OR fracture
#4 [cochrane review]/lim OR [systematic review]/lim OR [meta analysis]/lim
#5 #1 or #2
#6 #3 and 5
#7 #4 and #6
COCHRANE:
#1 calcium:ti,ab,kw
#2 vitamin d:ti,ab,kw
#3 fracture:ti,ab,kw
#4 #1 or #2
#5 #3 and #4






