Translate this page into:
Role of ATP-dependent K channels in the effects of erythropoietin in renal ischaemia injury
Reprint requests: Dr Tonguç Utku Yilmaz, Kocaeli University, Faculty of Medicine, Department of General Surgery 41380, Umuttepe, Kocaeli, Turkey e-mail: utku.yilmaz@kocaeli.edu.tr
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Erythropoietin (EPO) has cytoprotective and anti-apoptotic effects in pathological conditions, including hypoxia and ischaemia-reperfusion injury. One of the targets to protect against injury is ATP-dependent potassium (KATP) channels. These channels could be involved in EPO induced ischaemic preconditoning like a protective effect. We evaluated the cell cytoprotective effects of EPO in relation to KATP channel activation in the renal tubular cell culture model under hypoxic/normoxic conditions.
Methods:
Dose and time dependent effects of EPO, KATP channel blocker glibenclamide and KATP channel opener diazoxide on cellular proliferation were evaluated by colorimetric assay MTT [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide] under normoxic and hypoxic conditions in human renal proximal tubular cell line (CRL-2830). Evaluation of the dose and time dependent effects of EPO, glibenclamide and diazoxide on apoptosis was done by caspase-3 activity levels. Hypoxia inducible factor-1 alpha (HIF-1 α) mRNA levels were measured by semi-quantative reverse transcription polymerase chain reaction (RT)-PCR. Kir 6.1 protein expresion was evalutaed by Western blot.
Results:
Glibenclamide treatment decreased the number of living cells in a time and dose dependent manner, whereas EPO and diazoxide treatments increased. Glibenclamide (100 μM) treatment significantly blocked the anti-apoptotic effects of EPO (10 IU/ml) under both normoxic and hypoxic conditions. EPO (10 IU/ml) and diazoxide (100 μM) treatments significantly increased (P<0.01) whereas glibenclamide decreased (P<0.05) HIF-1 α mRNA expression. Glibenclamide significantly (P<0.01) decreased EPO induced HIF-1 α mRNA expression when compared with the EPO alone group.
Interpretation & conclusions:
Our results showed that the cell proliferative, cytoprotective and anti-apoptotic effects of EPO were associated with KATP channels in the renal tubular cell culture model under hypoxic/normal conditions.
Keywords
Diazoxide
erythropoietin
glinbenclamide
hypoxia inducible factor-1α
KATP channels
Renal ischaemia is a common cause of acute renal failure. Ischaemia/reperfusion (I/R) is an inevitable event in renal transplantations and surgical revascularizations of the renal artery. Renal dysfunction following I/R, the leading cause of delayed graft function and chronic allograft nephropathy in transplantation, is a frequent complication (15 to 22%) after aortic surgery1. Many mechanisms such as, hypoxia, apoptosis, inflammatory response, oxidative damage, loss of intercellular and cell-to-extracellular matrix interactions, and depletion of adenosine triphosphate (ATP), take part in the pathophysiology of renal ischaemia2. These are associated with increased mortality, morbidity, prolonged hospitalization, and sometimes organ failure3.
Several agents have been studied for the prevention of cellular injury, among those erythropoietin (EPO) is a haematopoietic cytokine and growth factor that stimulates erythropoiesis4. It has been shown that EPO has cytoprotective, anti-apoptotic, anti-inflammatory, and antioxidant functions under different stresses and pathological conditions456. Hypoxia is the major stimulus for the EPO synthesis. EPO induces a preconditioning-like effect and protects tissues against I/R injury7. The opening of ATP-dependent potassium (KATP) channels or calcium-activated potassium channels, nitric oxide synthesis, increased hypoxia inducible factor-1 alpha (HIF-1α) expressions, activation of Ras/MAPK (mitogen activated protein kinase), protein kinase C (PKC) and phosphoinositide-3-kinase/Akt pathways, and mTOR are the important mechanisms that are involved in EPO-induced cytoprotection789101112. It has been shown in cardiac and renal ischaemic models that blockage of KATP channels can abolish the cytoprotective effects of EPO410.
KATP channels have important functions in the neuroendocrine system, pancreatic beta cells, smooth and skeletal muscles, heart, and kidneys, and have been shown to be important in the ischaemic preconditioning response. The focus has been shifted on the mitochondrial rather than the sarcolemmal KATP channel, due to its energy-modulating property. KATP channels are also present in the kidneys. Blockers of KATP channels, e.g. glibenclamide (Gli) and repaglinide, stimulate the release of insulin and increase susceptibility to ischaemic injuries. On the other hand, openers of KATP channels, e.g. Diazoxide have important roles in cardioprotection and spinal ischaemia through the ischaemic preconditioning effect1314. Diazoxide has been shown to have both ischaemic preconditioning and postconditioning protective effects through predominantly KATP channels13141516. In animal myocardial ischaemia/reperfusion models, activation of the mitochondrial KATP channels by their pharmacological openers has been shown to attenuate myocardial infarction and endothelial dysfunction. Conversely, blockage of the KATP channels aggravates myocardial necrosis and the no-reflow phenomenon after ischaemia/reperfusion1516.
Although I/R has been shown to cause an increase in reactive oxygen species, activated leukocytes, and pathological changes, cellular mechanisms are the triggers. There are some missing links in the relationship between KATP and EPO, including a probable intranuclear mechanism. İn this study we aimed to evaluate the cell proliferation, cytoprotective, and anti-apoptotic effects of EPO in relation to KATP channels by cell proliferation index, caspase-3 levels, Kir 6.1 protein and HIF-1α mRNA expression in the renal tubular cell culture model under hypoxic/normoxic conditions.
Material & Methods
The experiments were performed in Molecular Biology and Technology Research and Development Unite Laboratory Ankara University.
Cell culture and experimental protocol: Human renal tubular cell line CRL-2830 was obtained from the American Type Culture Collection (ATCC; Rockville, USA) and was grown in a monolayer culture in Dulbecco's modified Eagle's medium-F12 (DMEM-F12; Biological Industries, Israel) supplemented with 10 per cent heat-inactivated foetal calf serum (PAA, Austuria), 2mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma, USA).
For hypoxic conditions, a special chamber in which the oxygen level was continuously kept at three per cent O2 concentration with a gas tank and in which the oxygen level was continuously controlled by a sensor was prepared. Depending on the groups, different concentrations of erythropoietin (rHuEPO alpha, CILAG, Australia, EPO 1, 5, 10, 50 IU/ml), glibenclamide (Sigma, USA, Gli, 10, 100 μM) and diazoxide (Sigma, USA Dia, 10, 100 μM) were added to the medium for 48 h; and evaluated on the 2, 24 and 48 h. For evaluation of the effects of EPO during hypoxia; EPO was used at a dosage of 10 IU/ml.
Caspase-3 levels were evaluated in EPO, Gli and/or Dia treated cells at the 48 h. Cell proliferation was measured by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT, Sigma, USA) at the 2, 24 and 48 h as mentioned in previous study17.
After supernatants were removed, the cell surface was washed with sterile phosphate buffered saline (PBS) at physiological pH and the cells were harvested with lysis solution (20 µl of 1% triton-X lysis solution). The caspase-3 levels of the cell groups including control, EPO, Gli and Dia were measured in the cell lysates.
Evaluation of cellular proliferation: MTT, a colorimetric assay based upon the ability of living cells to reduce MTT into formazan, was used for the evaluation of dose and time dependent effects of EPO, Gli and Dia on cellular deaths or proliferations (2, 24, 48 h) under normoxic and hypoxic conditions. Cell number (index) % was calculated as the ratio of the cell number of the affected group vs control group × 100 at the determined hour.
Measurement of apoptosis: The presence of apoptosis was determined by caspase-3 levels. Equal numbers of cells were used for caspase-3 level measurements. Cells were lysed with an assay buffer (50 mmol/l HEPES, pH 7.4, 100 mmol/l NaCl, 0.1% CHAPS, 10 mmol/lM DTT, 2 mmol/l EDTA, 2 mmol/l EGTA, triton X-100, 0.1%). Caspase-3 levels were measured in cell lysates by amino acid sequence Asp-Glu-Val-Asp DEVD-R110 Fluorometric HTS assay kit (Biotium, Hayward, CA, USA). The fluorogenic substrate (Ac-DEVD)2-R110 was used for this assay. It was completely hydrolyzed by the enzyme in two successive steps. Cleavage of the first DEVD peptide resulted in the monopeptide Ac-DEVD-R110 intermediate, which had absorption and emission wavelengths similar to those of R110 (λabs/λabs= 496/520 nm). Hydrolysis of the second DEVD peptide released the dye R110, leading to a substantial fluorescence increase. R110 was used for generating a standard curve to calculate the amount of the substrate conversion.
HIF-1α mRNA expression: Total RNA was extracted by guadinium thiocyanate extraction method. By using cDNA synthesis kit (Invitrogen, Carlsbad, California, USA), RNA was converted to cDNA. Reverse transcription (RT)-PCR was used for mRNA expression analysis using the following primers:
HIF-1 α forward 5’- TGCTTGGTGCTGATTTGTGA-3’,
HIF-1 α reverse 5’- GGTCAGATGATCAGAGTCCA-3’,
ß-Actin forward; 5’- GTAGCCATCCAGGCTGTGTT-3’,
ß-Actin reverse; 5’- CCCTCATAGATGGGCACAGT-3’ (Santa Cruz, CA, USA).
Cycle protocol was: 30 sec at 94°C, 1 min at 60°C for, and 2 min at 70˚ for 34 cycles. β-actin was used as an internal control18.
Kir 6.1 protein expression analysis: Cells in log-phase growth were collected and lysed as previously described19 and Western blot analysis was performed using standard methods. Antibodies used were: Kir 6.1 and secondary antibodies (1/200 and 1/1000 dilution Abcam, MA, USA); β-actin (1/1000 dilution, Pierce, Rockford, USA) antibodies. Protein bands were visualized with the enhanced chemiluminescence (ECL) Advance Western blot detection reagents (GE Healthcare Life Sciences, NJ, USA) and quantified by the Image J image processing program (National Institutes of Health, Bethesda, MD).
Statistical analysis: Parametric data were analyzed by one way ANOVA (post hoc Tukey), and nonparametric data were analyzed by Kruskal-Wallis followed by a multiple comparison test using SPSS 13.0 (SPSS, Inc. Chicago, IL, USA). P<0.05 was considered as significant. Results were given as mean ± SEM of 40 observations each in 2nd, 24th and 48th h of study.
Results
Cell proliferation: In the cell culture model, with the appropriate media the cells proliferate, while death occurs as a result of end of cell life. The difference between proliferation and apoptosis determined the effects of our experiment. Effects of EPO, Gli, and Dia on proximal tubular cell proliferation were evaluated as functions of time in normoxia/hypoxia. EPO treatment increased cell proliferation in a dose- and time-dependent manner. Under normoxia, the number of cells increased in the course of time in all cell culture groups except Gli. A significant increase was seen in groups EPO 10 and EPO 50 between 2 and 48 h (P<0.001). The number of cells significantly increased at 48 h in groups EPO10 and EPO 50 when compared with the control (P<0.001). There was no significant difference between EPO 10 and EPO 50 groups. With the increase of dosage, the proliferative effect of EPO became more prominent at the 48th h (P<0.001) (Fig. 1). Under hypoxic conditions, the number of cells decreased over time in the control, EPO 1, and EPO 5 groups, but the differences were not significant. However, in the EPO 10 and EPO 50 groups, the number of cells increased over time but the differences were not significant. When we compared the number of cells at 48 h, EPO 10 and EPO 50 had a significantly increased number of cells as compared to the control group (P<0.05) (Fig. 1).
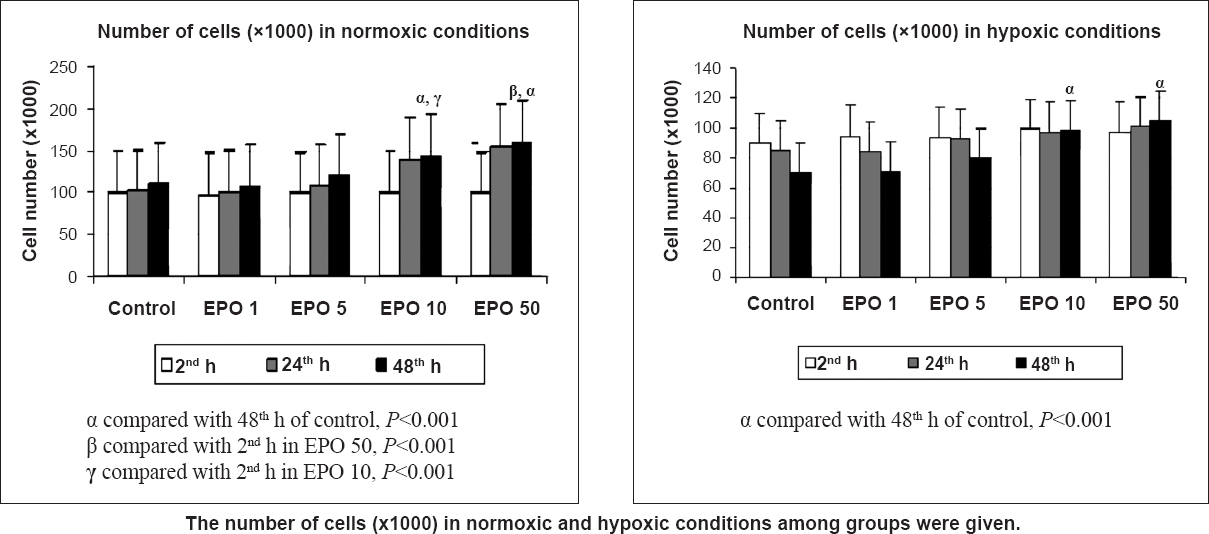
- Effects of different concentrations (1,5,10, 50 IU/ml) of erythropoietin (EPO) treatment on cell proliferation index in normoxia and hypoxia at 2, 24 and 48 h by the MTT assay. Data are presented as mean ± SE (n=40 observations).
The Gli treatment decreased the living cell number in a time- and dose-dependent manner. The number of cells in groups Gli 10 and 100 significantly decreased at the 48th h when compared with the control group (P<0.001). While the number of cells in the control group increased under normoxia, the number of cells decreased in groups treated with Gli. On the other hand, Dia treatment increased the number of cells in a time- and dose-dependent manner. The number of cells in Dia 100 were significantly increased at the 48th h when compared with the control group (P<0.001). Hypoxia decreased cell proliferation to 89, 85, and 69 per cent of the control group at 2, 24 (P<0.05), and 48 (P<0.01) h, respectively. In hypoxic conditions, the number of cells decreased significantly in group Gli 100 when compared with the control group (P<0.001). However, the number of cells treated with Dia significantly increased in a time-dependent manner under hypoxic conditions when compared with the control group (P<0.001) (Fig. 2). Glibenclamid also decreased the proliferative effect of EPO in both normoxic (P<0.001) and hypoxic (P<0.05) conditions. There were significant differences between the EPO 10 and EPO 10+ Gli 100 groups at 48 h (Fig. 2).
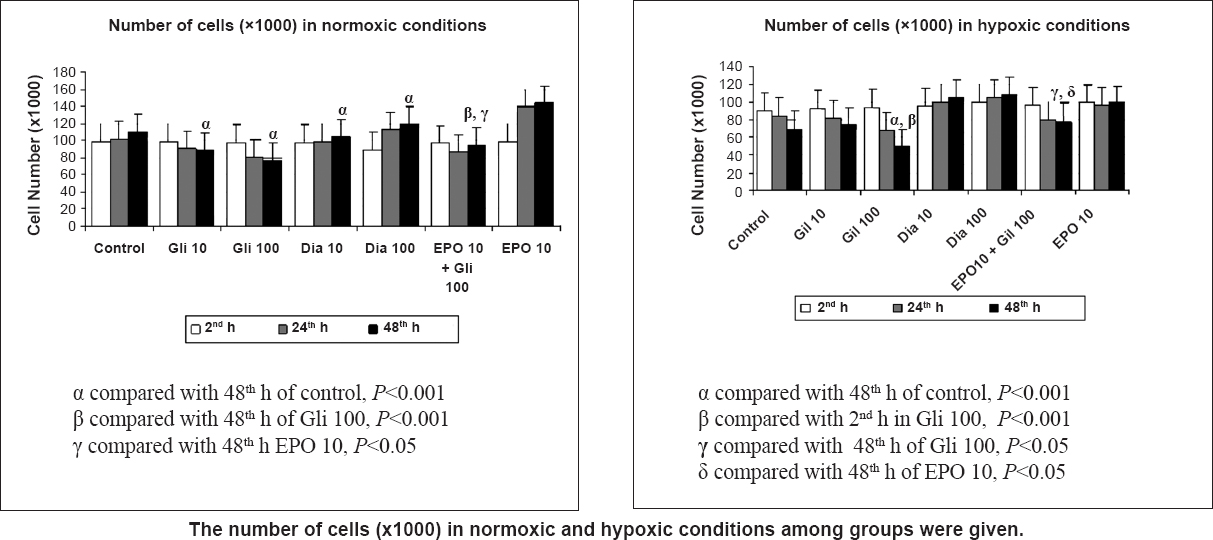
- Effects of glibenclamide (Gli 10,100 μM) and diazoxide (Dia 10,100 μM) treatment on cell proliferation index in normoxia and hypoxia at 2, 24 and 48 h by the MTT assay. Data are presented as mean ± SE (n=40 observations).
Determination of apoptosis: The apoptosis levels in the proximal renal tubule cells were determined by measuring the caspase-3 activity levels. In cell culture media, while cell proliferation continued, caspase activation led to apoptotic cell death. In normoxic conditions, apoptosis decreased with increased EPO amount over time (P<0.05). Similarly, treatment with Dia decreased the apoptosis over time in a dose-dependent manner (P<0.05). Glibenclamide treatment significantly increased apoptosis at 48 h as compared with the control group (P<0.05). Also, addition of Gli to EPO significantly increased the apoptosis when compared with the EPO 10 group (Fig. 3). Hypoxia significantly (P<0.01) increased the apoptosis at 48 h when compared with 2 h. EPO treatment decreased the caspase-3 levels when compared with the control group (P<0.01). Similarly, treatment with Dia decreased the apoptosis within time in a dose-dependent manner (P<0.01). Glibenclamide treatment increased the caspase-3 levels at 48 h when compared with the control group. Also, treatment with Gli significantly increased the caspase-3 levels over time (P<0.01). Addition of Gli to EPO significantly increased the apoptosis when compared with the EPO 10 group in hypoxic conditions (P<0.01) (Fig. 4).
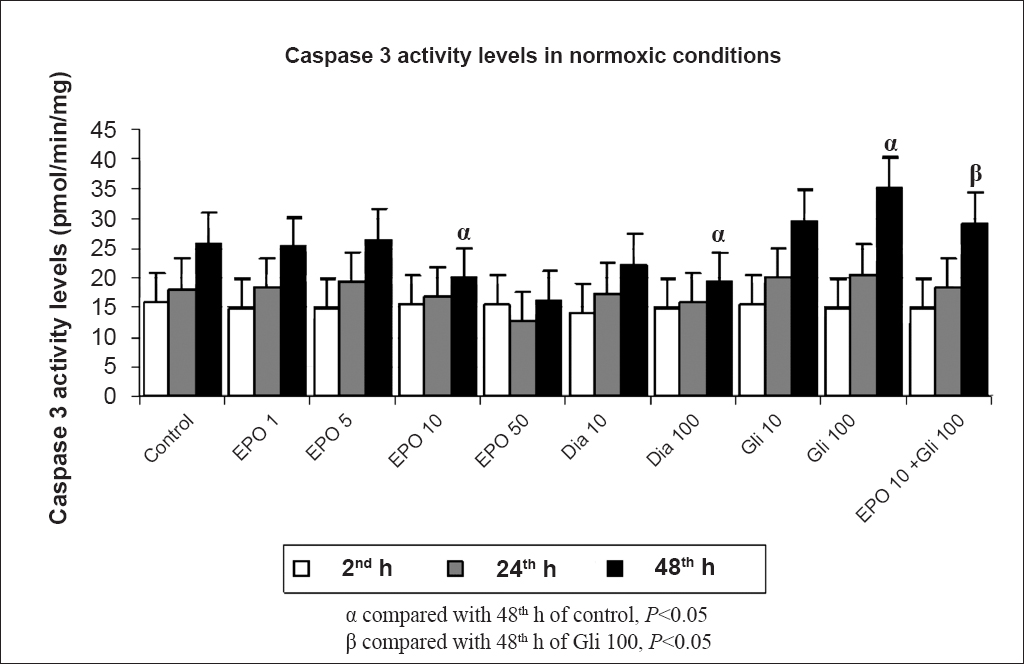
- Effects of different concentrations (1, 5, 10, 50 IU/ml) of erythropoietin (EPO) treatment, glibenclamide (Gli 10,100 μM) and diazoxide (Dia 10,100 μM) on apoptosis in normoxic conditions at 2, 24 and 48 h by the caspase-3 activity levels. Data are presented as mean ± SE (n=40 observations).
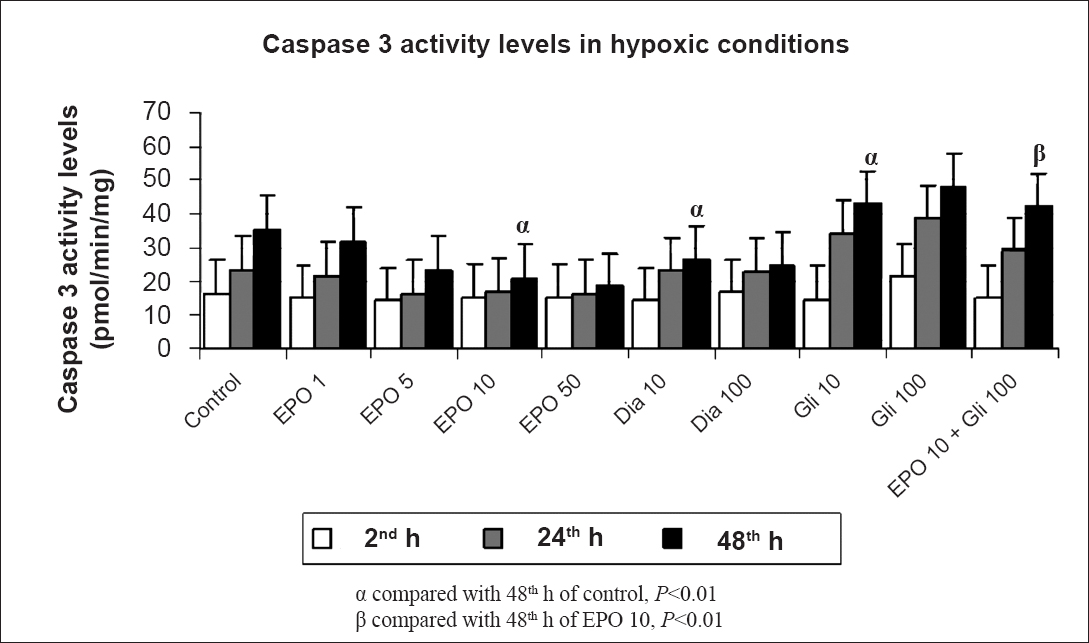
- Effects of different concentrations (1,5,10,50 IU/ml) of erythropoietin (EPO) treatment, glibenclamide (Gli 10,100 μM) and diazoxide (Dia 10,100 μM) on apoptosis in hypoxic conditions at 2, 24 and 48 h by the caspase-3 activity levels. Data are presented as mean ± SE (n=40 observations).
HIF-1 α mRNA expression: HIF-1 α mRNA expression was evaluated by semi-quantitative RT-PCR. EPO (10 IU/ml) and Dia (100 μM) treatment significantly increased (P<0.01) HIF-1 α mRNA expression, whereas Gli decreased it (P<0.05). Glibenclamide significantly (P<0.01) decreased EPO-induced HIF-1 α mRNA expression when compared with the EPO-only group (Fig. 5). In hypoxic conditions, HIF-1 α mRNA expression significantly increased when compared with the normoxic control group (P<0.01). Treatment with EPO in hypoxic conditions significantly increased HIF-1 α mRNA expression when compared with the expression of HIF-1 α mRNA in normoxic control, normoxic EPO, and hypoxic conditions (P<0.01) (Fig. 5).
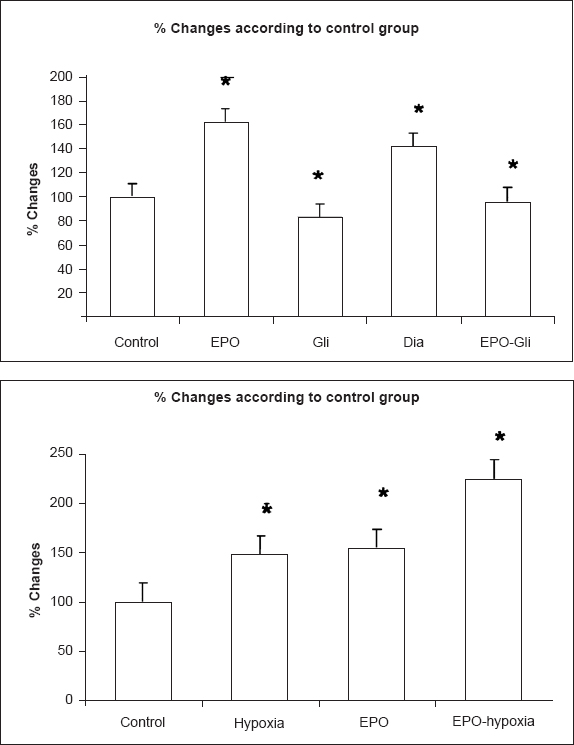
- HIF-1 α mRNA expression changes in control, EPO, diazoxide, glibenclamide groups under normoxic and hypoxic conditions. Results are given as % change compared with control. β-actin was used as internal control. (Con: control group, EPO: erytropoietin (10 IU/ml) group, Gli: glibenclamide (100 μM) group, Dia: diazoxide (100 μM) group, EPO-Gli: erytropoietin (10 IU/ml) and glibenclamide (100 μM) group) (n=40 observations). *P<0.01 compared with control.
Kir 6.1 protein expression levels: Kir 6.1 channel protein expression level was evaluated by Western blot. Anti-Kir 6.1 antibody was used and multiple bands between 40-55 kDa were obtained. According to datasheet of ab (Abcam) predicted band size is 48 kDa. Additional bands were also mentioned between 34 and 45 kDa. The bands of EPO (10 IU/ml) and Dia (100 µM) were more prominent than the others (P<0.01). KATP channel blocker Gli decreased Kir 6.1 (P<0.01). Glibenclamide significantly (P<0.01) decreased EPO and Dia-induced Kir 6.1 expression (Fig. 6).
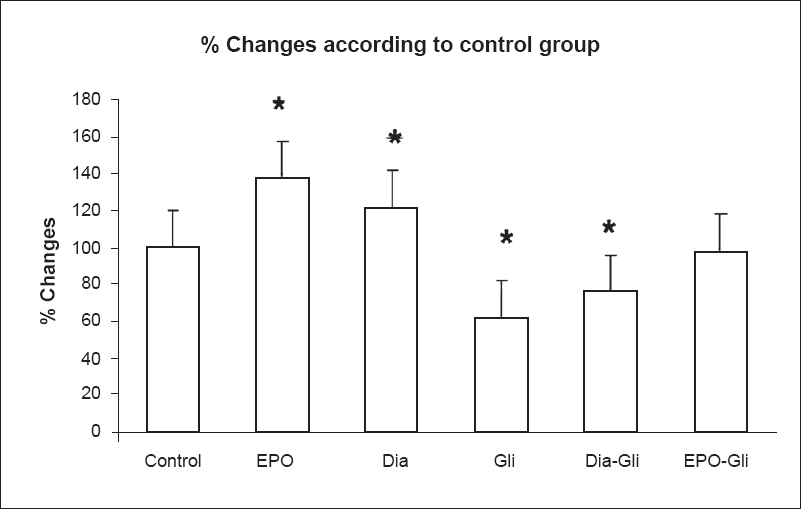
- Kir 6.1 protein expression changes in control, EPO, diazoxide, glibenclamide groups. Results are given as % change compared with control. β-actin was used as internal control (Con: control group, EPO: erytropoietin (10 IU/ml) group, Gli: glibenclamide (100 μM) group, Dia: diazoxide (100 μM) group, EPO-Gli: erytropoietin (10 IU/ml) and glibenclamide (100 μM) group) (n=40 observations). *P<0.01 compared with control.
Discussion
In the present study, EPO treatment was observed to protect the proximal tubular cells under normoxic and hypoxic conditions. EPO impaired apoptosis by reducing caspase-3 activity and increasing HIF-1 α mRNA expression. The effects of EPO were decreased by the KATP channel blockers.
Molecular cloning of the KATP channel subunit proteins Kir 6.x and SUR has shown that this channel consists of a number of subtypes (Kir 6.1, Kir 6.2, etc.); investigators have studied the regulation and function of this channel in different organs as well as under different pathophysiological situations. Previous studies showed that Kir 6.1 and Kir 6.2 protein levels change during myocardial ischaemia and ATP depletion and in response to the KATP channel openers pinacidil, cromakalim, and Dia. Glibenclamide completely abolished the increase in Kir 6.1 levels produced by pinacidil20. But the effect of EPO on Kir 6.1 channel protein expression levels was not known before.
KATP channels have important roles in cell membrane voltage protection, energy salvage, protection of cells against irreversible changes after ischaemia, the inhibition of calcium (Ca) deposition, control of intracellular Ca levels and cell volume, and the inhibition of apoptosis14. It has been shown that in vivo recombinant human erythropoietin (rhEPO) administration could initiate preconditioning-like mechanisms such as iNOS activation and KATP channel opening, and also that rhEPO administration would cause cardioprotection against ischaemic injury5. EPO has a close relationship with KATP channels. Activation of the EPO receptor causes activation of Janus kinase (JAK) and related phosphokinase, which is associated with caspases and protein kinases, and leads to activation of signal transducer and activator transcription 3 (STAT3), which is related to nuclear bcl gene activation and the NF-κB pathway8910. Intracellular Ca and voltage changes are the first to appear after I/R, followed by other intracellular mechanisms, activation of genes, and then systemic results. The opening of KATP channels is related to PKC activation, potentially through mitogen activated protein kinase (MAPK) activation, and these kinases are also activated by rhEPO10. We achieved similar results with our previous in vivo IR model4, in which the closure of KATP channels increased apoptosis in the cell culture model, an effect that was more prominent under hypoxic conditions. However, addition of EPO probably activates the proliferative and anti-apoptotic pathways and shows an ischaemic preconditioning-like effect by increasing HIF-1 α mRNA expression and related gene pathway. Glibenclamide abolishes the anti-apoptotic effect of EPO and the increase in HIF-1 α mRNA expression. Our results confirmed that activation of KATP channels was a part of the cytoprotective mechanisms of EPO treatment.
Previous studies have shown that long-term EPO usage decreased the toxicity of cisplatin and cylosporine, improved the tubular functions, and also decreased the caspase-3 levels even in haemorrhagic shock112122. Solling et al23 have shown that EPO can improve glomerular filtration rate after I/R via immunomodulatory effects manifested as decreased TNF-α and IL-10 in renal biopsies and reduced TNF-α in plasma. Also, it was shown in a renal IR model that EPO treatment decreased the serum creatinine concentration significantly when compared with saline treatment24. EPO treatment also decreased the transcription of inflammatory cytokines (IL-1β, IL-6, TNF-α, and IL-10) and chemokines (MCP-1)24. In addition to the anti-apoptotic and anti-inflammatory effects, EPO has anti-oxidative effects. EPO has been shown to decrease the MDA, superoxide dismutase, and catalase levels of rats exposed to aortic I/R renal injury1. These effects are thought to be mediated primarily through the activation of EPO receptor-mediated intracellular signalling pathways, including transcription factor NF-κB, phospatidylinositol 3-kinase, extracellular signal-regulated kinase 1 and 2, anti-apoptotic and pro-regenerative factor BMP-7, and HSP72-mediated Na+/K+ ATPase-α12526. Diazoxide 10-100 μM showed a cytoprotective effect in our study. The proliferative effect of Dia was significant under both normal and hypoxic conditions. In contrast to the KATP channel blocker Gli, Dia protected the renal tubular cell from apoptosis, which pointed to the effects of KATP channels in renal tubular cell ischaemia. The activation of KATP channels with EPO might be direct or indirect, through other signalling pathways downstream. EPO might have activated the mitochondrial KATP channels by activating PKC. In previous studies, Dia was shown to open the KATP channels, allowing calcium to exit the mitochondria, thereby protecting the mitochondria from the injury27. Diazoxide showed its effects by changing the membrane potentials and inhibiting the excess production of free oxygen radicals. The activated pathway was PKC, the activator of Janus-kinase / signal transducer and activator transcription (JAK/STAT) that was related to the EPO activity1516. The protective role of Dia obtained by PKC, (NO), and ion channels was the common pathway for EPO, as has been pointed out in several studies45627. The new recombinant EPO forms that are lacking in haematopoietic activity were shown to have cytoprotective effect via supression of caspase-3 activity and phosphorylation of Janus kinase (JAK2)28.
In addition to KATP channel activation, our results showed that EPO and Dia had a direct effect on Kir 6.1 protein expresion levels. Glibenclamide application inhibited both channel activation and Kir 6.1 protein expression levels in renal proximal tubule cells.
HIF-1 α gene expression is an important response to cellular stress conditions such as hypoxia and glucose deprivation. This gene activation starts several adaptation reactions, metabolic changes, induction of EPO production, and gene activation. As expected, under hypoxic conditions, HIF-1 α mRNA expression increased29, but in our study EPO administration also increased HIF-1 α mRNA expression. This effect could probably be due to the ischaemic preconditioning-like effect or activation of HIF-1 α via intracellular secondary messengers related to KATP channels29. While HIF-1 α mRNA expression increased, the cell proliferation increased but the caspase-3 levels decreased. Blockage of KATP channels by Gli decreases HIF-1 α mRNA expression even when EPO is present. This may be explained by the protein kinase, which is the common intracellular pathway for both KATP and EPO effects. Our results show that there is an internal feedback mechanism between EPO and HIF-1 α gene expression. In addition to KATP channels and HIF-1 α interactions, other ion channels like the Ca- and voltage-dependent K channels take part in hypoxic responses, and HIF-2 stabilization is very important in preconditioning mechanisms29.
Our data supported previous studies that EPO treatment significantly reduced renal apoptosis under hypoxic/ischaemic conditions. KATP channel activator Dia and blocker Gli have roles in Kir 6x subgroup channel expression levels. EPO has a similar and more significant role in Kir 6x channel activation and expression levels. Our results show that Kir 6x channels take part in the protective effects of EPO, and this may be important in warm ischaemia. Adaptive mechanism-related HIF-1 α gene activation also takes part in the protective role of EPO. In conclusion, ion channels play important roles in renal adaptation to ischaemic conditions. HIF-1 α activation is one of the critical components of this homeostatic mechanism. Understanding the mechanisms of signalling pathways that are involved in the protection of the kidney from I/R damage might be helpful in deciding on more effective and less harmful treatment protocols.
Acknowledgment
The study was supported by Gazi Universtiy Scientific Research Project Unit (SBE-01/2008-42) and TUBITAK (SBAG-108S242).
References
- The protective effect of erythropoietin on renal ýnjury ýnduced by abdominal aortic-ýschemia-reperfusion in rats. J Surg Res. 2008;149:206-13.
- [Google Scholar]
- Long-term effects of acute ischemia and reperfusion injury. Kidney Int. 2004;66:523-7.
- [Google Scholar]
- Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40:3279-88.
- [Google Scholar]
- The effect of K-ATP channel blockage during erythropoietin treatment in renal ischemia-reperfusion injury. J Invest Surg. 2008;21:340-7.
- [Google Scholar]
- Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009;42:554-61.
- [Google Scholar]
- Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J. 2003;17:1754-5.
- [Google Scholar]
- Erythropoietin protects the infant heart against ischaemia-reperfusion injury by triggering multiple signaling pathways. Basic Res Cardiol. 2005;100:187-97.
- [Google Scholar]
- Mechanisms of erythropoietin-mediated cardioprotection during ischemia-reperfusion injury: role of protein kinase C and phosphatidylinositol 3-kinase signaling. FASEB J. 2005;19:1323-5.
- [Google Scholar]
- Acute cardioprotective effects of erythropoietin in infant rabbits are mediated by activation of protein kinases and potassium channels. Basic Res Cardiol. 2004;99:173-82.
- [Google Scholar]
- Erythropoietin enhances recovery from cisplatin-induced acute renal failure. Am J Physiol. 1994;266:360-6.
- [Google Scholar]
- Pretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivo . Kidney Int. 2004;66:983-9.
- [Google Scholar]
- Mitochondrial ATP-sensitive potassium channel plays a dominant role in ischemic preconditioning of rabbit heart. Eur Surg Res. 2001;33:57-63.
- [Google Scholar]
- Protection of the spinal cord from ischaemia: comparative effects of levosimendan and iloprost. Eur Surg Res. 2008;41:1-7.
- [Google Scholar]
- Mechanism by which K(ATP) channel openers produce acute and delayed cardioprotection. Vascul Pharmacol. 2005;42:253-64.
- [Google Scholar]
- Opening of mitochondrial K ATP channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460-6.
- [Google Scholar]
- Synergistic effect of CGS16949A and 5-fluorouracil on a human breast cancer cell line. Eur Surg Res. 2001;33:232-6.
- [Google Scholar]
- Angiotension II induces hypoxia inducible factor-1 alpha in PC 12 cells through a posttranscriptional mechanism: role of AT2 receptors. Am J Nephrol. 2004;24:415-21.
- [Google Scholar]
- Decreased therapeutic effects of noscapine combined with imatinib mesylate on human glioblastoma in vitro and the effect of midkine. Cancer Cell Int. 2011;11:18.
- [Google Scholar]
- Channel activators regulate ATP-sensitive potassium channel (KIR 6.1) expression in chick cardiomyocytes. FEBS Lett. 1997;412:121-5.
- [Google Scholar]
- Erythropoietin enhances angiogenesis in an experimental cyclosporine A-induced nephrotoxicity model in the rat. Clin Exp Pharmacol Physiol. 2007;34:866-9.
- [Google Scholar]
- Erythropoietin protects severe haemorrhagic shock-induced organ damage in conscious rats. Injury. 2010;41:724-30.
- [Google Scholar]
- Erytropoietin administration is associated with short term improvement in glomerular filtration rate after ischemia/reperfusion injury. Acta Anaesthesiol Scand. 2001;55:185-95.
- [Google Scholar]
- Erythropoietin ameliorates renal ischemia and reperfusion injury via inhibiting tubulointerstitial inflammation. J Surg Res. 2012;176:260-6.
- [Google Scholar]
- Renoprotective effect of erythropoietin in rats subjected to ischemia/reperfusion injury: gender differences. Surgery. 2011;150:39-47.
- [Google Scholar]
- Erythropoietin regulates apoptosis, inflammation and tissue remodelling via caspase-3 and IL-1â in isolated hemoperfused kidneys. Eur J Pharmacol. 2011;660:420-30.
- [Google Scholar]
- Multiplicity of effectors of the cardioprotective agent, diazoxide. Pharmacol Ther. 2013;140:167-75.
- [Google Scholar]
- Cytoprotective effect of recombinant human erythropoietin produced in transgenic tobacco plants. PloS One. 2013;8:e76468.
- [Google Scholar]
- Regulation of gene expression by the hypoxia-inducible factors. Mol Interv. 2002;2:229-43.
- [Google Scholar]






