Translate this page into:
Role of anti-tissue transglutaminase IgA+IgG antibodies in detection of potential celiac disease in patients with type 1 diabetes
For correspondence: Dr Ranjana W. Minz, Department of Immunopathology, Postgraduate Institute of Medical Education & Research, Chandigarh 160 012, India e-mail: rwminz.minz88@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Celiac disease (CD) can exist in various forms in type 1 diabetes (T1D) patients and can remain undetected, leading to severe complications. This study was aimed to evaluate five commercially available anti-tissue transglutaminase (tTG) ELISA kits with distinct formats for the detection of CD and potential CD in T1D patients. Clinical and demographic profiles of the patients with different disease subsets were also studied.
Methods:
Fifty T1D patients with classical and non-classical symptoms of CD and 100 T1D patients without any symptoms of CD were included in this study. Anti-tTG autoantibody levels were estimated by five ELISA kits followed by histological examination of duodenal biopsy. HLA DQ2-DQ8 and DRB1-DQB1 typing was done, and serum levels for transforming growth factor (TGF)-β1 were also estimated.
Results:
Assay format detecting anti-tTG IgA antibodies against recombinant antigens along with neopeptides of gliadin was most efficient in the detection of CD in symptomatic patients, and assay format detecting IgA+IgG helped in the detection of potential CD in asymptomatic T1D patients. These findings were supported by histological examination and human leucocyte antigen analysis. Patients with potential CD were found to have markedly deranged glycaemic control parameters and also had significantly raised serum levels of TGF-β1, (P<0.05) compared to T1D patients.
Interpretation & conclusions:
Potential CD can be frequently seen in T1D patients. This can be attributed to the dietary patterns prevalent in the subcontinent and the genetic basis of the disease. Anti-tTG IgA+IgG antibodies can be useful in the detection of these potential CD cases in T1D patients. Early intervention with gluten-free diet can be considered in these patients for better disease management.
Keywords
Celiac disease
ELISA
human leucocyte antigens
tissue transglutaminase
type 1 diabetes
Type 1 diabetes (T1D) is an immune-mediated chronic disorder characterized by the destruction of pancreatic β-cells, leading to absolute insulin deficiency, hence resulting in hyperglycaemia. T1D is frequently associated with other autoimmune diseases such as celiac disease (CD), autoimmune thyroid disease (AiTD), vitiligo and uveitis. The coexistence of T1D and CD has been known for more than 50 yr, implicating that CD is more prevalent in T1D patients than normal population, with the reported prevalence ranging from 5 to 12 per cent1.
CD or non-tropical sprue is a systemic, chronic enteropathy, which has autoimmune origin and is induced by intolerance to gluten protein. It is characterized by certain intestinal (mucosal inflammation leading to abdominal symptoms and malabsorption of nutrients) and extraintestinal symptoms (anaemia, dermatitis, delayed puberty, stunted growth, etc.) and is histologically marked by villous atrophy2. Gluten, a protein complex present in wheat, rye and barley, has been regarded as the major trigger for the disease, as a remission-relapse phenomenon is observed on gluten-free diet (GFD) compliance and reintroduction of gluten in diet. Immune responses in CD are largely elicited against alcohol-soluble fraction of gluten, i.e. gliadin, thus leading to the production of antibodies such as antigliadin antibodies, anti-endomysial antibodies (EMAs) and more specifically antibodies against the enzyme tissue transglutaminase (tTG) that catalyzes the cross linking of the glutamine and lysine residues in gliadin34. The diagnosis of CD is based on serological and histological investigations that comprise positive EMA/tTG testing by serology followed by histological examination of duodenal biopsy with abnormalities such as villous atrophy, crypt hyperplasia and increased density of inflammatory cells in the epithelium and lamina propria2.
Both T1D and CD are multifactorial diseases where an interplay of genetic and environmental factors determines the disease outcome. More than 40 genes have been identified to be associated with T1D as well as CD, but there is a robust and primary association of CD with human leucocyte antigen (HLA) DQ2 (DQA1* 05/DQB1* 02) and DQ8 (DQA1* 0301/DQB1* 0302) and thus DQ2-DQ8 typing has been recommended as a tool for diagnosis/exclusion of CD5. DRB1* 03 and DRB1* 04 have been reported to be strongly associated with T1D, and a strong linkage disequilibrium between DRB1* 03 and DQB1* 02 has also been reported6.
Other than the florid forms, CD also presents as silent or as potential disease (pot CD). Silent CD is defined as the one where an individual lacks the symptoms of CD, but tests positive for antibodies and shows histological abnormalities on duodenal biopsy7, whereas in pot CD there is absence of symptoms and histological abnormalities, but presence of tTG/EMA antibodies8. Such cases may or may not develop definitive CD later. The prevalence of CD has been reported and reviewed fairly well in the general population of the Indian subcontinent9. However, the prevalence, diagnosis and clinical presentation of overt CD and its other forms such as pot CD and silent CD need to be studied in depth in T1D patients of the region. The present study was therefore, undertaken to evaluate five commercially available kits for the detection of pot CD and CD in T1D patients from north India so as to delineate an apt diagnosis kit for high-risk group of patients. ELISA kits with distinct assay formats were evaluated followed by HLA typing to understand the underlying genetics and disease characterization. The comparative clinical and demographic profiles of the pot CD-T1D, T1D and T1D-CD patients were also evaluated.
Material & Methods
Fifty consecutive T1D patients with symptoms of CD (group I) and 100 consecutive T1D patients without any suspicion of CD (group II) were enrolled in this study from August 2009 to 2013. These patients were attending the Diabetes Clinic in the departments of Endocrinology and Pediatrics, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. A written informed consent was obtained from all the patients after explaining them the prospects of the study. Diagnosis of the patients for T1D and CD was done by following the American Diabetes Association (ADA) guidelines and European Society for Pediatric Gastroenterology, Hepatology, and Nutrition criteria1011. One hundred and fifty healthy, voluntary, age- and sex-matched controls were enrolled in the study for genetic analysis of the disease. The controls were first screened for autoantibodies and oral glucose tolerance test, and those testing negative for both were enrolled in the study. The pediatric controls were school going children attending a healthy living camp of friends and family members of departmental staff.
The Institutional Ethics Committee approved the study protocol. Sample size was calculated by Epi Info release 7.1.4 (Centers for Disease Control and Prevention, http://www.cdc.gov/epiinfo). After the division of participants into two subgroups, the power of study obtained was 85 per cent. Five millilitre of peripheral venous blood sample was withdrawn under sterile conditions; 2.5 ml for serum separation in a plain vial and 2.5 ml for DNA extraction in EDTA vial.
Serum transglutaminase (tTG) ELISAs: The patients were screened for tTG IgA by using the following four commercially available ELISA kits: ELiA Celikey IgA (Varelisa Phadia, USA), Celichek IgA (Aeskulisa, Germany), anti-tTG IgA from Orgentec, Germany, and anti-TGlu IgA from Xema, Russia. tTG IgA+IgG was detected in the patients using Celichek IgA and IgG new-generation kits from Aeskulisa, Germany. These five kits were selected because of the variations in their detection formats as follows: Aeskulisa IgA and IgG kit detected both IgA and IgG against recombinant tTG along with neoepitopes of gliadin, Aeskulisa IgA detected only tTG IgA against recombinant tTG along with neoepitopes of gliadin, Orgentec detected IgA antibodies against the new-generation recombinant antigen of tTG, Varelisa detected IgA against recombinant antigen of tTG and Xema detected IgA against crude tTG antigen.
Anti-endomysial antibody (EMA) antibodies: Anti-EMA IgA antibodies were detected in the serum of patients in groups I and II by indirect immunofluorescence (Euroimmun, Germany). Tissue sections of primate intestine were used as substrate, and positivity was defined as the detection of honeycomb-like fluorescence in serum dilution of 1/40.
IgA quantification and transforming growth factor (TGF)-β ELISA: Semi-quantitative estimation of IgA was done in the samples by radial immunodiffusion assay (Bindiraid, Binding Site, USA). Serum levels of transforming growth factor (TGF)-β were estimated by ELISA set procured from BD Biosciences, USA.
DNA extraction and HLA DQ2- DQ8- and DRB1, DQB1 typing: DNA extraction was done using column-based kits from Axygen Biosciences, USA. Polymerase chain reaction-sequence-specific primer (PCR-SSP) typing was done for DQ2 (DQA1* 05/DQB1* 02) and DQ8 (DQA1* 0301/DQB1* 0302) by using kits from Innotrain, Germany. Complete HLA typing for DRB1, DRB3/4/5 and DQB1 loci was done using PCR-SSP-based kits procured from Texas Biogene, Taiwan.
Statistical analysis: Quantitative data were expressed as mean±standard deviation and frequency as applicable. Data were analyzed by receiver operating characteristic (ROC) curve, Chi-square test with Yates correction and Mann-Whitney test, taking healthy controls as reference by using SPSS software (Statistical Package for the Social Sciences, version 10.0, Chicago, IL, USA).
Results
In this prospective study, patients were included in two groups. Group I (n=50) included T1D patients with symptoms of CD and group II (n=100) included T1D patients without any symptoms of CD. The symptoms seen in 50 patients in group I included both intestinal and extraintestinal manifestations and these were found to be positive for anti-tTG antibodies by using four ELISA kits and duodenal biopsy histology of all these patients showed partial or subtotal villous atrophy, thus confirming the diagnosis of CD. All symptomatic cases were positive for anti-tTG antibodies and were diagnosed with overt CD. However, in seven of these samples, the titres obtained by Varelisa kit were much lower (i.e. 20-30 U/ml) than that by other kits (70-80 IU/ml), indicating a lesser sensitivity of this kit in the detection of CD. This was also evident from the AUC of 0.828 for this kit compared to that of 0.970 for Aeskulisa IgA kit and 0.950 for Orgentec kit. The Xema ELISA kit detected only 39 patients as positive, and 11 of these confirmed CD patients were found to be negative. Particulars of the kits used along with their detection formats, ROC cut-offs and AUCs are mentioned in Table I. Of these confirmed cases of CD, only 19 (38%) were positive for anti-EMA IgA antibodies.
| ELISA kit source | tTG antigen | Serum dilution | Incubation times (min) | Number of calibrators | Conjugates | Substrate | Proposed cut-off (U/ml) | Measuring range (U/ml) | ROC cut-off (U/ml) | AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| Aeskulisa, Germany | Human recombinant tTG crosslinked with gliadin specific peptides displays neo-epitopes of tTG | 1:101 | 30; 15; 15; 5 | 6 | Anti-human immunoglobulins conjugated to HRP and thimerosal 0.01 per cent | TMB | 15 | 0-300 | 14.93 | 0.970 |
| Aeskulisa, Germany | Human recombinant tTG cross linked with gliadin specific peptides displays neo-epitopes of tTG | 1:101 | 30; 15; 15; 5 | 6 | Anti-human immunoglobulins conjugated to HRP and thimerosal 0.01 per cent | TMB | 20 | 0-300 | 16.01 | 0.950 |
| Varelisa Phadia, USA | Recombinant human tTG | 1:101 | 30; 30; 10 | 6 | Anti-IgA HRP conjugate | TMB | 8 | 0.1-100 | 4.9 | 0.828 |
| Xema, Russia | tTG | 1:101 | 30; 30; 15 | 6 | Murine monoclonal antibodies to IgA coupled with HRP | TMB | 20 | 0-200 | 12.82 | 0.684 |
| Orgentec, Germany | Recombinant human tTG | 1:100 | 30; 15; 15; 5 | 6 | Anti-human IgA antibodies labelled with HRP | TMB | 10 | 0-200 | 14.4 | 0.963 |
HRP, horse radish peroxidase; TMB, 3,3’,5,5’-tetramethylbenzidine; tTG, transglutaminase; ROC, receiver operating characteristics; AUC, area under the curve
In group II, all the 100 patients were negative for anti-tTG IgA by ELiA Celikey IgA kit but four of these patients were detected positive by Celichek IgA, two were positive by Orgentec, three were detected positive by Xema and 37 patients were positive by Celichek IgA+IgG, Aeskulisa. To ensure that the patients detected positive by tTG IgG+IgA were not negative for tTG IgA due to IgA deficiency, these 37 patients were tested for IgA levels by radial immunodiffusion assay, but none of the patients were found to have IgA deficiency. Thereafter, duodenal biopsy was carried out in only five of these patients, and non-specific changes such as mild lymphoplasmacytic infiltrate and intraepithelial lymphocytes were observed. Due to the non-specific changes noticed in these five patients and absence of symptoms, duodenal biopsies were not done in the remaining 32 patients. These patients were screened for anti-EMA antibodies, and five of the 37 (13.51%) patients were detected positive (4+ grading).
These 37 patients exhibiting ambiguity on the status of CD were screened for genetic markers, i.e. DQ2-DQ8 haplotypes. All the 37 patients were positive for DQ2-DQ8 haplotypes, thus indicating a pot CD status. These patients were followed up for 24 months for the symptoms of CD. Only four of these patients reported of vague abdominal symptoms, and the rest 33 patients continued to be asymptomatic on the 24 month follow up. To determine the frequency of DQB1* 02 and DQB1* 03 distinctly, HLA typing for DRB1, DRB3/4/5 and DQB1 was done in all the patients. The frequencies obtained are mentioned in Table II. None of the allele was present at significantly increased or decreased frequency in either of the disease category. However, the susceptibility alleles for T1D and CD i.e. DRB1* 03, DRB1* 04, DRB3, DQB1* 02 and DQB1* 03 were present at higher frequency in pot CD cases compared to T1D cases without pot CD, although the difference was not significant. The protective alleles such as DRB1*15 and DRB1*10 were present at lower frequency in pot CD cases compared to T1D patients without CD.
| Allele | T1D (n=63) | T1D-pot CD (n=37) | T1D-CD (n=50) |
|---|---|---|---|
| Allele frequency (AF) in % | |||
| DRB1*01 | AF=3.1, NS | AF=5.6, NS | AF=0, P=0.04 |
| DRB1*03 | AF=89.1, χ2=76.4, P=6.9×10−20, OR=26.7 (11.2-64) | AF=92, χ2=47.2, P=3.5×10−12, OR=20.3 (7.3-56.3) | AF=92, χ2=74.5, P=1.1×10−19, OR=51.5 (15-75) |
| DRB1*04 | AF=28.1, χ2=5.1, P=0.019, OR=2.4 (1.1-4.9) | AF=38, χ2=4.4, P=0.02, OR=2.7 (1.1-6.2) | AF=32, χ2=8.4, P=0.003, OR=3.1 (1.4-4.2) |
| DRB1*07 | AF=4.7, χ2=6.9, P=0.006, OR=0.196 (0.05-0.67) | AF=11.1, NS | AF=4, χ2=6, P=0.01, OR=0.166 (0.03-0.72) |
| DRB1*08 | AF=1.6, NS | AF=2.8, NS | AF=8, NS |
| DRB1*09 | AF=1.6, NS | AF=5.6, NS | AF=4, NS |
| DRB1*10 | AF=3.1, χ2=6.7, P=0.006, OR=0.153 (0.03-0.66) | AF=0, P=0.005 | AF=0, P=0.003 |
| DRB1*11 | AF=3.1, χ2=6.7, P=0.006, OR=0.153 (0.03-0.66) | AF=8.3, NS | AF=4,χ2=4.4, P=0.03, OR=0.19 (0.04-0.86) |
| DRB1*13 | AF=3.1, χ2=5, P=0.01, OR=0.23 (0.06-0.80) | AF=2.8, χ2=5.88, P=0.005 | AF=2, χ2=8.49, P=0.003 |
| DRB1*14 | AF=4.7, χ2=7.9, P=0.004, OR=0.18 (0.05-0.616) | AF=2.8, χ2=3.8, P=0.02, OR=0.217 (0.04-0.95) | AF=2, χ2=8.82, P=0.001, OR=0.07 (0.01-0.5) |
| DRB1*15 | AF=10.9 χ2=16.2, P=2 × 10−5, OR=0.184 (0.07-0.43) | AF=3, χ2=11.6, P=2×10−4, OR=0.136 (0.04-0.46) | AF=6, χ2=12, P=4×10−4, OR=0.204 (0.08-0.5) |
| DRB1*16 | AF=0, NS | AF=0, NS | AF=2, NS |
| DRB3 | AF=89.1, χ2=9.4 P=0.001, OR=4.1 (1.6-10.29) | AF=97.2, χ2=6.6, P=0.006, OR=6.4 (1.4-28) | AF=94, χ2=10.56, P=0.001, OR=6.7 (2-22.7) |
| DRB5 | AF=15.6, χ2=13.8, P=1×10−4, OR=0.232 (0.106-0.504) | AF=5.6, χ2=14.9, P=6×10−5, OR=0.08 (0.02-0.36) | AF=12, χ2=13.1, P=2×10−4, OR=0.19 (0.07-0.48) |
| DQB1*02 | AF=90.6, χ2=57, P=1×10−15, OR=22.2 (8.4-58.8) | AF=92, χ2=39.3 P=1.4×10−11, OR=32 (7.4-138.6) | AF=96, χ2=54, P=2×10−15, OR=45.2 (10.5-193.5) |
| DQB1*03 | AF=29.7, NS | AF=37.8, NS | AF=34, χ2=4.9, P=0.02, OR=2.3 (1.1-4.7) |
| DQB1*05 | AF=17.2, χ2=24.9, P=1.8×10−7, OR=0.167 (0.08-0.34) | AF=13.9, χ2=18.38, P=4.8×10−6, OR=0.13 (0.04-0.35) | AF=8, χ2=32, P=2.1×10−9, OR=0.07 (0.02-0.2) |
| DQB1*06 | AF=9.5, χ2=31.5, P=6.8×10−9, OR=0.098 (0.04-0.24) | AF=13.9, χ2=20.18, P=3.5×10−6, OR=0.08 (0.02-0.29) | AF=10, χ2=24.8, P=3.7×10−7, OR=0.1 (0.39-0.28) |
AF, allele frequency; χ2, χ2 with Yates correction; OR, OR with 95% CI; OR, odds ratio; CI, confidence interval; T1D, type 1 diabetes; CD, celiac disease; NS, not significant
DRB1*03:01-DQB1*02:01 and DRB1*04:01-DQB1*03:02 haplotypes that were significantly associated with T1D and also with concomitant presence of T1D and CD were found to be predominantly present in the patients with pot CD. The presence of these haplotypes in the pot CD patients is shown in Fig. 1.
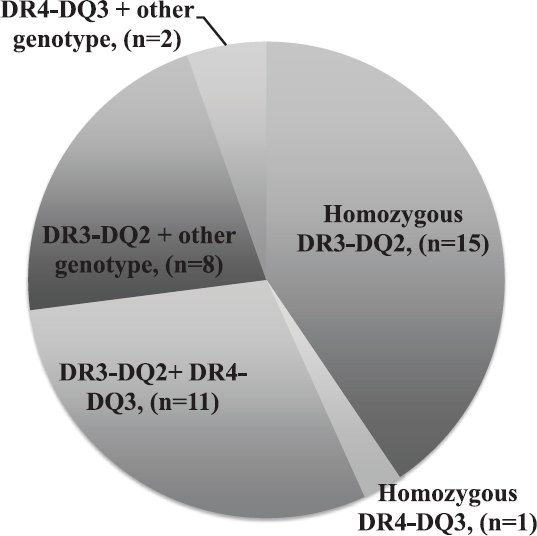
- Distribution of DR3-DQ2 and DR4-DQ3 haplotypes in patients with potential celiac disease.
The profile of these pot CD cases compared to other patients (i.e. patients with T1D and definitive CD and T1D patients without pot CD) is shown in Table III. It was observed that the parameters related to hyperglycaemia were more deranged in T1D patients with pot CD compared to patients with T1D only. These included higher HbA1c levels, lower body mass index, higher incidence of diabetic ketoacidosis and significantly (P<0.05) higher serum levels of TGF-β1 levels.
| Parameter | Group I (patients with T1D with symptoms of CD) (n=50) | Group II (patients with T1D without any symptoms of CD) (n=100) | |
|---|---|---|---|
| Patients with potential CD (n=37) | Patients without potential CD (n=63) | ||
| Mean age (yr) | 16.7±7.2 | 15.9±9.6 | 17.4±9.8 |
| Mean age of onset of diabetes (yr) | 11.5±4.67 | 11.4±4.9 | 12.1±5.6 |
| Age range (yr) | 3-45 | 3-45 | 4-60 |
| Sex ratio (male/female) | 1/1.9 | 1.3/1 | 1.5/1 |
| Mean duration of diabetes (months) | 63.70 | 54.69 | 64.4 |
| Diabetic ketoacidosis based hospitalization (%) | 67.5 | 60.0 | 46 |
| Mean C peptide levels (nmol/l) | 0.27±0.12 | 0.26±0.11 | 0.27±0.13 |
| Mean HbA1c (%) | 10.4±2.9 | 11.0±3.4 | 8.9±3 |
| Mean BMI (kg/m2) | 20±7.7 | 17.7±2.8 | 18.7±3.1 |
| Patients with family history of T1D | 2 | 6 | 3 |
| Patients with anti-GAD65 antibodies (%) | 72 | 56.75 | 60.9 |
| Patients with thyroid autoimmunity (%) | 32 | 21.62 | 21.8 |
| Patients with microvascular complications (%) | 38 | 29.7 | 23.12 |
| Serum TGF-β levels (pg/ml) | 1974.2±431.5* | 1231.6±368.1* | 797.3±119.3* |
| Patients presenting features for CD (%) | Abdominal symptoms-52 Osteopenia-6 Delayed puberty-16 Stunted growth-16 Anaemia-6 Miscellaneous-34 |
Abdominal symptoms-10.8 | None |
T1D, type 1 diabetes; CD, celiac disease; BMI, body mass index; HbA1c, haemoglobin A1c; GAD65, glutamate decarboxylase; TGF, transforming growth factor. *Mann-Whitney test compared to T1D patients without pot CD
Discussion
Several studies in the last decade have reported an increase in the prevalence of CD in the general population as well as in T1D patients in north India121314. However, these reports have shown data only from symptomatic CD cases, whereas a proportion of T1D patients may be asymptomatic or may exhibit modest/atypical symptoms for CD15.
The evaluation of the kits in symptomatic cases that were diagnosed with overt disease showed that Orgentec, Aeskulisa (both formats IgA only and IgA+IgG) and Varelisa Phadia had high specificity for the detection of CD, but amongst these, Varelisa Phadia was less sensitive. Fernández et al16 showed that for detection of CD in Saharawi population, kits by Aeskulisa and Orgentec were in the category of high specificity and low sensitivity. This conflict might be due to two main reasons. First, the study population in the two studies was different as general population in the former study and a high-risk group in our study. And second, the time gap between these two studies was large, during which the commercially available kits might have been improved for better sensitivity. In our study, the best kits for the detection of CD in symptomatic patients were Orgentec and Aeskulisa IgA. It also became evident that high titres of anti-tTG antibodies (5-10 times the cut-off) would suffice for the diagnosis of CD without any requirement of biopsy as these corroborated 100 per cent with the histology findings17.
Although detection of tTG IgA is widely accepted for the diagnosis of CD, and IgG is only used in individuals with IgA deficiency, in our study when tTG IgA+IgG was tested in the asymptomatic patients, 37 patients were detected positive1819. Picarelli et al20 have also shown that anti-EMA IgG1 antibody detection increases the prevalence of CD in T1D patients. Also, in our study, the titres obtained were 3-4 times the cut-off; therefore, could not be regarded as non-specific. These patients were further evaluated to check if they were pot CD cases or it was just an epiphenomenon. The duodenal biopsy findings in five of the patients were consistent with Marsh classification21, suggestive of either non-specific changes or an early/potential disease. The lack of duodenal biopsy in the other 32 patients was the major limitation of the study. EMA is considered to be more specific and less sensitive than tTG; and was present in only 38 per cent of the proven celiacs. In a study on the pot CD patients, 13.5 per cent were positive for anti-EMA IgA22. Anti-tTG IgG antibodies have been reported in several autoimmune diseases such as systemic lupus erythematous, ankylosing spondylitis and psoriatic arthritis23. The epitope specificity and mechanism of action of tTG IgG autoantibodies might be different than the classical IgA isotype. This helped to elucidate that the presence of anti tTG IgA+IgG antibodies was not an epiphenomenon and neither were the duodenal biopsy changes non-specific. A celiac-specific lower immune response was prevalent in these individuals, and it is already known that T1D patients often present with the subclinical form of CD24.
In our study, all the patients were DQ2-DQ8 positive, and no significant difference was found in the DR and DQ genotypes of the proven CD and pot CD patients. The high-risk genotypes of T1D, i.e. DR3-DQ2 and DR4-DQ3 were present in all of these individuals, and it is known that homozygosity for DR3-DQ2 carries 33 per cent risk for the presence of anti-tTG antibodies25. The five patients in whom EMA was detected positive had DR3-DQ2 genotypes, along with DR4-DQ3 in three and DRB1*14 and DRB1*11 in each of the other two. No major differences were seen in the demographic and clinical profile of the pot CD and CD patients, which indicated that these patients were not in the prodrome phase. Furthermore, the sex ratio in pot CD patients showed male dominance contrary to CD, which showed female preponderance. The patients with T1D-pot CD had higher incidence of micovascular complications detected at an early age and also had more deranged glycaemic control parameters; therefore, the option of GFD in these patients needs to be considered and its long-term impact on clinical presentation should be evaluated. The TGF-β levels were significantly different in the three groups of the patients, being the highest in CD patients, moderate in pot CD and lower in only T1D patients. TGF-β plays a major role in celiac lesion26. The levels reflected the same as the highest levels were detected in patients with the worst lesion, i.e. subtotal or partial villous atrophy.
Our study emphasized that the strategies for screening CD in low-risk and high-risk groups should be different. We put forth the screening algorithm for CD in high-risk group of T1D patients as shown in Fig. 2. In low-risk groups, ELISAs based on tTG IgA targeted against recombinant human tTG antigen with neopeptides of gliadin should be employed. The high titres (5-10 times the cut-off value) obtained in these patients will be in accordance with partial/subtotal villous atrophy on duodenal biopsy findings. The individuals with low titres should be closely followed up for rising titres. In high-risk group of T1D, presence of CD, pot CD and silent CD should be screened in a prudent manner. This can be done by employing tTG IgA+IgG-based kits with neopeptides of gliadin, followed by HLA typing for DQ2-DQ8. It is also recommended to follow up such patients at regular intervals to keep check on their progression to overt CD.
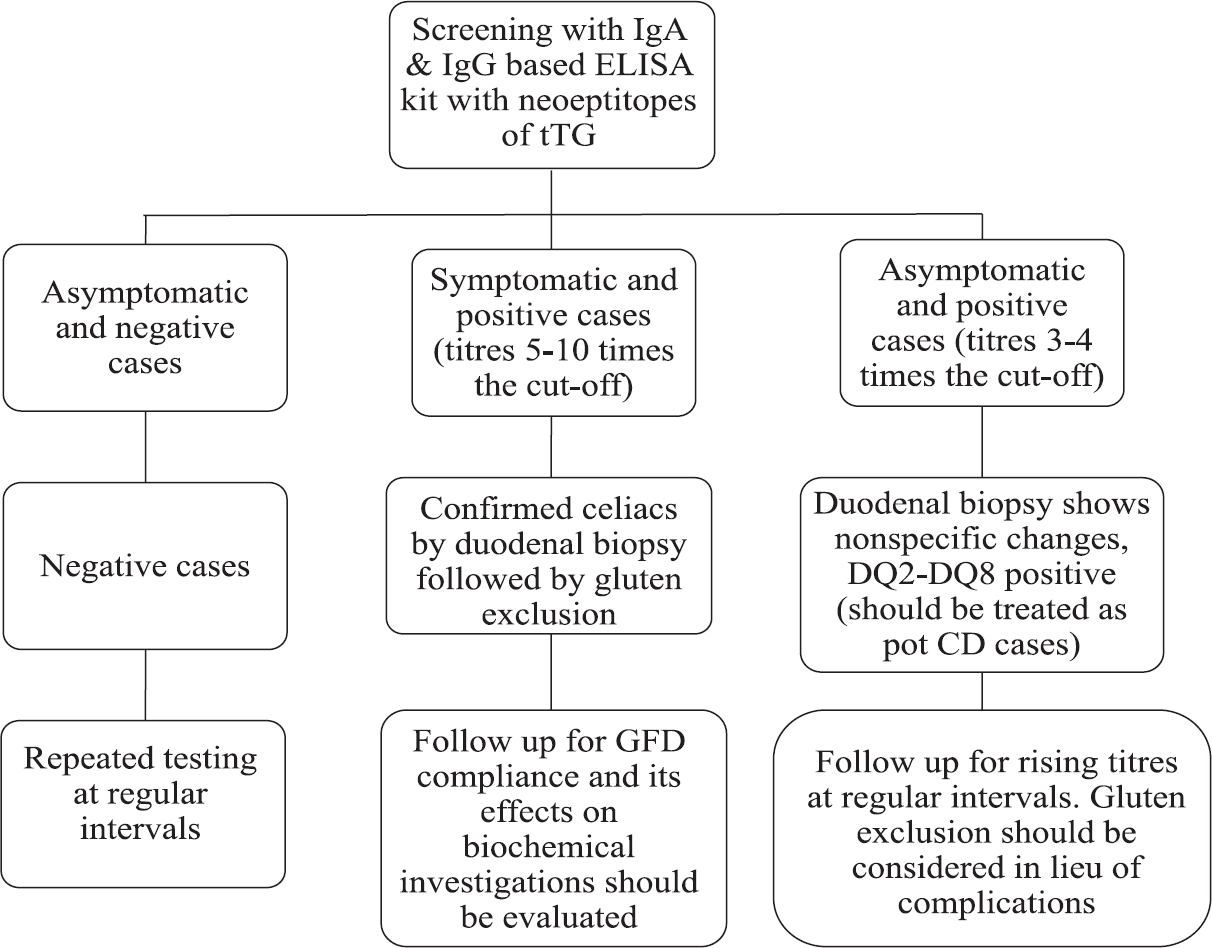
- Screening algorithm for celiac disease in high-risk group of type 1 diabetes patients. tTG, tissue transglutaminase; CD, celiac disease; GFD, gluten free diet.
Larger multicentric studies are needed to formulate the diagnosis and treatment strategies in these patients. Research should also be done in this subset of patients in terms of impact of GFD on gut inflammation, improvement of immunological parameters and better glycaemic control.
Financial support & sponsorship: The authors acknowledge Institute's research grant and the Indian Council of Medical Research, New Delhi, for financial assistance.
Conflicts of Interest: None.
References
- Celiac disease: Pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv Pediatr. 2008;55:349-65.
- [Google Scholar]
- Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234-42.
- [Google Scholar]
- Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713-7.
- [Google Scholar]
- Current approaches to diagnosis and treatment of celiac disease: An evolving spectrum. Gastroenterology. 2001;120:636-51.
- [Google Scholar]
- The spectrum of celiac disease: Epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol. 2010;7:204-13.
- [Google Scholar]
- Celiac disease: Can we avert the impending epidemic in India? Indian J Med Res. 2011;133:5-8.
- [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes-2013. Diabetes Care. 2013;36(Suppl 1):S11-66.
- [Google Scholar]
- European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-60.
- [Google Scholar]
- Prevalence of celiac disease in the northern part of India: A community based study. J Gastroenterol Hepatol. 2011;26:894-900.
- [Google Scholar]
- Prevalence and clinical profile of celiac disease in type 1 diabetes mellitus in North India. J Gastroenterol Hepatol. 2011;26:378-81.
- [Google Scholar]
- IgA anti-transglutaminase autoantibodies at type 1 diabetes onset are less frequent in adult patients and are associated with a general celiac-specific lower immune response in comparison with nondiabetic celiac patients at diagnosis. Diabetes Care. 2012;35:2083-5.
- [Google Scholar]
- Comparison of six human anti-transglutaminase ELISA-tests in the diagnosis of celiac disease in the Saharawi population. World J Gastroenterol. 2005;11:3762-6.
- [Google Scholar]
- Intestinal biopsy is not always required to diagnose celiac disease: A retrospective analysis of combined antibody tests. BMC Gastroenterol. 2013;13:19.
- [Google Scholar]
- IgG(1) antiendomysium and IgG antitissue transglutaminase (anti-tTG) antibodies in coeliac patients with selective IgA deficiency. Working Groups on Celiac Disease of SIGEP and Club Del Tenue. Gut. 2000;47:366-9.
- [Google Scholar]
- Anti-endomysial antibody of IgG1 isotype detection strongly increases the prevalence of coeliac disease in patients affected by type I diabetes mellitus. Clin Exp Immunol. 2005;142:111-5.
- [Google Scholar]
- The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-94.
- [Google Scholar]
- Endomysial antibody in the diagnosis and management of coeliac disease. Postgrad Med J. 2000;76:466-8.
- [Google Scholar]
- IgG anti-tTG responses in different autoimmune conditions differ in their epitope targets and subclass usage. Mol Immunol. 2015;67:369-76.
- [Google Scholar]
- Screening for celiac disease in children with type 1 diabetes: Two views of the controversy. Diabetes Care. 2003;26:1932-9.
- [Google Scholar]
- One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143-8.
- [Google Scholar]






