Translate this page into:
Role of 72 kDa protein of Leptospira interrogans as a diagnostic marker in acute leptospirosis
Reprint requests: Dr Mehdi Riazi, School of Pharmaceutical Sciences, Universiti Sains Malaysia 11800 Penang, Malaysia e-mail: mehdi@usm.my
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Leptospirosis is a widespread zoonotic disease and a public health problem, particularly in tropical and subtropical countries. Varied clinical manifestations of the disease frequently lead to misdiagnosis resulting in life-threatening multi-organ complications. Therefore, early laboratory investigation using an appropriate diagnostic approach is crucial. In the present study, a potential protein marker was identified and evaluated for its usefulness in the serodiagnosis of acute leptospirosis.
Methods:
Leptospira interrogans serovar Icterohaemorrhagiae (L44), which represents a commonly prevalent serovar in Malaysia, was cultivated for preparation of sequential protein extract (SEQ). SDS-PAGE and immunoblotting were performed with a serum panel comprising confirmed cases of leptospirosis and controls (n=42 each). Identification and characterization of the highest scoring protein from the antigenic band was performed. Subsequently based on the nucleotide coding sequence of the protein, the corresponding recombinant protein was custom-produced. It was then evaluated for sensitivity and specificity by testing against 20 serum samples from leptospirosis patients and 32 from controls.
Results:
Among the antigenic components, a 72kDa protein band demonstrated significant sensitivity (83.3%) and specificity (95.2%) for the detection of specific anti-leptospiral IgM antibodies. The protein was identified by mass-spectrometry analysis as heat shock protein DnaK of L. interrogans. Recombinant form of the protein (r72SEQ) showed 85 per cent sensitivity and 81 per cent specificity for the detection of specific anti-leptospiral IgM antibodies.
Interpretation & conclusions:
The findings of our study indicate that a protein (72kDa) of L. interrogans has the potential utility of being used for the diagnosis of acute leptospirosis. Further studies need to be done to confirm these findings.
Keywords
Acute leptospirosis
diagnosis
mass-spectrometry
recombinant protein
sequential protein extract
Leptospirosis is a zoonotic disease caused by spirochetes from the genus Leptospira. It is a major public health concern in tropical and subtropical countries, affecting about one billion people in urban slums. More than half a million severe cases are reported annually with mortality rate exceeding 10 per cent1. In Malaysia the seroprevalence has been reported to be 12 per cent23. There are more than 250 serovars identified worldwide, and 37 of these have been isolated in Malaysia3. Several outbreaks in Malaysia have been recorded particularly during flooding caused by monsoon or heavy rain4. The clinical symptoms of leptospirosis are non-specific particularly during the early phase of the disease, thus making it almost impossible to confirm the diagnosis without laboratory investigations. In addition, early diagnosis of leptospirosis is essential in regions where other febrile illnesses such as dengue, malaria, and typhoid are endemic. Misdiagnosis or delayed diagnosis may lead to wrong or delayed treatment. Institution of timely antibiotic therapy is critical in preventing serious multi-organ complications resulting in kidney and liver failure and a high mortality56.
Despite various modalities available for laboratory diagnosis of leptospirosis, antibody detection appears to be the preferred diagnostic tool. Antigen detection may not be advantageous in a clinical setting, mainly due to the organism's short transit time in the blood circulation (4-7 days) and its intermittent presence in other body fluids. Microscopic agglutination test (MAT) remains the standard reference method, however, as the method is technically demanding, requires maintenance of live culture and is time consuming, it is limited to reference laboratories. Other serological tests such as latex agglutination, indirect haemagglutination and ELISA have demonstrated unacceptable level of sensitivities (<72%), despite having good specificities (89-95%), particularly during the early phase of the infection78910. There is a need to identify new markers particularly those useful for timely diagnosis of acute leptospirosis. Thus, this study was aimed at identifying new protein marker(s) for detection of acute leptospirosis.
Material & Methods
This study was conducted in laboratory of School of Pharmaceutical Sciences, and Institute for Research in Molecular Medicine both at Universiti Sains Malaysia (USM), Penang, Malaysia. The study period was from September 2010 to December 2011. The study protocol was approved by the USM Human Research Ethics Committee.
Leptospiral culture and antigen preparation: A 100 ml culture of Leptospira interrogans serovar Icterohaemorrhagiae strain RGA (L44) was prepared using commercial liquid Ellinghausen-McCullough-Johnson-Harris (EMJH) medium (Difco, USA). The medium was prepared according to the manufacturer's instruction and placed in an incubator shaker at 30°C for seven days. Cells were harvested and washed twice with phosphate-buffered saline, pH 7.2 (PBS) by centrifugation at 10000 × g for 10 min at 4°C. The washed pellet was suspended in 40mM tris (pH 7.8) at a ratio of 1:2 and vortexed vigorously for 5 min. The cell suspension was then subjected to three cycles of freeze-thawing using liquid nitrogen and 37°C water bath for 5 and 10 min, respectively. The cell lysate was centrifuged and the pellet was mixed with 2ml of commercial sample buffer (Solution A, Agilent 3100 OFFGEL Fractionator, USA) containing urea, thiourea, dithiothreitol (DTT) and glycerol. The mixture was vortexed, centrifuged and the resulting supernatant was used as the protein antigen for subsequent analysis. Protein content of the leptospiral sequential protein extract (SEQ) antigen preparation was determined using a reducing agent and detergent compatible RC DC™ assay (BioRad, USA), aliquot and stored at -80°C.
Serum samples: All serum samples used in this study were those stored at USM serum bank and coded to maintain anonymity. A total of 42 serum samples collected from cases of acute leptospirosis within 10 days from onset of symptoms, were used. In addition to strong clinical diagnosis, these serum samples had been tested positive by three serological tests namely, microscopic agglutination test (MAT), latex agglutination (Tek Dri-Dot™,BioMerieux, France) and IgM lateral flow test (Omega Diagnostics, United Kingdom). These were performed to ensure that the samples had demonstrable IgM antibodies to Leptospira. Based on MAT, the serum samples used in this study reacted with serovars of the following serogroups: Icterohaemorrhagiae, Autumnalis, Pyrogenes, Bataviae, Grippotyphosa, Canicola, Australis, Pomona, Javanica, Sejroe, Djasiman, Tarassovi, and Hebdomadis.
Control samples comprised 42 serum samples, 24 were from patients with other febrile diseases [dengue (n=11), malaria (n=5), typhoid (n=3), toxoplasmosis (n=4) and amoebic liver abscess (n=1)], and the remaining 18 samples were taken from healthy blood donors. The control serum samples were also tested with the same three tests to ensure that these had no IgM antibodies to Leptospira. Prior to use, all serum samples were pre-absorbed with (RF) absorbent (Virion-Serion, Germany) to remove rheumatoid factors, thus excluding non-specific reactions.
SDS-PAGE: SDS-PAGE was performed using Mini-PROTEAN 3 apparatus (Bio-Rad, Hercules, CA, USA) and Laemmli's standard protocol11. Briefly, 16 μl of the liquid protein preparation was mixed in 4 μl of 5× final sample buffer, placed in 37°C incubator for 10 minutes, then centrifuged. The supernatant was loaded onto 10 per cent resolving gels at 100 volts. For gel staining, Coomassie brilliant blue (BioRad, USA), or mass spectrometer-compatible silver stain were used (Thermo Fisher Scientific, USA).
Two dimensional electrophoresis: The first dimension electrophoresis was performed using 3100 OFFGEL fractionator (Agilent Technologies, USA) and run according to the manufacturer's instructions. Two mililiters of the leptospiral SEQ antigen preparation containing 2 mg protein was loaded onto 12 well-frame using immobilized pH gradient (IPG) strips of pH 4-7. Fractionation process was started at 500V, 50 μA and 200 mW followed by separation at maximum 8000 V, 100 μA and 300 mW until 50 kVh was reached. Each fraction was then concentrated to half its volume using Speed Vac (Thermo Fisher Scientific, USA). The second dimension electrophoresis by SDS-PAGE was performed as described above.
Immunoblotting: The electrophoresed SDS-PAGE gel was transferred onto a nitrocellulose paper (NCP) with 0.45μm pore size (GE Osmonic, USA) using a semi-dry transblot (BioRad, USA). The NCP strips were blocked by Super Block® (Pierce, USA) and incubated overnight with diluted serum (1:50) at 4°C. After a washing step, the strips were incubated with monoclonal anti-human IgM conjugated with horseradish peroxidase (HRP) (Zymed, USA) at 1: 8000 or monoclonal anti-human IgG-HRP dilution (Zymed, USA) at 1:2000. The reactivity was detected using chemiluminescence substrate (Roche Diagnosrtic, Germany) and developed on X-ray film (Kodak, USA). The molecular weights (MW) of the antigenic bands were determined using molecular weight markers (BioRad, USA) and image analyzer (SynGene, USA). Sensitivity was determined based on the number of leptospirosis serum samples (n=42) that were reactive with an antigenic band. Specificity was based on the number of serum sample from other infections and healthy controls (n=42) which were reactive with the antigenic band.
Protein preparation for mass spectrometry analysis: The identified silver-stained protein band was manually excised and subjected to destaining by transferring it into a 1.5 ml microfuge tube containing 100 mM sodium thiosulphate and 30 mM potassium ferricyanide (1:1 ratio) for 15-20 min. After a brief centrifugation, the supernatant was removed, 100 μl of 200 mM ammonium bicarbonate was added and left for 15-20 min at room temperature and the supernatant was removed. The washing and de-staining steps were repeated twice until the silver stain was completely removed. The sample was sent to the Proteomic Center, National University of Singapore for mass-spectrometry analysis using ABI 4800 Proteomic Analyzer MALDI-TOF/TOF mass-spectrometer (Applied Biosystems, Foster City, USA). Identification and characterization of the 72kDa protein was performed by comparing their peptide mass against the built-in MASCOT search engine with Protein Pilot proteomic software (4800 Proteomics Analyzer, Applied Biosystems, USA), a computer generated database of tryptic peptides of known proteins.
Western blot analysis of custom-produced recombinant protein: The nucleotide coding sequence of highest scoring protein identified from the antigenic band was sent to a scientific company (GenScript, USA) for custom production of recombinant protein by gene synthesis, cloning and expression using their proprietary expression system.
SDS-PAGE, protein transfer and IgM immunoblot were performed as described earlier using serum samples as the primary antibody and anti-human IgM-HRP as the secondary antibody. Determination of sensitivity and specificity of the recombinant protein was performed using 20 serum samples from leptospirosis patients and 32 randomly selected control serum. The latter comprised five from patients with dengue, three with malaria, two with typhoid and the rest were from healthy individuals.
Results
Gel electrophoresis and immunoblotting: The protein content of the leptospiral SEQ antigen preparation was determined to be 2.8-3.4 mg per 100 ml bulk culture. SDS-PAGE profiles were observed in gels stained with Coomassie blue and mass spectrometry-compatible silver stain. Most bands were not clearly seen with the former, while the latter showed a profile of complex pattern of bands with molecular weights ranging from <15 to >150 kDa. The immunoblot assay using the panel of positive and control leptospirosis serum samples showed strong consistent seroreactivity to a 72 kDa band in the IgM immunoblot assay (Fig. 1), however, no reactivity was observed in IgG immunoblot (data not shown). Although other reactive bands could be seen in the IgM blots, they were not consistently present when probed with the panel of sera from leptospirosis pateints. The overall sensitivity and specificity of the 72 kDa band to detect specific anti-leptospiral IgM antibodies were found to be 83.3 per cent (35/42) and 95.2 per cent (40/42), respectively.

- A representative IgM Western blot of leptospiral SEQ antigen using serum samples from leptospirosis patients with different serogroups, as well as control serum samples. Lanes (1) Djasiman serogroup, (2) Australis serogroup, (3) Icterohaemorrhagiae serogroup, (4) Bataviae serogroup, (5) dengue patient serum, (6) normal serum, (7) typhoid patient serum, (8) toxoplasmosis patient serum, M: molecular weight marker.
Two-dimensional electrophoresis: The 72kDa protein bands mainly appeared in fractions 6-8, which corresponded to the pH 5.25 - 6.00. The fractions were then subjected to Western blot assay using confirmed leptospirosis and control serum samples. Intense reactivity at 72 kDa was observed in IgM immunoblot of fraction 6.
Mass-spectrometry analysis: Analysis of the 72 kDa protein by mass-spectrometer revealed a significant homology match (P<0.05) of a top scoring protein to be heat shock protein DnaK of L. interrogans (score of 124; cut-off score =85), gi 2735761.
Western blot evaluation of antigenicity of the recombinant protein form of the 72 kDa (r72SEQ): Evaluation using serum samples from 20 leptospirosis patients and 32 controls (remaining serum from the panel previously used for the native protein) showed that r72SEQ was sensitive (85%, 17/20) and specific (81%, 26/32). Fig. 2 shows representative IgM immunoblot results using patients’ serum samples containing heterologous anti-leptospiral antibodies, and control serum.
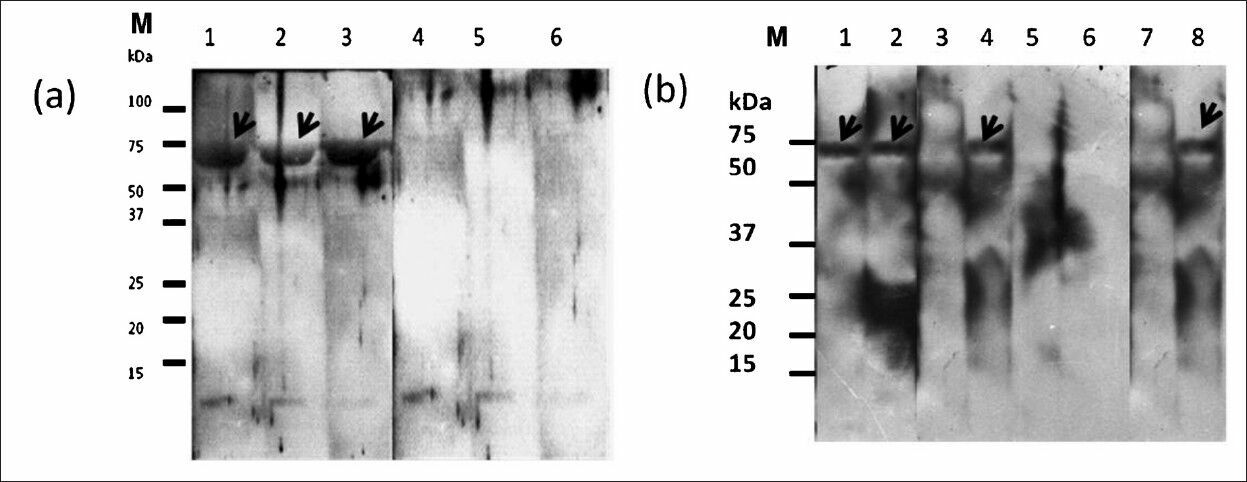
- Representative IgM Western blot results of r72SEQ probed with serum samples from leptospirosis patients with various serogroups and control serum samples. (a) Lane (1) Djasiman serogroup, (2) Bataviae serogroup, (3) Autumnalis serogroup, (4) dengue patient serum, (5) amoebic liver abscess patient serum, (6) typhoid patient serum, M, molecular weight markers. (b) Lane (1) Icterohaemorrhagiae serogroup, (2) Javanica serogroup, (3) Toxoplasmosis patient serum (4) Australis serogroup, (5) healthy blood donor serum, (6) dengue patient serum, (7) typhoid patient serum, (8) Pomona serogroup, M, molecular weight markers.
Discussion
Despite the availability of a number of serological methods of diagnosing leptospirosis, the definitive serological investigation remains the microscopic agglutination test (MAT)6. It is highly specific but is not adequately sensitive for clinical management of early infection910. The diagnostic value of MAT is strongly linked to the time of the sample collection and the serogroups in the MAT panel used for each locality. DNA-DNA hybridization and PCR have also been used for Leptospira detection but are laborious, expensive and time consuming, thus unlikely to be implemented for routine work particularly in developing countries71012.
There are many surface exposed proteins such as OmpL1, LipL32, LipL21, LipL36, and LipL41 that have been reported to stimulate specific antibody response in animal models13. Most of these protein antigens have shown a high sensitivity and specificity for detection of leptospirosis with convalescent-phase serum, however, these performed poorly with acute-phase serum1415. Thus, there is a need to identify new protein antigen(s) with improved sensitivity for acute leptospirosis.
In this study, protein preparation from one of the most prevalent pathogenic serovars in Malaysia was tested on a set of patients’ serum samples from various parts of Malaysia who were infected with a variety of Leptospira serogroups. In a previous report, heat shock protein DnaK has been reported as one of seven proteins that were reactive in immunoblots using 150 serum samples from Brazil and Barbados16. However, there are several differences noted between their study and the present findings. One difference was in the method of antigen preparation whereby in the previous study Leptospira organisms were directly added to sample buffer before electrophoresis. Further, the previous study used IgG instead of IgM immunoblot and the molecular weight of the band was reported to be 76 kDa. In addition, identification of the band as heat shock protein DnaK in that study was based on its reactivity with antisera to DnaK, and not by mass-spectrometry. By fractionation of the Leptospira proteins, the authors reported that 76 kDa protein was found in the cytoplasm and cytoplasmic membrane of the bacteria16. In this study heat shock protein DnaK was detected in the hydrophobic fraction of the protein preparation, thus it was probably derived from the membrane protein of the cytoplasm.
The patients serum samples used in the present study were from acute leptospirosis cases (≤ 10 days of symptoms), while 42 control samples were from patients with other related infections and from healthy individuals. Therefore, specific IgM antibody recognition of heat shock protein DnaK by the patients’ serum is a strong indication that this protein is specifically expressed during early stage of the infection. This is consistent with results reported by Guerreiro et al16.
Pol and Bharadwaj17 utilized a heat extracted antigen from non-pathogenic leptospires and purified it by high-performance liquid chromatography (HPLC). This 50 kDa protein fraction was reported to have a specificity and sensitivity of 93.3 and 85.0 per cent, respectively. In another study, MPL17 and MPL21 surface protein antigens were evaluated with serum samples from patients in the early and convalescent phases of leptospirosis. The prevalence of total IgG antibodies against MPL17 and MPL21 were 38.5 and 21.2 per cent, respectively. However, IgM-ELISA was positive with only MPL21 and the antibody level was not significantly different from that of MAT18.
Recombinant protein-based serological test can attain consistent high sensitivity and specificity because of high concentration of effective antigen, and the absence of non-specific moieties which are present in whole-cell preparations. In the present study, recombinant protein to L. interrogans heat shock protein DnaK (r72SEQ) showed 85 per cent sensitivity and 81 per cent specificity. The sensitivity of the native protein (83%) is comparable to the recombinant protein. However, the former seemed to have higher specificity than the latter, and the reason is not clear. It is possible that post-translational modification, which has been reported in Leptospira19, may be a reason for the higher specificity of the native protein. More accurate sensitivity and specificity rates can be obtained by testing a large number of samples.
There are some reported proteins antigens in the outer membrane of Leptospira with good diagnostic value for human infection. The notable ones are LipL32, LipL41 and OmpL1 and the first two are surface exposed proteins expressed only in pathogenic Leptospira spp2021. Flannery et al22 evaluated five recombinant antigens using IgG-ELISA for serodiagnosis of human leptospirosis namely LipL32, LipL41, HSP58, LipL36 and OmpL1, and their results showed that rLipL32 was the most useful antigen. The recombinant LipL32 IgG ELISA showed the highest sensitivity when tested with serum samples from acute and convalescent leptospirosis patients; and highest specificity with serum of healthy individuals and those with other diseases with similar symptoms. However, it is interesting to note that IgM antibodies to rLipL32, rHsp58, and rOmpL1 were not detected. This is in contrast to the study by Luo et al23 which detected IgM antibodies to rLip32 in more than 90 per cent of the samples. Another study showed improved sensitivity and specificity of recombinant Lig-based immunoblot assay as compared to other recombinant protein-based assays24.
Further studies on the r72SEQ need to be performed using a larger number of patients and control serum samples, and also including serum samples from convalescent patients. The diagnostic value of the protein would be high if there is IgG but no IgM reactivity when tested with serum from convalescent patients. In conclusion, the findings of this study showed that leptospiral 72kDa protein is a potential marker for acute infection, and the recombinant protein may be feasible to be used in developing a rapid test.
Acknowledgment
This study was funded by Universiti Sains Malaysia (USM) Research University grant No.1001/PFARMASI/813016 and USM short term grant No. 304/PFARMASI/636086. Authors thank the staff of the Institute for Research in Molecular Medicine (INFORMM) for providing assistance and research facilities.
References
- Estimating the burden of human leptospirosis. Int J Antimicrob Agents. 2010;36(Suppl 1):S5-S7.
- [Google Scholar]
- Seroprevalence of human leptospirosis in representative population in Malaysia. Trop Biomed. 2002;19:97-101.
- [Google Scholar]
- An epidemiological investigation of an outbreak of leptospirosis associated with swimming, Beaufort, Sabah. Med J Malaysia. 2004;59:455-9.
- [Google Scholar]
- New insights into the pathogenicity of leptospires: evasion of host defences. New Microbiol. 2010;33:283-92.
- [Google Scholar]
- Macroscopic agglutination test for rapid diagnosis of human leptospirosis. J Clin Microbiol. 1998;36:3138-42.
- [Google Scholar]
- Evaluation of the indirect hemagglutination assay for diagnosis of acute leptospirosis. J Clin Microbiol. 1998;36:11-4.
- [Google Scholar]
- Assessment of the efficacy of an IgM-ELISA and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am J Trop Med Hyg. 1999;61:731-4.
- [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-5.
- [Google Scholar]
- Proteomics in leptospirosis research: towards molecular diagnostics and vaccine development. Expert Rev Mol Diagn. 2008;8:53-61.
- [Google Scholar]
- Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect Immun. 2002;70:2311-8.
- [Google Scholar]
- Evaluation of the cross reacting leptospiral serovars by MAT, ELISA and MSAT in rabbit hyper immune sera. Indian J Anim Sci. 2003;73:957-9.
- [Google Scholar]
- Early diagnosis of leptospirosis by immunoglobulin M immunoblot testing. Clin Vaccine Immunol. 2008;15:492-8.
- [Google Scholar]
- Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect Immun. 2001;69:4958-68.
- [Google Scholar]
- Evaluation of high performance liquid chromatography purified leptospiral antigen for the diagnosis of leptospirosis. Jpn J Infect Dis. 2009;62:428-31.
- [Google Scholar]
- Evaluation of leptospiral recombinant antigens MPL17 and MPL21 for serological diagnosis of leptospirosis by enzyme-linked immunosorbent assays. Clin Vaccine Immunol. 2008;15:1715-22.
- [Google Scholar]
- High-coverage proteome analysis reveals the first insight of protein modification systems in the pathogenic spirochete Leptospira interrogans. Cell Res. 2010;20:197-210.
- [Google Scholar]
- Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect Immun. 1999;67:853-61.
- [Google Scholar]
- Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol Microbiol. 2003;49:929-45.
- [Google Scholar]
- Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. J Clin Microbiol. 2001;39:3303-10.
- [Google Scholar]
- Protein typing of major outer membrane lipoproteins from Chinese pathogenic Leptospira spp. and characterization of their immunogenicity. Vaccine. 2009;28:243-55.
- [Google Scholar]






