Translate this page into:
Risk factors associated with the development of overt nephropathy in type 2 diabetes patients: A 12 years observational study
Reprint requests: Dr Vijay Viswanathan, Prof. M. Viswanathan Diabetes Research Centre, WHO Collaborating Centre for Research, Education & Training in Diabetes 5, Main Road, Royapuram, Chennai 600 013, India e-mail: drvijay@mvdiabetes.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Diabetic nephropathy (DN) is the leading cause of chronic kidney disease and end-stage renal disease in developing countries. Early detection and risk reduction measures can prevent DN. The aim of the study was to determine the risk factors for the development of proteinuria over a period of 12 years of follow up in normoalbuminuric type 2 diabetes patients attending a specialized centre.
Methods:
Of the 2630 type 2 diabetes subjects newly registered in 1996, 152 (M:F;92:60) normoalbuminuric subjects had baseline and subsequent measurements of anthropometric, haemodynamic and biochemical details spanning 12 years. The subjects were divided into 2 groups based on the renal status during follow up visits. Group 1 (non-progressors) had persistent normoalbuminuria and group 2 (progressors) had persistent proteinuria. Presence of other diabetic complications during follow up and details on antidiabetic and antihypertensive agents were noted.
Results:
During median follow up of 11 years in subjects with normal renal function at baseline, 44.1 per cent developed proteinuria at follow up. Glucose levels, HbA1c, systolic blood pressure (SBP), triglycerides, and urea levels were significantly higher at baseline among progressors than non-progressors. Progressors had a longer duration of diabetes and significant fall in estimated glomerular filtration rate (eGFR) levels at follow up. In Cox's regression analysis, baseline age, duration of diabetes, baseline HbA1c and mean values of HbA1c, triglycerides, SBP and presence of retinopathy showed significant association with the development of macroalbuminuria.
Interpretation & conclusions:
Type 2 diabetes patients with uncontrolled diabetes and increase in blood pressure are at high risk of developing nephropathy. Age, long duration of diabetes, elevated BP, poor glycaemic control and presence of retinopathy were significantly associated with the progression of diabetic nephropathy.
Keywords
Diabetic nephropathy
Indians
macroalbuminuria
proteinuria
risk factors
type 2 diabetes
Diabetes and hypertension are the leading causes of end stage renal disease (ESRD)1. Diabetic kidney disease (DKD) is a life threatening and irreversible microvascular complication characterized by presence of persistent proteinuria, hypertension and progressive decline in renal function. It predisposes to excess morbidity and mortality resulting from renal failure and cardiovascular disease23. In developing countries like India, the high cost of treating ESRD precludes many such patients from availing optimal therapy. Early identification of patients at high risk for diabetic nephropathy (DN) is therefore, important to intensify the treatment and modify associated risk factors4.
Microalbuminuria is a predictor of DN5 and a risk factor for premature death from cardiovascular disease (CVD) in patients with diabetes6. The reported prevalence of microalbuminuria in India is 26.9 per cent among type 2 diabetes patients and the occurrence of proteinuria increases with duration of diabetes78. Evidence suggests that Asian ethnic group immigrants with type 2 diabetes had high incidence of end stage renal failure and a 40-fold increased risk for ESRD910.
The cross-sectional studies conducted among type 1 diabetes patients have described poor glycaemic control, high BP and excessive smoking habit to be associated with the development of proteinuria1112. Early treatment of hypertension is important in preventing CVD, progression of DKD and retinopathy13. Several studies demonstrated the effectiveness of angiotensin converting enzyme inhibitors (ACEI) in retarding the progression and slowing the rate of renal function decline in patients with proteinuria1415. Many prospective observational studies have reported the initiation and progression of incipient nephropathy and predictors in type 1 diabetes patients1617, but only limited data are available on type 2 diabetes patients. There is sparse information on the risk factors and conversion rate of normal renal function to proteinuria among type 2 diabetes patients from developing countries. Hence, the aim of this study was to determine the putative risk factors associated with the development of proteinuria over a follow up period of 12 years among type 2 diabetes patients attending a specialized diabetes centre in south India.
Material & Methods
Type 2 diabetes patients who attended a specialized diabetes care centre in Chennai, India for both baseline examination in 1996 and subsequent follow up visits till 2008 and who were free of DKD at baseline were included in the study. A total of 2630 (M: F; 1611:1019) type 2 diabetes subjects were newly registered for the evaluation of their glycaemic status in 1996. Of these, follow up data for 12 years (1996-2008) was available for 250 (M: F; 158:92) patients. Patients who were taking antihypertensive agents at baseline or had other diabetic complications, were excluded. Among 250 patients, 152 (M:F; 92:60) were having consecutive normal renal function with albumin to creatinine ratio (ACR) of <30 μg/mg creatinine (estimated by immunoturbidimetric method), normal BP of <120/80 mm Hg, with no diabetic complications like retinopathy, neuropathy, peripheral vascular disease (PVD) or coronary artery disease (CAD) at baseline. The data from these 152 study subjects were considered for further analysis (Fig. 1).
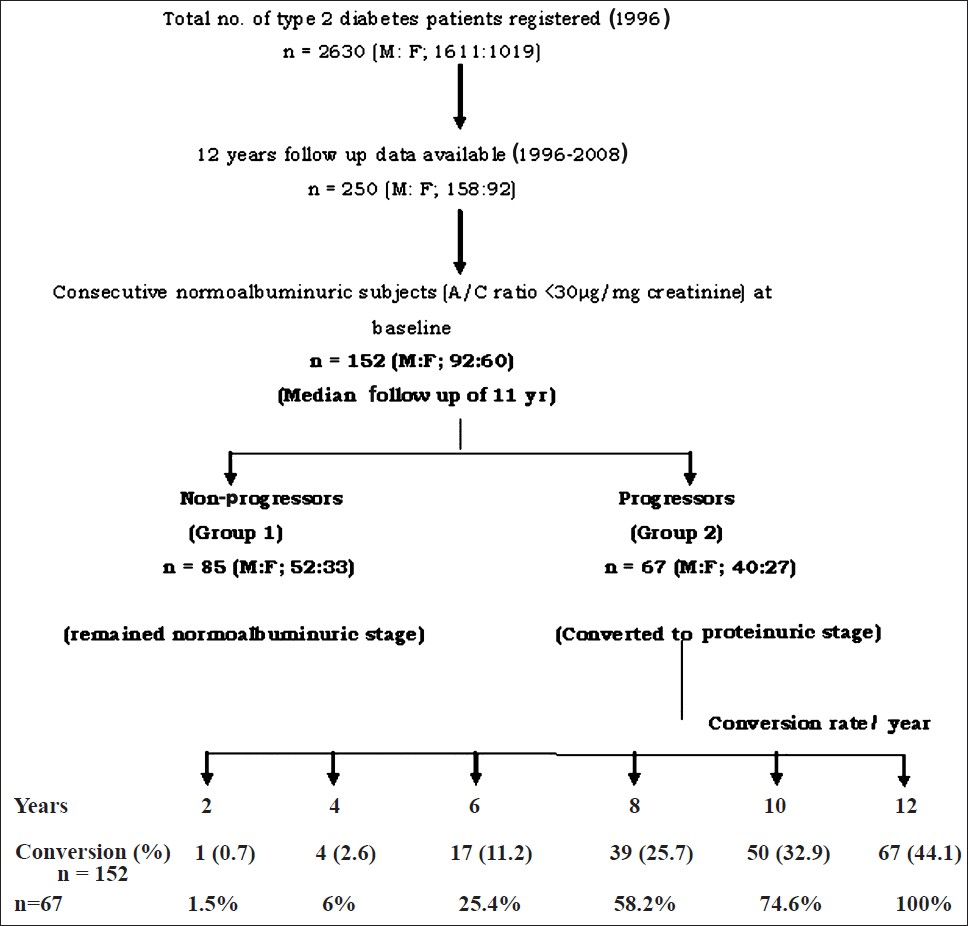
- Flow chart showing the patients’ recruitment and conversion rate.
Demographic, anthropometric and haemodynamic details like age, gender, height, weight, systolic and diastolic blood pressure (SBP and DBP), family history of diabetes and duration of diabetes were recorded at baseline. Body mass index (BMI) was calculated using standard formula. Biochemical details like fasting plasma glucose (FPG), post-prandial plasma glucose (PPG), HbA1c, total cholesterol, triglycerides, urea, creatinine and albumin creatinine ratio (ACR) or protein to creatinine ratio (PCR) were recorded. Estimated glomerular filtration rate (eGFR) was calculated using Cockcroft Gault formula18. Subsequent measurements spanning 12 year period were recorded. Biochemical, haemodynamic and anthropometric details of the subjects during the follow up visits were obtained from the medical records. Details of oral hypoglycaemic agents (OHA) prescribed and presence of other diabetic complications like diabetic retinopathy (DR), diabetic neuropathy, PVD and CAD occurred during the follow up visits were noted. Subjects who developed hypertension during the follow up were prescribed antihypertensive medications.
The study subjects were divided into two groups based on their attained renal status during follow up. Group 1 were the subjects who had persistent normal renal function (ACR <30 μg/mg creatinine) during the follow up of 12 years and were considered as non-progressors (n=85; M : F; 52:33); while group 2 subjects showed declining renal function with persistent proteinuria of expected protein excretion (EPE) >500 mg/day in the absence of any infection and were considered as progressors (n=67; M : F; 40:27). Time of onset of overt nephropathy during follow up was defined as the first recorded positive urine sample with proteinuria. However, during follow up conversion from normoalbuminuria to microalbuminuria was confirmed by consecutive three positive readings of ACR ≥30 μg/mg creatinine in the absence of any infection.
Statistical analysis: The analysis was performed using SPSS (version 16.0, Illinois, USA) software. Unpaired Student's t test was used to compare continuous variables and Chi- square test was used to evaluate proportions between groups. Multiple logistic regression (Forward step-wise addition method) analysis was performed considering progressors as dependent variable. Age, gender, duration of diabetes, mean values (FPG, PPG, HbA1c, SBP, DBP and BMI), family history of diabetes and presence of DR were included as independent variables.
Baseline and long term i.e., 12 yr averaged risk factor models were explored. Cox's proportional hazard model (Forward step-wise addition method) was used to examine the baseline variables predictive of progression to proteinuria (Model 1). The model included those baseline variables that were prior considered to be potentially important predictors of developing kidney disease, or that were found to be significantly different at baseline on comparing two groups. These variables included age, gender, baseline FPG, PPG, HbA1c, SBP, DBP, BMI, triglycerides, creatinine, family history of diabetes and duration of diabetes. Another model (Model 2) was used considering the long term, i.e., time varying averaged risk factors that were predictive of kidney disease. For long term, averaged analysis involving continuous data, the mean of the biochemical and haemodynamic variables of all follow up visits such as mean FPG, PPG, HbA1c, BMI, creatinine, triglycerides, SBP, DBP were taken and age, gender, duration of diabetes, family history of diabetes, presence of DR were also included as independent variables. Median survival time of the progressors was estimated by Kaplan Meier survival analysis.
Results
The final analysis was done with the data of 152 type 2 diabetes patients having normal renal function, absence of hypertension and other associated diabetic complications at baseline and who had follow up data for a median of 11 yr (range: 4-12 yr). Of the 152 subjects, during follow up, 67 (44.1%) (progressors) developed proteinuria and 85 (55.9%) (non-progressors) remained normoalbuminuric. Among the progressors, around 60 per cent developed proteinuria at the end of 8 years while remaining 40 per cent developed by the end of 12 years (Fig. 1). Table I shows the comparison of demographic, biochemical and haemodynamic details at baseline and at follow up in the study groups. Progressors who developed kidney disease at follow up were more likely to be older (P<0.01). Progressors also had significantly (P<0.05) higher SBP levels and longer duration of diabetes (P<0.001) compared to non-progressors at baseline. There was no difference in baseline BMI, DBP and presence of positive family history for diabetes among the study groups.
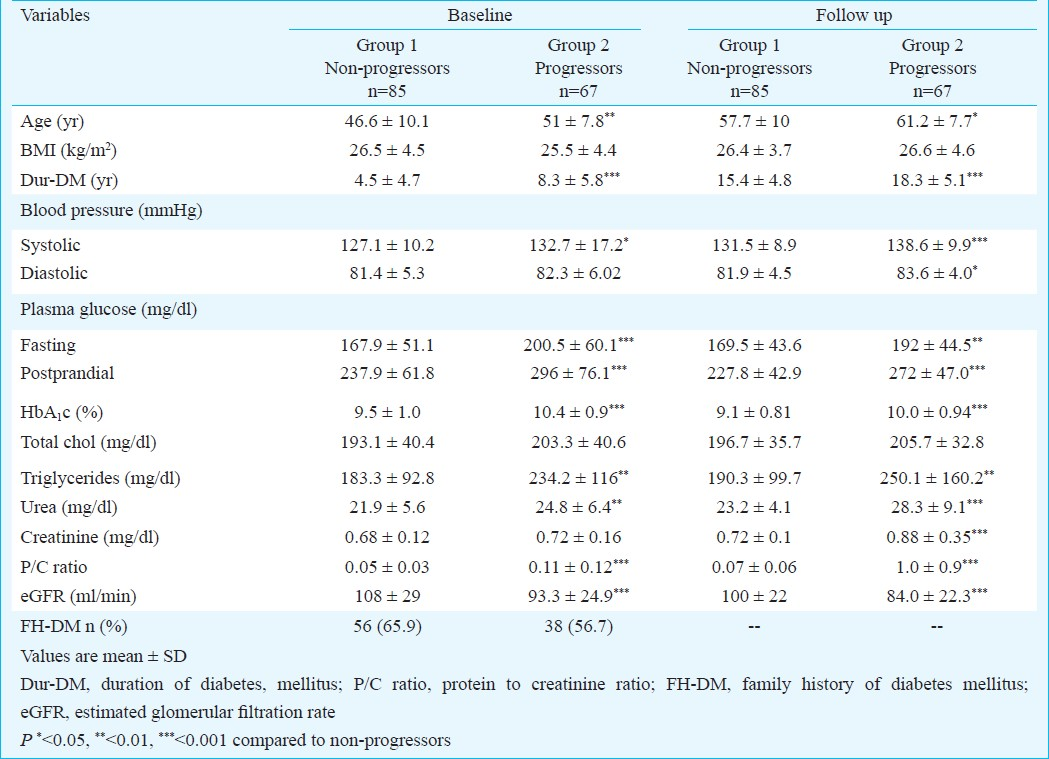
Fasting, post-prandial glucose levels and HbA1c per cent were significantly (P<0.001) higher at baseline among progressors compared to non-progressors. Baseline triglycerides and baseline urea levels were found to be significantly higher (P<0.01) among the progressors compared to non-progressors. eGFR was higher among non-progressors compared to progressors (P=0.001) but both the groups had normal eGFR levels of >90 ml/min at baseline.
At follow up, triglycerides (P<0.01), urea (P<0.001) and creatinine (P<0.001) were significantly higher among progressors compared to non-progressors. There was a significant (P<0.001) fall in eGFR levels among progressors compared to non-progressors from baseline to follow up visit. All the renal parameters among the progressors declined during the follow up.
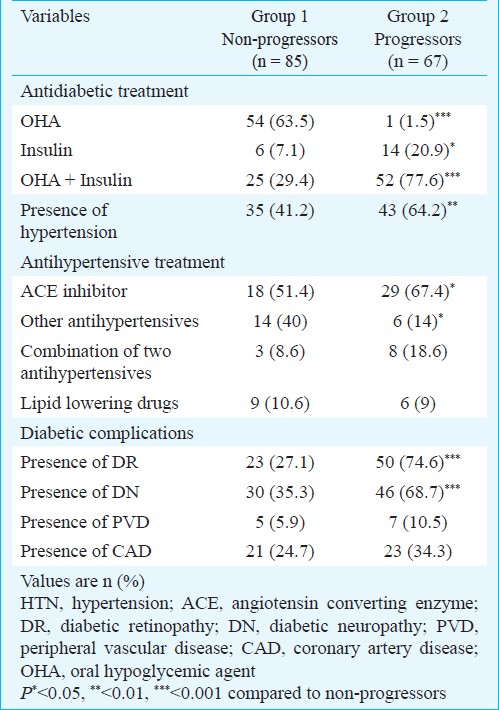
Majority of the subjects (63.5%) were on OHA alone in group 1 whereas 78 per cent were on combination of OHA and insulin treatment in group 2. Presence of hypertension (41.2 vs 64.2%, P<0.01), DR (27.1 vs 74.6%, P<0.001) and diabetic neuropathy (35.3 vs 68.7%, P<0.001) was significantly higher among the progressors compared to non-progressors (Table II). More than 50 per cent of the subjects with hypertension were on ACEI in both the study groups. Duration of antihypertensive treatment (ACEI) was also similar in both the groups.
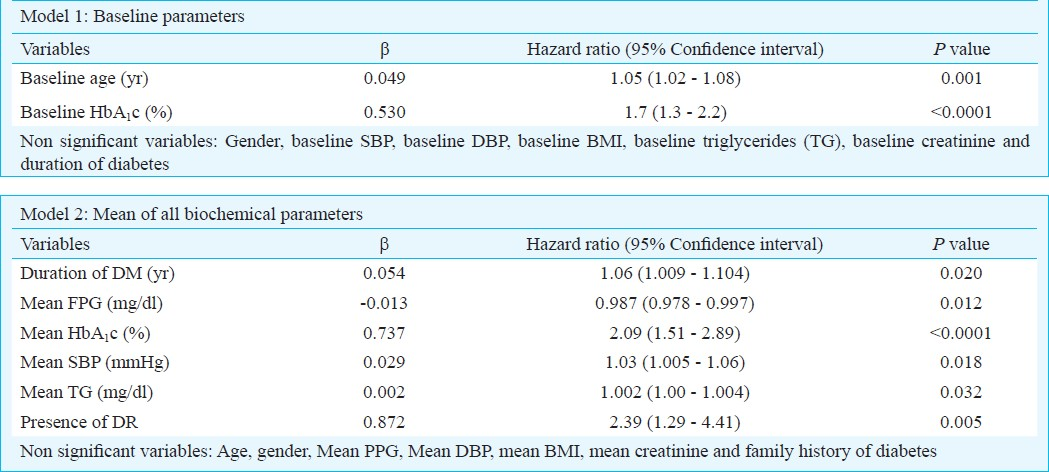
The putative risk factors for the development of macroalbuminuria among the progressors were examined by Cox's regression analysis (Table III). In the first model, baseline age with a hazard ratio (HR) of 1.05 (95% CI: 1.02 - 1.08; P=0.001) and baseline HbA1c with HR=1.7 (95% CI: 1.3 - 2.2; P<0.0001) emerged as significant determinants in the development of macroalbuminuria. Another model considering mean of biochemical and haemodynamic variables of follow up visits showed that mean HbA1c [HR=2.09;95%CI (1.51-2.89); P<0.0001], mean triglycerides [HR=1.002; 95% CI(1.00-1.004); P=0.032], mean SBP [HR=1.03; 95% CI (1.005-1.06); P=0.018], duration of diabetes [HR=1.06; 95% CI(1.009-1.104); P=0.02] and presence of DR [HR=2.39;95% CI (1.29-4.41); P=0.005] were significantly associated with the development of macroalbuminuria among the progressors. Non-progressors had a significantly lower FPG levels at follow up compared to progressors and FPG emerged as a protective factor against the development of proteinuria.[β=-0.013; HR=0.987; 95%CI (0.978-0.997); P=0.012].
Results of multiple logistic regression analysis also confirmed significant association of mean HbA1c [OR=2.3, 95% CI (1.5-3.6); P<0.0001], mean SBP [OR=1.08; 95% CI (1.03-1.13); P=0.001] and presence of DR [OR=3.9; 95% CI (1.7-9.2); P=0.001] with the development of proteinuria among the progressors. According to the Kaplan Meier survival analysis, progressors had the median survival time of 12 yr, range (11.6-12.4 yr). There was a minimum survival period of 4 yr and decline in renal function occurred after 4 yr and majority of the subjects ended up with proteinuria by the end of 8 years (Fig. 2).
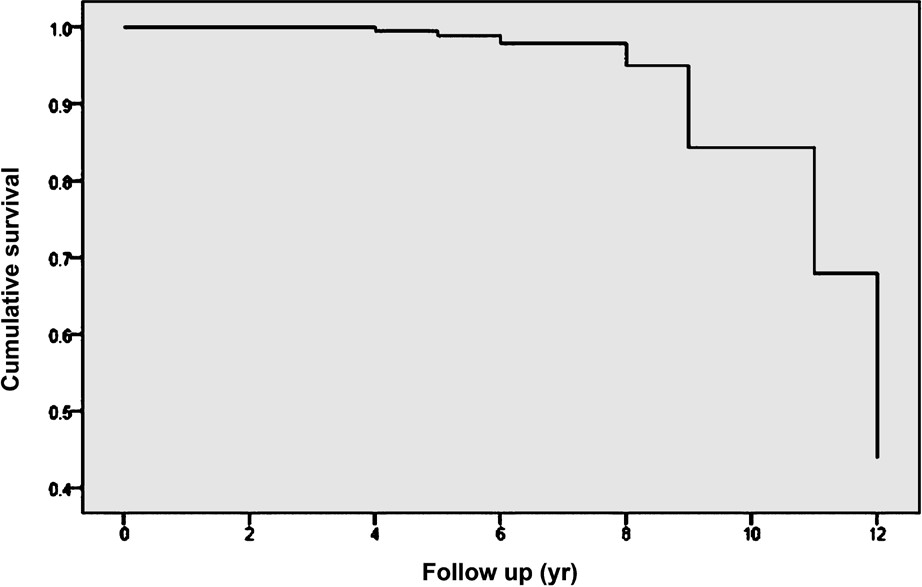
- Kaplan Meier survival analysis of progressors.
Discussion
The present study investigated the risk factors associated with the development of overt nephropathy among type 2 diabetes patients who had normal renal function and absence of hypertension and other associated diabetic complications at baseline. In a median follow up period of around 11 years, 44 per cent of subjects developed macroalbuminuria. This was higher than the 25 per cent progression to persistent microalbuminuria or macroalbuminuria reported in a 10 year prospective observational follow up study17. This difference could be because of the ethnic variations (risk factors vary between different populations) and type 1 diabetes subjects studied by Rossing et al17. We have explored the effect of long term follow up with multiple interim visits which allowed us to compute long term, averaged risk factors which are included in this analysis.
Baseline age and HbA1c were found to be the major predictors for the development of macroalbuminuria among progressors in our study. The long term, averaged risk factors such as HbA1c, triglycerides and SBP showed a significant association with the development of macroalbuminuria. Duration of diabetes and presence of DR also emerged as significant risk factors in the current study. The baseline risk factor model clearly indicated the risk factors which precede outcome, whereas the long term, averaged model allowed us to assess the changes in risk factors over time. Similarly, a prospective, observational study of type 2 diabetes patients followed for a median period of 5.8 yr revealed the presence of retinopathy, increased lipid level, HbA1c per cent and age to be associated with the development of incipient or overt diabetic nephropathy19. Another study on south Indians reported the similar risk factors for overt nephropathy and microalbuminuria7. This was a population based cross-sectional study from India on the prevalence and risk factors of diabetic nephropathy whereas our study was hospital-based observational study reporting the conversion rate along with risk factors of diabetic nephropathy.
Uncontrolled diabetes was the major determinant of progression to macroalbuminuria in our study. DN was associated with high BP, which is known to worsen renal function. SBP was higher at baseline among the progressors and mean SBP emerged as a significant factor for the development of proteinuria. A significantly higher proportion of patients starting antihypertensive therapy during follow up among progressors also indicated importance of rising BP in the development of DN. Since the changes in BP are small and may be pronounced at night, 24 h ambulatory BP measurements may be more precise, which was not done in our study. Our study showed that BP and glycaemic control had a significant role in the initiation and acceleration of DN. Many landmark studies have reported the impact of uncontrolled blood glucose and BP on the development of diabetic complications1620. The development and progression of DN can be prevented by improving glycaemic control21, control of BP and restriction of protein intake2223 and by use of ACE inhibitors and/ or angiotensin II receptor blocker antagonists (ARB)1524.
Rossing et al17 reported that the presence of retinopathy predicts the onset of microalbuminuria. Similar observation was noted in our study also. Presence of retinopathy at baseline was reported to be a putative risk factor for the development of proteinuria in the above study17, but in our study, subjects developed retinopathy during the progression of proteinuria and still presence of retinopathy emerged as a significant and important factor associated with the development of proteinuria. This is another sign of long exposure to poor glycaemic control which was evident in our study. Another reason might be the susceptibility to the development of diabetic complications in this ethnic group.
When a patient develops persistent proteinuria and elevated arterial BP, kidney function starts to decline. Similar pattern was observed in the present study. There was a greater fall in eGFR among progressors with elevated SBP compared to non-progressors. Although baseline eGFR was lower among the progressors, it still remained within normal limits of >90 ml/min and declined significantly during the median 11 yr follow up. Median time for the patients to be free from the occurrence of proteinuria was 4 years and the survival time was found to be 12 years irrespective of poor glycaemic control and uncontrolled BP.
Progression from microalbuminuria to macroalbuminuria occurred despite antihypertensive therapy in our subjects. A higher proportion of subjects were on ACE inhibitors but still progressed to more advanced stages of DN. Similar observation was noted in other studies also1415. This could be due to incomplete adherence to therapy or lack of sufficient biologic efficacy of prescribed medications. Our study supports the role of chronic hyperglycaemia as a major predictor of glomerular damage even in the presence of treatment with ACE inhibitors. There were some limitations to our study. The GFR was estimated by using the Cockcroft Gault formula instead of using the gold standard method. Details of smoking and alcohol consumption could not be obtained since the data were collected retrospectively. Finally, since this was a hospital-based study, this could have introduced a referral bias and generalizability of results therefore, might be limited.
Many of the subjects developed diabetic complications with time, so this study also highlights the need to initiate multi-disciplinary approach to improve care of diabetic patients with nephropathy. Further longitudinal studies in general population with an appropriate study design are necessary to better understand the association of these risk factors with DN and whether modification in these risk factors can slow the progression of kidney disease. Improved glycaemic control, early introduction to appropriate treatment strategies and reduction of renal parameters to attain normal status may prove to be key factors in the prevention of progression to DN.
In conclusion, type 2 diabetes patients with small increase in BP and with uncontrolled diabetes are at high risk for the development and progression to DN. Raised SBP, poor glycaemic control, age, longer duration of diabetes and presence of DR are the important initiators as well as accelerators for the progression of macroalbuminuria.
References
- Risk of end stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. JAMA. 1997;278:2069-74.
- [Google Scholar]
- Predictors of mortality in insulin dependent diabetes: 10 year follow up study. BMJ. 1996;313:779-84.
- [Google Scholar]
- Diabetic nephropathy. In: Brenner BM, ed. Brenner & Rector's The kidney Vol II. (7th ed). Philadelphia: Sauders; 2004. p. :1777-818.
- [Google Scholar]
- Prevalence and risk factors of albuminuria and chronic kidney disease in Chinese population with type 2 diabetes and impaired glucose regulation: Changhai diabetic complications study (SHDCS) Neph Dialy Transp. 2009;24:3724-31.
- [Google Scholar]
- Microalbuminuria as a predictor of clinical nephropathy in insulin dependent diabetes mellitus. Lancet. 1982;i:1430-2.
- [Google Scholar]
- Cohort study of predictive value of urinary albumin excretion for atherosclerotic vascular disease in patients with insulin dependent diabetes. BMJ. 1996;312:871-4.
- [Google Scholar]
- Prevalence and risk factors of diabetic nephropathy in an urban south Indian population. Diabetes Care. 2007;30:2019-24.
- [Google Scholar]
- Microalbuminuria: A major risk factor in non insulin dependent diabetes.A 10 year follow up study of 503 patients. Diabet Med. 1988;5:126-34.
- [Google Scholar]
- Increased end-stage diabetic nephropathy in Indo-Asian immigrants living in the Netherlands. Diabetologia. 2002;45:337-41.
- [Google Scholar]
- Increased incidence of end-stage renal failure secondary to diabetes mellitus in Asian ethnic groups in United Kingdom. Diabet Med. 1992;9:641-5.
- [Google Scholar]
- Microalbuminuria in type 1 (insulin-dependent) diabetic patients: prevalence and clinical characteristics. Diabetes Care. 1992;15:495-501.
- [Google Scholar]
- Glycaemia, arterial pressure and microalbuminuria in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1984;26:402-5.
- [Google Scholar]
- Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomized study. Lancet. 1999;353:617-22.
- [Google Scholar]
- Randomized, controlled trial of long term efficacy of captopril on preservation of kidney function in normotensive patients with insulin dependent diabetes and microalbuminuria. BMJ. 1999;319:24-5.
- [Google Scholar]
- The effect of angiotensin converting enzyme inhibitor on diabetic nephropathy. N Engl J Med. 1993;329:1456-62.
- [Google Scholar]
- Predictors of the development of microalbuminuria in patients with type 1 diabetes mellitus: a seven year prospective study. Diabet Med. 1999;16:918-25.
- [Google Scholar]
- Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients. Diabetes Care. 2002;25:859-64.
- [Google Scholar]
- Risk factors for development of incipient and overt diabetic nephropathy in patients with non insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314:783-8.
- [Google Scholar]
- The effect of intensive therapy on the development and progression of diabetic nephropathy in the diabetes control and complication trial. Kidney Int. 1995;47:1703-20.
- [Google Scholar]
- Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837-53.
- [Google Scholar]
- Therapeutic efficacy of different antihypertensive drugs in human diabetic nephropathy: an update meta analysis. Nephrol Dial Transplant. 1995;10:39-45.
- [Google Scholar]
- Blood pressure control, proteinuria and the progression of renal disease: the Modification of Diet in Renal Disease study. Ann Intern Med. 1995;123:754-62.
- [Google Scholar]
- The effect of irbesartan on the development and progression of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870-8.
- [Google Scholar]






