Translate this page into:
Revisiting the susceptibility testing of Mycobacterium tuberculosis to ethionamide in solid culture medium
Reprint requests: Dr Vanaja Kumar, Centre for Drug Discovery & Development (3D), Sathyabama University, Jeppiaar Nagar, Rajiv Gandhi Salai, Chennai 600 119, Tamil Nadu, India e-mail: vanaja_kumar51@yahoo.co.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Increase in the isolation of drug resistant phenotypes of Mycobacterium tuberculosis necessitates accuracy in the testing methodology. Critical concentration defining resistance for ethionamide (ETO), needs re-evaluation in accordance with the current scenario. Thus, re-evaluation of conventional minimum inhibitory concentration (MIC) and proportion sensitivity testing (PST) methods for ETO was done to identify the ideal breakpoint concentration defining resistance.
Methods:
Isolates of M. tuberculosis (n=235) from new and treated patients were subjected to conventional MIC and PST methods for ETO following standard operating procedures.
Results:
With breakpoint concentration set at 114 and 156 µg/ml, an increase in specificity was observed whereas sensitivity was high with 80 µg/ml as breakpoint concentration. Errors due to false resistant and susceptible isolates were least at 80 µg/ml concentration.
Interpretation & conclusions:
Performance parameters at 80 µg/ml breakpoint concentration indicated significant association between PST and MIC methods.
Keywords
Drug resistance
ethionamide
MIC method
Mycobacterium tuberculosis
proportion sensitivity testing
Ethionamide (ETO) is an efficacious, relatively non-toxic, second line anti-tuberculosis drug inhibiting the fatty acid synthesis in cell wall. The drug is structurally similar to isoniazid (INH) and pyrazinamide (PZA) and requires activation by a specific prodrug activator12. Efficacy of the drug is one of the reasons for development of resistance and should be considered before determination of minimum inhibitory concentration (MIC)3. An effective drug action can be observed whenever MIC of the drug is well below its therapeutic index. Drugs like isoniazid and rifampicin (RIF) have least likelihood of developing resistance, one of the reasons being that the MIC is well below their therapeutic index. The situation is different with ETO, where the MIC especially in solid Lowenstein-Jensen (L-J) medium is very near to its therapeutic index, thus increasing the chance of developing resistance3. It is difficult to determine ETO resistance accurately because change in MIC associated with resistance is small and the drug is thermolabile. Hence, the distributions of probable sensitive and probable resistant strains are not well separated leading to discrepancy between clinical outcome and laboratory susceptibility pattern34. We have previously established presence of discrepancy between methods used to define drug susceptibility of ETO with laboratory susceptible strain H37Rv5. Canetti et al3 reported that MIC value can differ with different testing laboratories and also with respect to time interval. Shift in the MIC value is attributed to variation in the level of “local” strain types of Mycobacterium tuberculosis circulating within the geographical region6. The breakpoint concentration to determine susceptibility profile for ethionamide on solid L-J medium was determined decades ago and subsequent re-evaluation of the same was not performed regularly7. In the current scenario with an increase in multidrug and extensively drug resistant tuberculosis (MDR and XDR-TB), the existing breakpoint concentration for susceptibility profile was re-evaluated for better discrimination of resistant and sensitive populations. An attempt was made to re-calibrate conventional MIC and proportion sensitivity testing (PST) methods using L-J solid medium and to find out the presence of shift in the breakpoint concentration defining resistance.
Material & Methods
Drug susceptibility testing (DST) of 235 clinical isolates of M. tuberculosis for ETO was performed according to standard procedure8 in the department of Bacteriology, National Institute for Research in Tuberculosis (NIRT), Chennai, India from 2010 to 2012. Conventional MIC method was performed using 4 mg moist weight per microlitre (ml) of culture suspension, whereas 1mg/ml of the culture suspension and its three serial decreasing 1 in 10 fold dilutions were used for PST method. Briefly, one-third loopful of 2-3 wk old culture on L-J medium was suspended in 1 ml of sterile distilled water and vortexed to obtain uniform suspension. The coarse particles or clumps in the suspension were allowed to settle at room temperature. For MIC method, 10 µl of the suspension was inoculated onto drug containing and drug free L-J medium. Concentrations of ETO used were 20, 28.5, 40, 57, 80, 114 and 156 µg/ml. Ten-fold dilution from 1mg/ml suspension was prepared by adding 0.2 ml to 1.8 ml sterile distilled water (S1, 10-1). Two further serial dilutions 10-2 (S2) and 10-3 (S3) were prepared in the similar manner. Ten microlitres each from the above dilutions were inoculated onto drug free and drug containing (at 40 µg/ml of ETO) media. Susceptibility testing was carried out at the same time point using the same batch of medium to avoid any error. Repeated sub-culturing of isolates was avoided to minimize clonal variation. Results were read after 28 and 42 days of incubation at 37°C for MIC and PST methods, respectively.
Interpretation of conventional methods: Isolates with ≥20 colony counts (1+ grading) were considered resistant to the particular drug concentration of ETO. Breakpoint MIC value for defining resistance in conventional MIC method used was ≥114 µg/ml and value less than that was considered susceptible. Variation in breakpoint MIC was assessed to obtain a “near” ideal value.
Interpretation of PST method: Isolates with more than one per cent of colony forming units (cfu) in drug containing L-J slopes in comparison with drug free L-J slopes were considered as resistant. Isolates with values less than one per cent criteria defining resistance were considered as susceptible8. As a modification, isolates with PST values of 0.9 and 1.1 per cent were termed as “borderline”.
Errors: In any method presence of false resistance (FR) or false susceptibility (FS) is considered as error9. False resistance is classified as major error (ME) which does not have any major implications with respect to treatment of patient. But false susceptible is considered to be very major error (VME) as it guides improper treatment for patient resulting in continued transmission of drug resistant organism.
Laboratory susceptible standard strain H37Rv was used as control on each batch of testing. Clinical isolates showing susceptible and resistant profile even upon repeated testing at different time points and by liquid culture systems were classified as resistant and susceptible clinical controls.
Statistical analysis: Results obtained were analyzed by Pearson chi-square test at 5 per cent level of significance using SPSS software version 17.0 (SPSS Inc., Chicago, USA).
Results
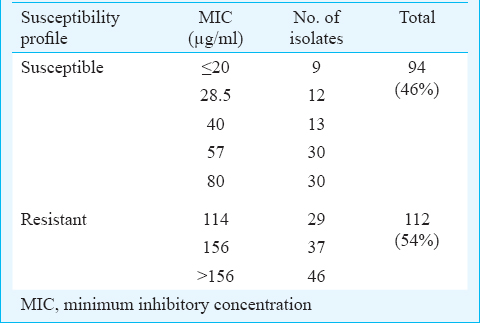
Among the 235 M. tuberculosis isolates subjected to DST procedures, 29 were either contaminated by single or both DST methods or showed no growth by either methods and hence were excluded from analysis. Therefore, 206 (88%) isolates with valid results for MIC and PST methods were analyzed. PST method was considered as gold standard. Resistance phenotype toward ETO was observed in 131 (64%) isolates by PST method whereas, 71 (34%) isolates had a susceptible phenotype. Four isolates (2%) were interpreted as “borderline” resistant at a concentration of 40 µg/ml. Conventional MIC method classified, 94 (46%) and 112 (54%) isolates as resistant and susceptible to ethionamide, respectively (Table I).
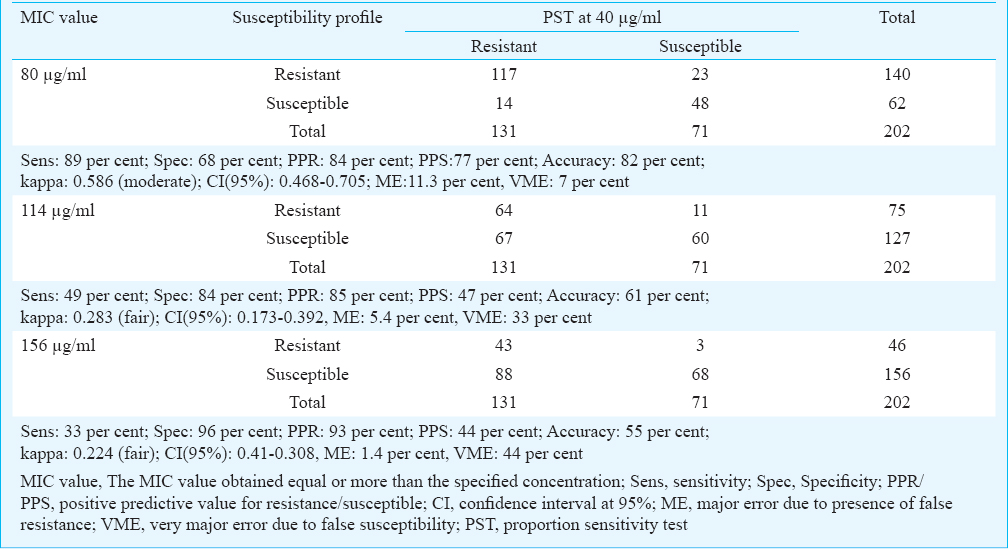
Isolates exhibiting an MIC of ≥114 µg/ml were considered resistant to ethionamide according to the current standard procedure being followed at the laboratory8. An attempt was made to determine the susceptibility profile by shifting the breakpoint concentration one above (156 µg/ml) and below (80 µg/ml) the set value of 114 µg/ml. The comparison of MIC was performed with PST method (Table II). When the breakpoint MIC was set at 80 µg/ml, an increased sensitivity (89%) was observed than that of 114 µg/ml (49%) and 156 µg/ml (33%). The specificity decreased to 68 per cent at 80 µg/ml where as 84 per cent was observed at 114 µg/ml and 96 per cent at 156 µg/ml. Although a marginal increase in the predictive value for resistance was observed with increase in the breakpoint, predictive value for susceptibility by conventional MIC method at 80 µg/ml (77%) was higher than the other concentrations. Major error due to presence of false resistant isolates was 11 per cent at 80 µg/ml. Use of higher breakpoint concentration reduced the rate of ME to 5.4 per cent at 114 µg/ml and 1.4 per cent at 156 µg/ml. The presence of false susceptible isolates depicted as VME was found to be least at 80 µg/ml (7%) (Table II).
Discussion
Ethionamide is being used in the programmatic management of drug resistant tuberculosis (PMDT) in India10. Determination of drug susceptibility and standardization of the methodology becomes important in TB endemic setting like India with a high number of MDR-TB patients needing appropriate treatment. Susceptibility profile at the defined concentration in conventional MIC (≥114 µg/ml) and PST (40 µg/ ml) methods showed a marginally high percentage of ethionamide resistance among the isolates tested, a finding similar to that reported by Paramasivan et al11. Inclusion of a higher concentration revealed the presence of considerable number of isolates (20%) having MIC equal or more than 156 µg/ml.
Breakpoint concentration used to define susceptibility was evaluated using a lower (80 μg/ml) and a higher (156 μg/ml) concentration in addition to the regular value (114 μg/ml). Sensitivity was found to be higher (89%) when the breakpoint MIC concentration was set at 80 μg/ml. Increase in specificity at higher concentration can be attributed to additional number of isolates being defined as susceptible. Though specificity was higher with increased concentrations, performance parameters indicated consistent values only when MIC was set at 80 μg/ml.
Considering the presence of errors with respect to different breakpoint MIC values, variations of VME and ME were narrow with an MIC at 80 μg/ ml. Unequal distribution of VME and ME was observed at other concentrations. As the defined drug concentration was increased, more isolates were defined as false susceptible. Major errors do not pose any major implication in treatment regimen while false susceptible isolates, termed as VME can completely amend treatment leading to continued transmission of drug resistant clones in the population. Susceptibility results at 80 μg/ml concentration almost fulfilled the above criteria and might be considered as breakpoint MIC for conventional MIC method in the present situation.
Selection of susceptibility method also plays a major role in resistance definition. Meager variation between methods is observed with drugs with stable activity. When testing drugs like ethionamide, thermolabile nature of the drug should also be considered. Being thermolabile, there could be loss or complete deterioration during inspissation and with prolonged incubation while performing different DST procedures12.
Presence of clumps plays a major role in altering the susceptibility pattern413. Significant association between clumping and calculation of proportion, as used in PST method, was observed leading to erroneous results. Thus, facts such as inoculum preparation, incubation and interpretation tend to introduce false results that constitute error. But these are inherent to the method and are thus inevitable. To overcome these hindrances rapid tests in solid or liquid medium are ideal alternatives414.
Drug susceptibility testing is widely used as a tool for the selection of effective regimens to treat tuberculosis patients successfully and to develop strategies to cope with the problems of drug resistant tuberculosis. The test procedure has to be standardized or re-evaluated if necessary to yield results with acceptable reproducibility. In the present study, susceptibility testing for ETO by conventional MIC method was re-evaluated and necessary modification in the effective breakpoint concentration was recommended.
Acknowledgment
The authors acknowledge WHO for financial assistance provided through NIH/USAID and ICMR for infrastructure facilities. The first author (RL) thanks ICMR and WHO for providing financial assistance. The authors thank Staff, Department of Bacteriology for technical support.
Conflicts of Interest: None.
References
- Mechanism of thioamide drug action against tuberculosis and leprosy. J Exp Med. 2007;204:73-8.
- [Google Scholar]
- Activation of the pro-drug ethionamide is regulated in mycobacteria. J Biol Chem. 2000;275:28326-31.
- [Google Scholar]
- Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41:21-43.
- [Google Scholar]
- Consistency of standard laboratory strain Mycobacterium tuberculosis H 37 Rv with ethionamide susceptibility testing. Indian J Med Res. 2012;135:672-4.
- [Google Scholar]
- Comparison of methods for testing the sensitivity of Mycobacterium tuberculosis to ethionamide. Tubercle. 1966;47:250-61.
- [Google Scholar]
- Can inhA mutation predict ethionamide resistance? Int J Tuberc Lung Dis. 2013;17:129-30.
- [Google Scholar]
- Standard operating procedure for mycobacteriology laboratory: version 1.1. Chennai: Department of Bacteriology, Tuberculosis Research Centre (ICMR); 2010. p. :86-96.
- [Google Scholar]
- Current perspectives on drug susceptibility testing of Mycobacterium tuberculosis complex: the automated nonradiometric systems. J Clin Microbiol. 2006;44:20-8.
- [Google Scholar]
- Revised National Tuberculosis Control Programme (RNTCP). RNTCP response to challenges of drug resistant TB in India. January 2012 (update) Available from: http://www.tbcindia.nic.in/pdfs/RNTCP%20Response%20DR%20TB%20in%20India%20-%20Jan%202012%20update.pdf
- [Google Scholar]
- First- and second-line drug resistance patterns among previously treated tuberculosis patients in India. Int J Tuberc Lung Dis. 2010;14:243-6.
- [Google Scholar]
- Effect of temperature on storage of ethionamide during susceptibility testing. J Microbiol Infect Dis. 2013;3:128-32.
- [Google Scholar]
- Evaluation of automated BACTEC MGIT 960 System for testing susceptibility of Mycobacterium tuberculosis to four major antituberculous drugs: Comparison with the radiometric BACTEC 460TB method and the agar plate method of proportion. J Clin Microbiol. 2002;40:607-10.
- [Google Scholar]
- Drug susceptibility testing of Mycobacterium tuberculosis against second-line drugs using the BACTEC MGIT 960 System. Int J Tuberc Lung Dis. 2008;12:1449-55.
- [Google Scholar]






