Translate this page into:
Retrospective study of the effect of sarcopaenia on post-operative outcomes in patients undergoing thoracic surgery
For correspondence: Dr Kemal Karapınar, Department of Thoracic Surgery, Yedikule Chest Diseases and Thoracic Surgery Health Application and Research Center, University of Health Sciences, Kazlıçeşme Mh, Belgrat Kapı yolu Cad No:1, 34020, Zeytinburnu, Istanbul, Turkey. e-mail: kemalkarapinar55@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Sarcopaenia refers to the pathological loss of muscle mass that may be observed in malnutrition, immobility, chronic disease, particularly chronic obstructive pulmonary disease and malignancies. A relationship has been identified between sarcopaenia and thoracic surgery. The aim of the present study was to investigate the relationship between density and area of the psoas major muscle (PSM), the pectoralis major and minor muscles (PEC) and the post-operative morbidity, mortality and survival of patients undergoing anatomic lung resection.
Methods:
A retrospective review of the medical record data of the patients who underwent lung resection was conducted in between 2009 and 2018. The study included patients who underwent upper abdominal computed tomography (CT) for the measurement of PSM and thoracic CT for PEC. The demographic data, laboratory test results, radiological findings and the survival data of the patients were recorded.
Results:
Evaluation was made of 161 patients with available CT data. With the exception of mean PEC density, the PEC parameters (P=0.013-0.026), and PSM density (P=0.015) were significantly lower in the non-survivors than in the survivors. In general, the mean measurements of the PSM and PEC were seen to affect mortality (P=0.001-0.024).
Interpretation & conclusions:
The mean area and density measurements in the PSM, and particularly in the PEC, were determined to be significantly higher in patients who survived after lung cancer surgery, suggesting that sarcopaenia could be a useful predictor of post-operative mortality risk and survival.
Keywords
Lung cancer
morbidity
mortality
sarcopaenia
thoracic surgery
The term sarcopaenia refers to the pathological loss of muscle mass that may be observed in malnutrition, immobility, chronic diseases, particularly chronic obstructive pulmonary disease (COPD), and malignancies12. Although lobectomies are associated with lower morbidity in thoracic surgery practice, an increase in the rate of patients referred for surgery is known to increase post-operative morbidity and mortality345. Suzuki et al6 reported that sarcopaenia is a poor prognostic factor in patients undergoing surgery for non-small cell lung cancer (NSCLC). It has also been demonstrated that sarcopaenia affects post-operative morbidity and mortality in major surgeries, such as the surgical treatment of lung cancer or transplantation78. Previous studies have identified a linear relationship between survival, density and area measurements of the psoas major muscle (PSM), the pectoralis major and minor muscles (PEC) and the erector spinae muscle (ESM) in patients with COPD7,9-11. There have also been studies investigating the effects of these muscles on early post-operative morbidity and mortality in patients undergoing lobectomy for lung cancer, and studies which have reported the effects of ESM7. The ESM erector spinae muscle consists of three muscles (spinal thoracis muscle, iliocostalis thoracis muscle and, longissimus thoracis muscle), the functions of which are to keep the spine erect. The pectoralis muscles that act as the accessory respiratory muscles are expected to be a more accurate indicator of post-operative morbidity and mortality than ESM in thoracic surgery910 . The psoas major muscle is a group of muscles extending from the thorax into the pelvis that can be visualized with computed tomography (CT) scans of the thoracic cavity.
The aim of the present study was to measure the density and area of the PSM and PEC, and to evaluate their relationship with post-operative morbidity, mortality and survival in patients undergoing anatomic lung resection.
Material & Methods
This study was approved by the Ethics Committee of Istanbul Training and Research Hospital. A retrospective review of the medical records of patients who underwent lung resection and lymph node dissection (standard) and extended lung resection for the four most common indications in Yedikule Training and Research Hospital for Chest Diseases and Surgery, Istanbul, Turkey, between 2009 and 2018. From these patients, those who underwent upper abdominal CT for the measurement of PSM and thoracic CT for the measurement of PEC were included in the study.
The age (old age limit adopted by the social security institutions in Turkey is 65 yr), gender, diagnosis and indication for surgery, type of surgery and objective indicators of post-operative morbidity (number of surgeries and whether or not re-operation was performed for reasons such as post-operative haemorrhage or major bronchopleural fistula), operation time, number of days and number of admissions to surgical ıntensive care unit (SICU), number of nutritional assessments (high-protein oral nutritional supplement given daily under the supervision of a dietician) in the post-operative period, length of hospital stay, mode of discharge from the SICU and blood transfusions, latest condition and follow up were analyzed. Pre-operative biochemical parameters, including albumin, glucose, haemoglobin (HB), glomerular filtration rate (GFR) and blood gases [pH, base excess (BE)] were all reviewed. The risk factors were examined and co-variable analysis modelling was applied to include the factors that may affect mortality, such as the number of surgeries, operation time in hours, blood transfusion, length of hospital stay (days), number of admissions and days in SICU and, number of nutritional assessments.
The exclusion criteria were surgery other than lung resection and mediastinal lymph node dissection or extended lung resection, not having had thoracic and upper abdominal CT scans in the pre-operative period and incomplete data of biochemical parameters.
Radiological evaluation: The densities of PSM and PEC and their areas on axial images were measured by a radiologist. The area of the skeletal muscles was measured by drawing a polygonal region of interest on a single axial image captured with the CT device (Philips, The Netherlands, Ingenuity Elite model, 128-detector, 2 mm slice interval). PSM and PEC were evaluated bilaterally. The number of upper abdominal CT images was extremely low, and so, all images were acquired from the L1-L2 intervertebral disc level to standardize the PSM measurements. The pectoral muscle area measurement was performed in a superior axial section in a caudal direction at the level of the aortic arch. The areas of the right and left PSM and right and left PEC were measured (in cm2), and the total density was calculated in Hounsfield units (HU). The survival data of the patients and mortality in the first 30 post-operative days were documented from the national database.
Statistical methods: Data obtained in the study were analyzed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA) software. Descriptive statistics included number and percentage for categorical variables, as well as mean, standard deviation, and minimum and maximum values for numerical variables. A Student’s t test was used for the comparison of two independent groups if the numerical variables met the assumptions of normal distribution, otherwise, Mann-Whitney U test was used for the analysis. Kruskal–Wallis test was used for the comparison of multiple groups in which the numerical variables did not meet the assumptions of normal distribution. Mann-Whitney U test was used for the sub-group analyses, and the results were interpreted with Bonferroni correction. Spearman’s correlation coefficient was used in the analysis of relationships, as the assumptions were not met for the parametric tests. Survival analyses were made using Kaplan–Meier analysis. Determinants were examined with Cox regression analysis. The level of statistical significance was set at an alpha level of <0.05.
Results
Data from a total of 161 patients with available CT and biochemical records was considered for evaluation in this study. The mean age of the patients was 58.6±12.8 yr (45-89), 82 per cent of the patients were male and 68.9 per cent were aged 65 yr or younger (Table I). Of the patients, 73.3 per cent underwent lung resection and lymph node dissection and 26.7 per cent underwent, extended lung resection, with a mean operation time of 4.9 h. The pre-operative diagnosis of the patients was mostly lung cancer (85.1%). Of the patients with lung cancer, 33 per cent underwent pneumonectomy (45/137), and of these patients, 93.7 per cent were discharged with full recovery, and 10 male patients (6.3%) succumbed during hospitalization.
| Parameters assessed | n (%) |
|---|---|
| Gender | |
| Male | 132 (82.0) |
| Female | 29 (18.0) |
| Age (yr), mean±SD | 58.6±12.8 |
| Age (yr) | |
| ≤65 | 111 (68.9) |
| >65 | 50 (31.1) |
| Type of surgery | |
| Extended | 43 (26.7) |
| Standard | 118 (73.3) |
| Operation time (h), mean±SD | 4.9±2.5 |
| Diagnosis and indication for surgery | |
| Malignant neoplasm of bronchus or lungs (lobectomy) | 92 (57.1) |
| Malignant neoplasm of the main bronchus (pneumonectomy) | 45 (28.0) |
| Haemoptysis (lobectomy) | 21 (13.0) |
| Bronchiectasis (lobectomy) | 3 (1.9) |
| Mode of discharge | |
| Full recovery | 151 (93.7) |
| Death (surgical mortality) | 10 (6.3) |
| Latest condition | |
| Death | 33 (20.5) |
| Survival | 128 (79.5) |
| Duration of follow up (months), mean±SD | 21.8±86.2 |
SD, standard deviation
Surgical mortality was defined as death occurring during hospitalization or within the first 30 days of discharge, and the rate of surgical mortality in the present study was found to be 6.3 per cent. The surgical mortality rate did not differ significantly between patients with benign and malignant diseases (P = 0.286). From the whole sample, 23 patients succumbed due to non-surgical causes (metastases in 7, pneumonia secondary to adjuvant therapy in 5, tumour recurrence in 4, myocardial infarction in 5, diabetes mellitus in 1 and renal failure in 1) (Table I). During the follow up period of mean 21.8 months, the three and five year survival rates were 69.4 and 56.1 per cent respectively, and the median survival was estimated to be 73 months (95% confidence interval CI 25, 3-100 months). Both the three and five year survival rates for patients with non-malignant neoplasm were 91.3 per cent (log rank P = 0.164) (Figure). For those with malignant neoplasm of the bronchi and lungs and a malignant neoplasm of the main bronchus, the three and five year survival rates were 79.1/79.1 per cent and 69.1/ 46.1 per cent, respectively, and the differences were not significant. The right and left pectoralis minor area values and the density of the psoas major were found to be significantly lower in non-survivors (0.013-0.026) (Table II).
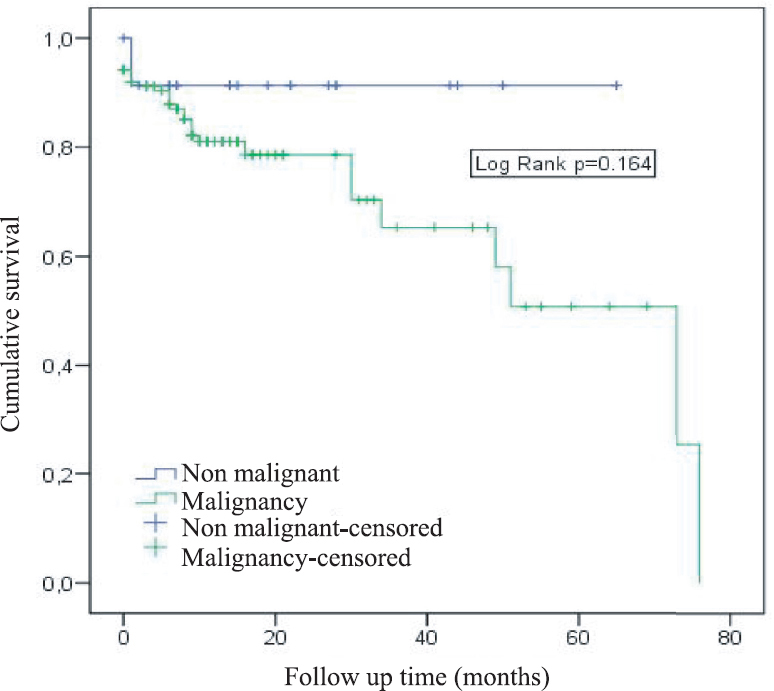
- Survival curve of malignant and non-malignant diseases.
| Parameters assessed | Cumulative survival (%) | Log rank P | |
|---|---|---|---|
| Three years | Five years | ||
| Gender | |||
| Men | 64.8 | 48.6 | 0.057 |
| Female | 93.1 | 93.1 | |
| Malignity | |||
| Negative | 91.3 | 91.3 | 0.164 |
| Positive | 70.3 | 50.8 | |
| Type of surgery | |||
| Lobectomy | 79.1 | 79.1 | 0.884 |
| Pneumonectomy | 69.1 | 46.1 | |
| Mean areas and densities (cm2-HU) | |||
| Right pectoralis major 10.22 | |||
| Low | 68.7 | 57.3 | 0.506 |
| High | 69.3 | 52.0 | |
| Right pectoralis minor 3.50 | |||
| Low | 56.7 | 35.5 | 0.013 |
| High | 85.9 | 85.9 | |
| Left pectoralis major 10.25 | |||
| Low | 59.6 | 49.7 | 0.093 |
| High | 83.9 | 62.9 | |
| Left pectoralis minor 3.66 | |||
| Low | 55.3 | 44.2 | 0.026 |
| High | 88.6 | 69.3 | |
| Total area 27.7 | |||
| Low | 59.3 | 47.6 | 0.164 |
| High | 82.7 | 66.2 | |
| Density area 52 | |||
| Low | 73.0 | 50.1 | 0.686 |
| High | 66.3 | 66.3 | |
| Right psoas 2.12 | |||
| Low | 74.7 | 54.5 | 0.735 |
| High | 62.0 | 62.0 | |
| Left psoas 2.20 | |||
| Low | 73.6 | 56.1 | 0.964 |
| High | 62.3 | 62.3 | |
| Total 4.22 | |||
| Low | 73.7 | 56.2 | 0.998 |
| High | 62.2 | 62.2 | |
| Density 48 | |||
| Low | 63.1 | 42.0 | 0.015 |
| High | 73.6 | 73.6 | |
HU, Hounsfield unit
The mean total PSM and PEC values were significantly lower in the female patients than in the male patients (Tables III and IV). The mean density of PSM was lower in patients aged >65 yr than in patients aged ≤65 yr. The mean density of PSM did not differ significantly among the diagnosis groups. The mean density of PSM was significantly lower in patients with malignant neoplasm of the main bronchus than in patients with haemoptysis. The mean density of PSM was significantly lower in the non-survivors than in the survivors (Table III).
| Parameters assessed | Right psoas | Left psoas | Total PSM | Density of PSM |
|---|---|---|---|---|
| Gender | ||||
| Male | 2.56±1.20 (2.24) | 2.64±1.35 (2.32) | 5.20±2.47 (4.63) | 47.6±7.2 (48) |
| Female | 1.63±0.65 (1.46) | 1.77±0.77 (1.61) | 3.40±1.30 (3.08) | 47.7±7.1 (51) |
| P | <0.001 | <0.001 | <0.001 | 0.926 |
| Age (yr) | ||||
| ≤65 | 2.46±1.30 (2.13) | 2.57±1.43 (2.15) | 5.02±2.66 (4.10) | 49.1±6.1 (51) |
| >65 | 2.22±0.79 (2.12) | 2.28±0.95 (2.20) | 4.50±1.61 (4.32) | 44.2±8.2 (45) |
| P | 0.815 | 0.457 | 0.630 | <0.001 |
| Number of surgeries | ||||
| 1 | 2.36±1.16 (2.12) | 2.45±1.31 (2.20) | 4.81±2.39 (4.22) | 47.5±7.2 (48) |
| 2 | 2.71±1.35 (2.18) | 2.82±1.23 (2.37) | 5.53±2.51 (4.55) | 48.7±6.0 (48.5) |
| P | 0.388 | 0.259 | 0.294 | 0.804 |
| Type of surgery | ||||
| Extended | 2.63±1.43 (2.10) | 2.60±1.41 (2.27) | 5.23±2.76 (4.17) | 47.4±7.7 (49) |
| Standard | 2.30±1.07 (2.12) | 2.44±1.28 (2.18) | 4.74±2.27 (4.24) | 47.7±6.9 (48) |
| P | 0.369 | 0.804 | 0.551 | 0.920 |
| Diagnosis and indication for surgery | ||||
| Malignant neoplasm of bronchus or lungs (lobectomy) | 2.48±1.19 (2.20) | 2.59±1.36 (2.28) | 5.07±2.45 (4.55) | 47.8±7.3 (50) |
| Malignant neoplasm of the main bronchus (pneumonectomy) | 2.20±1.28 (1.98) | 2.19±1.30 (1.91) | 4.39±2.55 (3.91) | 46.1±5.8 (46)* |
| Haemoptysis (lobectomy) | 2.23±0.77 (2.08) | 2.50±1.03 (2.21) | 4.73±1.74 (4.10) | 51.6±7.8 (53) |
| Bronchiectasis (lobectomy) | 3.05±0.63 (3.33) | 3.18±0.07 (3.20) | 6.23±0.58 (6.53) | 45.0±10.6 (41) |
| P | 0.107 | 0.067 | 0.073 | 0.026* |
| Blood transfusion | ||||
| Yes | 2.69±2.58 (1.59) | 3.24±3.35 (2.21) | 5.93±5.90 (3.80) | 48.4±7.1 (44) |
| No | 2.37±1.07 (2.12) | 2.44±1.14 (2.19) | 4.81±2.13 (4.26) | 47.6±7.1 (48) |
| P | 0.454 | 0.831 | 0.605 | 0.907 |
| Latest condition | ||||
| Dead | 2.39±1.17 (2.01) | 2.26±1.19 (1.80) | 4.65±2.26 (3.87) | 43.9±8.4 (42) |
| Survival | 2.39±1.18 (2.15) | 2.53±1.34 (2.21) | 4.92±2.44 (4.31) | 48.6±6.5 (49) |
| P | 0.869 | 0.138 | 0.383 | 0.004 |
*Lower than haemoptysis. PSM, psoas major muscle
The mean density of the right and left pectoralis major, the mean total PEC and the mean density of PEC were significantly lower in patients aged >65 yr than in patients aged ≤65 yr (Table IV). The mean areas of the right and left pectoralis minor muscles were significantly lower in patients undergoing extended lung resection than in patients undergoing a standard resection. With the exception of mean density, all the PEC parameters were significantly lower in the non-survivors than in the survivors (Table IV).
| Parameters assessed | Right pectoralis major | Right pectoralis minor | Left pectoralis major | Left pectoralis minor | PEC total | Density of PEC |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 11.38±3.65 (11.2) | 3.85±1.36 (3.7) | 11.21±3.57 (10.9) | 3.91±1.42 (3.8) | 30.3±9.0 (29.1) | 53.4±8.0 (53.5) |
| Female | 7.84±1.80 (7.8) | 2.89±0.88 (2.7) | 8.04±2.22 (7.7) | 3.35±0.89 (3.4) | 22.1±4.8 (21.4) | 46.9±7.7 (47) |
| P | <0.001 | <0.001 | <0.001 | 0.053 | <0.001 | <0.001 |
| Age (yr) | ||||||
| ≤65 | 11.17±3.87 (10.7) | 3.77±1.44 (3.7) | 11.08±3.80 (10.7) | 3.92±1.39 (3.7) | 29.9±9.5 (28.9) | 53.0±8.1 (53) |
| >65 | 9.78±2.93 (9.3) | 3.46±1.04 (3.3) | 9.65±2.82 (9.5) | 3.58±1.27 (3.6) | 26.5±7.1 (25.2) | 50.3±8.6 (49.5) |
| P | 0.039 | 0.357 | 0.029 | 0.176 | 0.031 | 0.040 |
| Number of surgeries | ||||||
| 1 | 10.72±3.70 (9.9) | 3.70±1.37 (3.5) | 10.70±3.61 (10.3) | 3.85±1.37 (3.7) | 29.0±9.1 (27.5) | 52.0±8.5 (52) |
| 2 | 10.98±3.18 (11.8) | 3.38±0.84 (3.7) | 9.90±3.18 (9.7) | 3.36±1.08 (3.3) | 27.6±7.7 (28.5) | 54.5±5.2 (54.5) |
| P | 0.532 | 0.565 | 0.457 | 0.248 | 0.892 | 0.225 |
| Type of surgery | ||||||
| Extended | 10.35±3.69 (10.6) | 3.37±1.22 (3.1) | 10.18±3.89 (9.9) | 3.48±1.39 (3.3) | 27.4±9.2 (27.6) | 53.0±9.3 (53) |
| Standard | 10.88±3.65 (10.1) | 3.79±1.36 (3.7) | 10.80±3.46 (10.6) | 3.93±1.33 (3.7) | 29.4±8.9 (27.9) | 51.9±8.0 (52) |
| P | 0.480 | 0.038 | 0.333 | 0.041 | 0.182 | 0.398 |
| Diagnosis and indication for surgery | ||||||
| Malignant neoplasm of bronchus or lungs (lobectomy) | 10.78±3.76 (10.2) | 3.65±1.38 (3.4) | 10.85±3.82 (10.5) | 3.93±1.45 (3.8) | 29.2±9.4 (28.2) | 52.7±7.7 (53) |
| Malignant neoplasm of the main bronchus (pneumonectomy) | 10.67±3.40 (10.6) | 3.58±1.17 (3.6) | 10.46±3.29 (10.3) | 3.70±1.28 (3.6) | 28.4±8.4 (26.6) | 51.8±9.6 (52) |
| Haemoptysis (lobectomy) | 10.76±4.09 (9.7) | 3.90±1.54 (3.6) | 10.04±3.35 (10.1) | 3.54±1.12 (3.3) | 28.2±9.1 (26.6) | 51.9±8.5 (50) |
| Bronchiectasis (lobectomy) | 10.57±1.34 (10.9) | 4.32±0.64 (4.0) | 11.02±2.12 (11.6) | 3.88±1.15 (3.7) | 29.8±3.8 (31.5) | 45.0±3.6 (46) |
| P | 0.998 | 0.497 | 0.784 | 0.473 | 0.929 | 0.344 |
| Blood transfusion | ||||||
| Yes | 12.24±4.53 (11.3) | 4.49±2.07 (4.0) | 12.12±4.43 (11.3) | 4.81±2.08 (4.2) | 33.7±12.1 (30.2) | 46.8±6.8 (46.5) |
| No | 10.66±3.60 (10.2) | 3.63±1.28 (3.5) | 10.56±3.53 (10.2) | 3.76±1.30 (3.7) | 28.6±8.8 (27.7) | 52.5±8.3 (52) |
| P | 0.377 | 0.316 | 0.230 | 0.164 | 0.273 | 0.062 |
| Latest condition | ||||||
| Dead | 9.24±3.24 (9.3) | 3.08±0.87 (3.0) | 8.95±3.24 (8.7) | 3.22±1.05 (3.2) | 24.5±7.6 (23.1) | 49.9±8.0 (49) |
| Survival | 11.13±3.67 (10.5) | 3.83±1.39 (3.7) | 11.07±3.54 (10.8) | 3.96±1.39 (3.8) | 30.0±9.0 (28.4) | 52.8±8.3 (52) |
| P | 0.013 | 0.004 | 0.002 | 0.004 | 0.002 | 0.075 |
PEC, pectoralis major and minor muscles
Age, albumin, number of days in SICU, blood haemoglobin (HBG), psoas muscle density and PEC muscle area were determined to have an effect on mortality (P= 0.001-0.024), and of these, the number of days in SICU, glucose, and PEC muscle area were observed to the determinants of mortality (Table V).
| Parameters assessed | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR | P | HR | P | |
| Age (reference: <65 yr) | ||||
| ≥65 yr | 2.412 | 0.016 | 0.344 | 0.228 |
| Gender (reference: Female) | ||||
| Male | 3.655 | 0.078 | 0.178 | 0.146 |
| Albumin (reference: >3.4) | ||||
| ≤3.4 | 5.118 | 0.003 | 1.753 | 0.561 |
| Number of days in SICU | 1.087 | <0.001 | 1.075 | 0.009 |
| Malignancy | 2.634 | 0.186 | 2.941 | 0.355 |
| Type of surgery (reference: lobectomy) | ||||
| Pneumonectomy | 1.058 | 0.885 | 0.921 | 0.911 |
| HGB (Reference: >11) | ||||
| ≤11 | 3.039 | 0.002 | 3.595 | 0.078 |
| pH | 1.886 | 0.799 | 5.788 | 0.855 |
| Blood gases BEecf | 1.063 | 0.149 | 1.786 | 0.489 |
| Blood gases Beb33 | 1.08 | 0.110 | 0.584 | 0.596 |
| Glucose | 0.987 | 0.163 | 1.032 | 0.031 |
| GFR | 1.006 | 0.460 | 0.992 | 0.572 |
| Psoas muscle area | 1.004 | 0.958 | 1.284 | 0.097 |
| Psoas muscle density | 0.914 | 0.001 | 0.961 | 0.388 |
| PEC muscle area | 0.949 | 0.024 | 0.859 | 0.004 |
| PEC muscle density | 0.968 | 0.153 | 0.913 | 0.071 |
SICU, surgical ıntensive care unit; HGB, haemoglobin, GFR, glomerular filtration rate; HR, hazard ratio
In the examination of the factors that determine mortality, the number of admissions and the number of days in SICU were found to be the most significant factors in the model. In the risk factors and co-variable analysis, the number of admissions to SICU, density of PSM and volume of right pectoralis minor were found to be the most significant factors.
Discussion
Post-operative morbidity and mortality can occur in patients undergoing anatomic lung resection12. Although the rates have decreased in number with advances in surgical techniques over the years, post-operative complications still remain a challenge due to the increased number of such surgeries345, and accordingly, new parameters can be found to predict the risk.
Suzuki et al6 demonstrated previously that sarcopaenia is a poor prognostic factor in patients undergoing surgery for NSCLC. Post-operative morbidity and mortality can be observed in our hospital, where the focus is predominantly on the surgical treatment of lung cancer (85.1%). Mortality is often associated with non-surgical causes and occurs in the long term, whereas surgical mortality occurs during the hospitalization period or within 30 days of discharge13. In the present study, 6.3 per cent of the patients died during their stay in hospital, but none within 30 days of discharge. During the mean follow up period of 21 months, the three and five year survival rates were 69.4 and 56.1 per cent, respectively. In the present study, which included not only isolated NSCLC, but also all patients who had undergone anatomic lung resection, analysis was made of the survival of patients mostly with malignant diseases. In the current study, 85 per cent of the study patients had cancer, and survival data reported in the literature in general relates to patients operated on for cancer3. The survival rates (both 3 and 5 yr) for malignant neoplasm of the lungs were 70.3 and 50.8 per cent, respectively. For malignant disease, extended surgery and pneumonectomy may be necessary due to oncological principles. Therefore, pneumonectomy was performed and surgical mortality was 6.3 per cent. This may have been due to the difference in the ratio between the groups (85.1-14.9%), but the surgical mortality rate did not differ significantly between patients with benign and malignant diseases. This value indicated that patients with malignant diseases can be operated upon as safely as patients with benign diseases in this study, which mostly involved patients who underwent surgery due to malignant causes.
The fact that malignancy or the type of surgery is not associated with mortality demonstrated that surgery is safe. The present study found in particular that changes in the area of PEC minor were directly and proportionally correlated with survival. Psoas muscle density and PEC muscle area were seen to have an effect and determined mortality. The number of admissions and days in SICU and blood albumin and HBG were determined to be related to mortality. This indicates that sarcopaenia affects survival and surgical mortality, contrary to the findings of Miller et al7. Similarly, the lack of difference in PSM and PEC measurements according to the extent of resection (standard vs. extension) shows that these two are unrelated. In a study by Hervochon et al9, pneumonectomy was reported to be associated with high post-operative mortality and a low five-year survival rate. Similarly, 28.9 per cent of the patients that underwent pneumonectomy due to lung cancer and 19.6 per cent of the patients who underwent lobectomy died in the long term, which can be attributed to the advanced disease stage in patients undergoing pneumonectomy. While gender, malignancy or type of surgery is not associated with survival, a significantly lower area and density of PSM and PEC in female patients aged 65 yr and older is an expected finding due to the decreased anabolism above this age. Collins et al14 found that sarcopaenia becomes more evident with advancing age in males, that it affects morbidity in patients with lung cancer and that it serves as a better marker of morbidity than cachexia. Similar results were noted in the present study, although no significant effect of sarcopaenia on morbidity was found, contrary to expectations, most likely due to the small patient sample. Therefore, further studies on a larger patient group for whom only thoracic CT scans are available in order to investigate the effect of pectoral muscle loss on mortality. The finding of a lower total area of the pectoralis minor muscles in patients undergoing extended rather than standard surgery was thought to be incidental considering that the extent of surgery is determined by the oncology principles. This type of surgery is, however, known to be associated with increased morbidity and mortality. Parameters indicating morbidity, such as the number and duration of surgeries, blood transfusions, length of hospital stay, duration of follow up, and number of nutritional assessments did not differ to any significant degree. suggesting that the differences between the muscle groups had no effect on morbidity. Considering that the size of the pectoral muscles is rapidly affected by physical activity, it was decided that it would be more accurate to measure both muscle density and area. However, a lack of a direct association between the changes in the area and density of the muscle groups and the changes in hemoglobin and albumin values, expectedly associated with morbidity suggests that a linear relationship could exist between anabolic activity and the muscle groups. The finding that the pectoralis minor, which is relatively less affected by physical activity than the pectoralis major, had an effect on survival was an important finding in this study. The psoas muscle density measurements were lower in patients with malignant neoplasm of the main bronchus than in patients with haemoptysis, and unlike the other groups, the rate of extended surgery was lower in the pectoralis minor muscle group, although these differences, although not significant.
The mean density of PSM (48 HU) had a significant effect on survival, whereas other parameters of the PSM (right psoas, left psoas, psoas total) had no significant effect. In the pectoralis muscle group areas, the right pectoralis minor (3.50 cm2), and left pectoralis minor (3.66 cm2) had a significant effect on survival, whereas density measurements had no effect. This suggests that the PEC similar to the findings by McDonald et al10 is a better indicator of survival than PSM. There are studies which have generally investigated sarcopaenia in PEC, PSM and ESM71011. One of the two studies investigating the pectoral muscle groups on the same subjects was conducted by Miller et al7, in which only ESM was found to have an effect on survival. Furthermore, Hübner et al15 reported that a decrease in serum albumin levels was influential in the development of post-operative complications and morbidity. It is a remarkable finding of the present study that the changes in the measurements in the PEC were directly proportional to the serum albumin levels. The authors believe that this finding may suggest that PEC has an effect on morbidity. Patients who are to undergo lung resection and lymph node dissection, or extended surgery, should certainly undergo a pre-operative CT scan of the thorax16. Sarcopaenia detected in the area of PEC minors and SICU time were determined to have an effect on mortality, although no effect on morbidity was detected, and this effect was greater than the effect of the density of PSM. Hervochon et al9 suggested that body mass index (BMI) and PSM measurements can be useful in prognosis. While BMI evaluation was initially considered in the present study, this idea was later abandoned due to the unavailability of pre-operative weight or height measurements of the patients for which the upper abdominal CT scans were available. This may represent a limitation of the study.
Overall, the present study has shown in particular that the pectoral minor muscle groups are an indicator of survival and mortality over the psoas major muscle. The change in pectoral muscle mass being directly proportional to albumin levels suggests that it can also reflect morbidity. Therefore further studies on a larger group of patient aimed to study pectoral muscle groups and BMI in patients undergoing thoracic surgical procedures can demonstrate the relationship with both morbidity and mortality.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Sarcopenia:European consensus on definition and diagnosis:Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-23.
- [Google Scholar]
- Sarcopenia in resected NSCLC:Effect on postoperative outcomes. J Thorac Oncol. 2018;13:895-903.
- [Google Scholar]
- Preoperative prediction of pulmonary complications following thoracic surgery. Chest. 1993;104:155-9.
- [Google Scholar]
- Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88:1093-9.
- [Google Scholar]
- Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016;101:92-7.
- [Google Scholar]
- Sarcopenia is a predictor of outcomes after lobectomy. J Thorac Dis. 2018;10:432-40.
- [Google Scholar]
- Sarcopenia of the psoas muscles ıs associated with poor outcomes following lung transplantation. Ann Thorac Surg. 2019;107:1082-8.
- [Google Scholar]
- Body mass ındex and total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg. 2017;103:287-95.
- [Google Scholar]
- Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11:326-34.
- [Google Scholar]
- Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography-derived ındex for prognosis. Ann Am Thorac Soc. 2016;13:334-41.
- [Google Scholar]
- The 30-day mortality and hospital mortality after chest surgery described in the annual reports published by the Japanese Association for Thoracic and Cardiovascular Surgery. Gen Thorac Cardiovasc Surg. 2015;63:279-83.
- [Google Scholar]
- Use of structured presentation formatting and NSQIP guidelines ımproves quality of surgical morbidity and mortality conference. J Surg Res. 2019;233:118-23.
- [Google Scholar]
- The assessment and impact of sarcopenia in lung cancer:a systematic literature review. BMJ Open. 2014;4:e003697.
- [Google Scholar]
- Postoperative albumin drop ıs a marker for surgical stress and a predictor for clinical outcome:A pilot study. Gastroenterol Res Pract 20162016:8743187.
- [Google Scholar]
- Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
- [Google Scholar]






