Translate this page into:
Relationship between increased serum & synovial fluid decorin levels & knee osteoarthritis
For correspondence: Dr Kenan Ozler, Department of Orthopedics, Konya Beysehir State Hospital, Esentepe Highroad, Street No: 1, Beyşehir 40525, Konya, Turkey e-mail: kenozler@hotmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Decorin is a proteoglycan that plays a role in the binding of collagen and has an important role in the pathogenesis of osteoarthritis (OA). This study was aimed to determine serum and synovial fluid decorin levels in patients with knee OA and to investigate whether these levels were associated with OA and the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) score.
Methods:
In this prospective study 88 participants were included (44 knee OA and 44 with other knee joint diseases) in the study and control groups. Knee function was assessed using the WOMAC score. The serum and synovial fluid decorin levels were analyzed using a human decorin ELISA. Binary logistic regression with a single and multi-categorical predictor was used to determine the possible risk factors for OA.
Results:
The serum decorin levels were significantly higher in the OA group than the control group (P<0.002). The synovial fluid decorin levels were not significantly different between OA and control groups. WOMAC score [odds ratio (OR)=1.073, 95% confidence interval (CI): 1.032-1.116, P<0.001] and high serum decorin levels (OR=1.114, 95%CI: 1.030–1.205, P=0.007) were found to be significant in the determination of OA. Serum decorin levels were positively correlated with the WOMAC score in OA.
Interpretation & conclusions:
An increased serum decorin levels may be indicative of changes in extracellular matrix structure. The positive correlation between serum decorin level and WOMAC score supports this result. Increased serum decorin levels and WOMAC score were found to be risk factors associated with OA. However, the decorin level in the joint fluid was not associated with OA.
Keywords
Knee osteoarthritis
osteoarthritis progression
serum decorin
synovial fluid
WOMAC score
Articular cartilage is hyaline cartilage, and the structure of its extracellular matrix (ECM) is composed of collagen, proteoglycans and chondrocytes, which carries the body load and provides flexibility and elasticity against tensile forces and resistance against compressive forces1. Proteoglycans provide bonds between the collagens in ECM2. In particular, small leucine-rich proteoglycans (SLRPs) play a role in the formation, bonding and morphology of the collagen fibril structure3.
Decorin is one of the extracellular proteoglycans belonging to the SLRP family; it belongs to class I along with biglycan and asporin4. The region where SLRPs are most abundant is the cartilage, and their abundance increases with age, especially that of decorin5. The role of decorin in fibrillogenesis is to limit the fibril diameter and regulate its structure6. Decorin has anti-fibrotic and pro-inflammatory activities7.
This study was undertaken to determine serum and synovial fluid decorin levels in patients with osteoarthritis (OA) and to investigate whether decorin levels were associated with OA and Western Ontario and McMaster Universities Osteoarthritis (WOMAC) score.
Material & Methods
A prospective study was performed between June and October 2018 in the Orthopaedics department, Konya Beysehir State Hospital, Konya, Turkey. The sample size was calculated to detect an anticipated effect size of 0.3 for the regression equation, at a power level of 0.95 (β =0.95) and a probability level of 0.05 (α =0.05). A total of 88 participants were included in the study. The diagnosis of knee OA was determined according to the radiographic features and magnetic resonance imaging. Forty four patients were diagnosed to have knee OA by the Kellgren–Lawrence scale8. Which required the presence of all five radiological criteria: osteophytes on the joint side, periarticular ossicles, joint area narrowing (JSN), small pseudocystic regions within the subchondral bone. OA patients were divided into five stages: stage 0 (no changes in X-ray), stage 1 (osteophyte and no JSN), stage 2 (osteophyte and JSN), stage 3 (medium multiple osteophytes, JSN, minimal sclerosis and deformity of bone ends) and stage 4 (giant osteophytes, evident JSN, severe sclerosis and deformity of bone ends)8. Forty four body mass index (BMI) and mean age-matched patients with other knee joint diseases (arthroscopic anterior cruciate ligament reconstruction, meniscus angioplasty or arthroscopy, non-OA group) were included as controls. Control group inclusion criteria: without radiological features (there was no osteophyte, JSN, sclerosis and bone deformity radiologically and/or clinically, no previous knee surgery, no intra-articular local and/or systemic injections, no septic arthritis), and clinically without knee pain in the past month (without crepitus on active joint motion, no morning stiffness, no bony enlargement of the knee on examination, no bony tenderness of the knee on examination and no palpable warmth)9. All participants included in the study were evaluated at the initial admission. Clinical examination was performed, and anthropometric measurements, as well as the previous radiographic features and medical history, were recorded. Knee function was assessed using the WOMAC. The WOMAC score is composed of 24 parameters that include pain (score range: 0–20), stiffness (score range: 0–8) and functional impairment (score range: 0–68)10.
Patients were excluded if any of the following disorders were present: infectious diseases, history of total knee arthroplasty or other types of knee surgery, septic arthritis, obesity, neurological or neuromuscular diseases, using antibiotics, bone tumours, osteoporosis- or trauma-related fractures, diabetes mellitus, Addison's disease and immune system disorder. Additional exclusion criteria were the previous use of systemic steroids, antibiotics and intra-articular hyaluronic acid injections.
All participants provided written informed consent. The study protocol was approved by the local Ethical Committee, University of Karatay, Konya, Turkey (approval date/number:05.06.2018/017).
The blood samples were obtained by venipuncture and serum was separated within one hour after withdrawal. The average synovial fluid volume of the normal knee joint is between 0.5 and 4 ml11. Different techniques evaluated the amounts of joint fluids12. In our study, a superolateral approach was used. Support was placed below the knee, the knee was flexed at 30°, then inserted into the side of the patella with a 5 ml sterile syringe, and the needle was advanced down and medially to the posterolateral direction of the patella, and the synovial space was reached13. Approximately 2 ml of synovial fluid was aspirated from the synovial space. The aspirated joint fluids were stored at −80°C without centrifugation. All serum samples were stored at −80°C until the day of analysis.
In the control group, joint fluid samples were collected before arthroscopy using a 22 gauge needle placed into the dorsomedial synovial pouch of the knee joint with the carpus flexed at approximately 70° in patients undergoing arthroscopy such as arthroscopic anterior cruciate ligament reconstruction, meniscus angioplasty or arthroscopy. The knee joint was distended with 10 ml of sterile saline using the needle previously inserted to collect the synovial fluid sample. The superolateral approach was used to obtain the synovial fluids of the other non-OA non-arthroscopic control group patients.
All joint fluid samples were studied in Algen Diagnostics Limited Biochemistry Laboratory, Ankara, Turkey. The serum and synovial fluid decorin levels were analyzed using a human decorin enzyme-linked immunosorbent assay (ELISA) kit (BioVendor Research and Diagnostic Products, Brno, Czech Republic) with an immunoassay (ALISEI) fully automatic ELISA device, and the results are presented as ng/ml.
Statistical analysis: Data analysis, was done using the IBM-Statistical Package for the Social Sciences version 22.0 (SPSS Inc., Chicago, IL, USA) and PASTE programs. The Kolmogorov–Smirnov test was used to determine the suitability of data for the normal distribution. parametric methods were used for analysis of the variables with a normal distribution. Student's t test was used for the comparison of BMI and age homogenized groups. Serum and WOMAC score receiver operator characteristic (ROC) curves were performed in OA patients for best cut-off value, sensitivity, specificity and area under the curve (AUC) for serum decorin and WOMAC scores in OA. Single and multi-categorical logistic regression analysis was used to determine the association between OA and serum decorin, synovial fluid decorin levels, decorin (synovial fluid)/decorin (serum), age, BMI and WOMAC score. Furthermore, single and multi-categorical logistic regression analysis was used to determine the association between WOMAC score and serum decorin, synovial fluid decorin levels, decorin (synovial fluid)/decorin (serum), age and BMI. Pearson's correlation and Spearman's rho tests were used to examine the correlations of variables and serum decorin levels in OA and control groups.
Results
The baseline demographic, clinical, and laboratory characteristics of study and control groups are shown in Table I. There were no significant differences among mean age and BMI between groups. WOMAC score was significantly higher in the OA group compared to the control group (P<0.001) (Table I). The serum decorin levels were significantly higher in the OA group than the control group (P<0.001). The serum decorin levels were significantly (P<0.001) higher than synovial fluid levels in OA patients.
| Characteristics | Osteoarthritis (n=44) mean±SD | Control (n=44) mean±SD |
|---|---|---|
| Age (yr) | 63.04±5.68 | 59.94±6.50 |
| BMI (kg/m2) | 29.76±6.44 | 28.64±6.20 |
| WOMAC score | 65.46±18.25*** | 46.13±13.65 |
| Decorin (serum) (ng/ml) | 19.39±8.57** | 12.67±6.89 |
| Decorin (synovial fluid) (ng/ml) | 17.41±8.42 | 11.94±7.47 |
| Decorin (synovial fluid) (ng/ml)/decorin (serum) (ng/ml) | 0.984±0.214 | 0.723±0.212 |
P **<0.01, ***<0.001 compared to controls. BMI, body mass index; WOMAC score, The Western Ontario and McMaster Universities Osteoarthritis score.
Serum decorin levels and WOMAC score were again evaluated with ROC analysis (Figure); cut-off levels were determined, and AUC values were calculated. The AUC, best cut-off values, sensitivity and specificity for distinguishing the groups for each parameter are given in Table II. Serum decorin levels and WOMAC score were found to be significant.
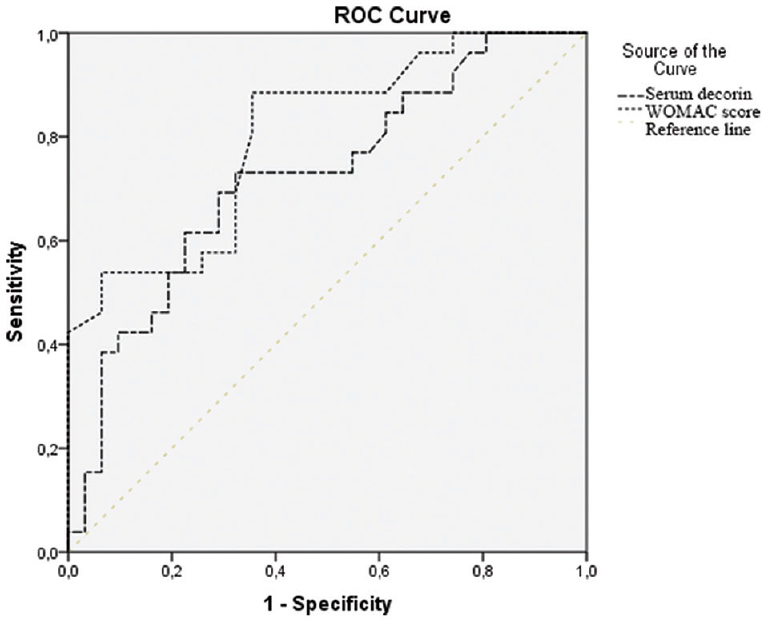
- Serum decorin levels and WOMAC (Western Ontario and McMaster Universities Osteoarthritis) score receiver operator characteristic (ROC) curve in osteoarthritis. Diagonal segments are produced by ties.
| Parameter | Cut-off | Specificity (%) | Sensitivity (%) | AUC (95% CI) |
|---|---|---|---|---|
| Decorin (serum) (ng/ml) | 15.71 | 73 | 68 | 0.723 (0.590-0.857)** |
| WOMAC score | 35 | 89 | 65 | 0.802 (0.688-0.916)*** |
P **<0.01, ***<0.001. WOMAC score, Western Ontario and McMaster Universities Osteoarthritis score; AUC, area under the curve; CI, confidence interval
Multivariate logistic regression analysis was used to determine if there was a relationship between groups and the defined cut-off levels of the laboratory parameters. WOMAC score [odds ratio (OR)=1.073, 95%CI: 1.032-1.116, P<0.001] and high serum decorin levels (OR=1.114, 95%CI: 1.030-1.205, P=0.007) were found to be significant in the determination of OA (Table III). In addition, we examined if there were any changes in synovial fluid decorin levels and synovial fluid decorin/serum decorin ratio according to the management of OA. No significant changes were observed when the OA patients were again evaluated (Table III).
| Variables | OR (95% CI) |
|---|---|
| Age (yr) | 1.087 (0.994-1.190) |
| BMI (kg/m2) | 1.030 (0.942-1.125) |
| WOMAC score | 1.073 (1.032-1.116)*** |
| Decorin (serum) (ng/ml) | 1.114 (1.030-1.205)** |
| Decorin (synovial fluid) (ng/ml) | 1.113 (0.981-1.263) |
| Decorin (synovial fluid) (ng/ml)/decorin (serum) (ng/ml) | 1.898 (0.407-8.8839) |
P **<0.01, ***<0.001. #Logistic regression model (binary logistic regression) was used to determine the possible risk factors for OA. BMI, body mass index; WOMAC score, Western Ontario and McMaster Universities Osteoarthritis score; CI, confidence interval; OR, odds ratio
Further analysis was also performed to determine whether there was a correlation between serum decorin levels and other variables (age, BMI, WOMAC score and synovial fluid decorin levels). Serum decorin levels were positively correlated with the WOMAC score in OA (r=0.514; P=0.007). No correlation was observed serum decorin levels and other variables [age, BMI, synovial fluid decorin and decorin (synovial fluid)/decorin (serum) ratio levels] in OA and control groups (Table IV).
| Variables | Decorin (serum) | |||
|---|---|---|---|---|
| Osteoarthritis | Control | |||
| r | P | r | P | |
| Age (yr) | −0.238 | 0.214 | −0.048 | 0.796 |
| BMI (kg/m2) | −0.152 | 0.290 | −0.069 | 0.727 |
| WOMAC score | 0.514 | 0.007 | 0.104 | 0.578 |
| Decorin (synovial fluid) (ng/ml) | 0.146 | 0.635 | 0.325 | 0.394 |
| Decorin (synovial fluid) (ng/ml)/decorin (serum) (ng/ml) | −0.532 | 0.061 | −0.484 | 0.187 |
Pearson correlation test was used. BMI, body mass index, WOMAC score, Western Ontario and McMaster Universities Osteoarthritis score
Discussion
Knee OA is a progressive chronic disease characterized by cartilage degeneration, subchondral bone formation, and changes in the synovium, and it causes clinical symptoms, such as pain, swelling, and restriction of movement14. Cartilage degeneration is a significant risk factor for the onset and progression of OA. The most important structure in ECM, particularly for load-bearing and elasticity, is fibril formation, which is formed by the binding of different collagens to each other15. In cases where pro-inflammatory and protease activity is increased, an increase in decorin levels indicates the presence of damage16.
Previous studies have revealed that decorin is anti-angiogenetic17, fibrillogenesis and pro-inflammatory18, and also associated with inflammatory diseases such as OA19, osteoporosis, and other conditions such as muscular dystrophy, Ehlers–Danlos syndrome and corneal diseases20. Decorin is also important in maintaining the biological activity of the ECM and proliferation and differentiation of the cell. It is particularly cleaved by extracellular matrix metalloproteinases (MMP) such as MMP-2, MMP-3 and MMP-721. Bock et al22 showed that levels of proteolytic levels of SLRPs such as decorin and big lycan were significantly increased in osteoarthritic patients compared to the healthy group. Further, decorin levels were higher in advanced OA, and thus the authors confluded that decorin contributed to the new structure of cartilage. Poole et al23 found that patients with OA had reduced the amounts of decorin and big lycan in cartilage in light microscopy. Manfort et al19 evaluated SLRP degradation in normal and OA cartilage tissues by Western blotting. They reported that there was no difference in decorin levels when healthy and early-stage OA cartilage tissue was incubated with MMP-13; however, the decorin was not detected due to complete degradation in advanced OA cartilage, and confirmed their results by sequence analysis. In our study, serum decorin level was higher in OA compared to the control group, and there was no difference in synovial fluid decorin levels. In the studies mentioned above, significantly higher levels of decorin were observed in OA patients; and our findings were consistent with earlier studies.
Although several factors have been proposed in the pathogenesis of OA, but the most essential factors are mechanical and inflammatory effects in the process leading to the deterioration of ECM structure in cartilage and consequently decreased cartilage elasticity and regeneration24. Mechanical and inflammatory factors cause the formation of proteoglycan and proinflammatory signals, resulting in the production and release of proteases25. Our study indicated that serum decorin was associated with pain and functional disability. However, we found that the synovial fluid decorin level was not associated with OA risk and WOMAC score. The WOMAC score is known to be related to the severity of OA. Wardale et al26 demonstrated increased cell migration and matrix proteins, including decorin in the ECM of osteoarthritic human cartilages. Zhang et al27 reported that tendon stretch properties were reduced in decorin-deficient mice. Dourte et al28 demonstrated decreased viscoelasticity and collagen content in the tendon of decorin heterozygosity patients. Although the cartilage oligomeric matrix protein29 and WOMAC score are related to the severity of OA, there is no study associated with decorin levels in serum and synovial fluid. In our study, a positive correlation was observed between serum decorin level and WOMAC score. The limitations of our study were the lack of evaluation of other proteases and proteoglycans, which are risk factors of OA, and a small number of patients and the controls had a history of joint injury and had been operated upon the knee for different reasons.
In conclusion, our findings showed that the increase in serum decorin level and WOMAC score were independent risk factors associated with OA risk. WOMAC score is known to be associated with OA progression. The increased serum decorin levels may indicate increased proteoglycan synthesis to provide and regulate cartilage regeneration in osteoarthritis. Also, the positive correlation between serum decorin level and WOMAC score is a finding that supports this result.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Fell-muir lecture: Proteoglycans and more-from molecules to biology. Int J Exp Pathol. 2009;90:575-86.
- [Google Scholar]
- Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319-23.
- [Google Scholar]
- Dermatansulfate in the synovial fluid of patients with knee osteoarthritis. Mod Rheumatol. 2007;17:301-5.
- [Google Scholar]
- Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729-43.
- [Google Scholar]
- Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget. 2018;9:5480-91.
- [Google Scholar]
- Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores. Semin Arthritis Rheum. 2005;35:197-201.
- [Google Scholar]
- Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-40.
- [Google Scholar]
- Advances in Molecular biomarker for early diagnosis of Osteoarthritis. Biomol Concepts. 2019;10:111-9.
- [Google Scholar]
- Standard reference values for musculoskeletal ultrasonography. Ann Rheum Dis. 2004;63:988-94.
- [Google Scholar]
- Joint aspiration and injection and synovial fluid analysis. Best Pract Res Clin Rheumatol. 2013;27:137-69.
- [Google Scholar]
- New findings in osteoarthritis pathogenesis: Therapeutic implications. Ther Adv Chronic Dis. 2013;4:23-43.
- [Google Scholar]
- Complexity of danger: The diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237-45.
- [Google Scholar]
- Relationships between transforming growth factor-beta1, myostatin, and decorin: Implications for skeletal muscle fibrosis. J Biol Chem. 2007;282:25852-63.
- [Google Scholar]
- Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci U S A. 2010;107:21671-6.
- [Google Scholar]
- Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: Identification of a new biglycan cleavage site. Arthritis Res Ther. 2006;8:R26.
- [Google Scholar]
- Mice deficient in small leucine-rich proteoglycans: Novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R-16R.
- [Google Scholar]
- MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009;46:541-50.
- [Google Scholar]
- The small proteoglycans decorin and biglycan in human articular cartilage of late-stage osteoarthritis. Osteoarthritis Cartilage. 2001;9:654-63.
- [Google Scholar]
- Contents and distributions of the proteoglycans decorin and biglycan in normal and osteoarthritic human articular cartilage. J Orthop Res. 1996;14:681-9.
- [Google Scholar]
- Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res Ther. 2005;7:R852-61.
- [Google Scholar]
- Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models. Mol Cells. 2008;25:1-6.
- [Google Scholar]
- An ex vivo model using human osteoarthritic cartilage demonstrates the release of bioactive insulin-like growth factor-1 from a collagen-glycosaminoglycan scaffold. Cell Biochem Funct. 2015;33:277-84.
- [Google Scholar]
- Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436-49.
- [Google Scholar]
- Influence of decorin on the mechanical, compositional, and structural properties of the mouse patellar tendon. J Biomech Eng. 2012;134:031005.
- [Google Scholar]
- Serum cartilage oligomeric matrix protein (sCOMP) is elevated in patients with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthritis Cartilage. 2011;19:1396-404.
- [Google Scholar]






