Translate this page into:
Regulation of toxic contents of smokeless tobacco products
For correspondence: Dr Amit Kumar, Data Management Laboratory, ICMR-National Institute of Cancer Prevention & Research, Noida 201 301, Uttar Pradesh, India e-mail: amitbioinfo@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Effective regulation of contents of tobacco products is one of the primary milestones to reduce negative health effects associated with the use of smokeless tobacco (SLT) products. As per the available sources, testing of some SLT products has been done on ad hoc basis, but there is a lack of comprehensive and periodic analysis of these products. In addition, the available results indicate huge variations among the levels of pH, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, N-nitrosonornicotine, benzo[a]pyrene, heavy metals and nicotine within different products as well as within different brands of the same product. This review was aimed to throw light on the variations and gaps in testing of SLT products and emphasize the need for strong policy regulation for monitoring the chemical constituents of these products.
Keywords
Carcinogen
regulation
smokeless tobacco
tobacco-specific nitrosamine
toxic
Introduction
Smokeless tobacco (SLT), a non-combustible form of tobacco, is consumed by 350 million people in 133 countries across the globe1. As per a recent survey, SLT use accounts to approximately 0.65 million deaths annually1. Consumption of these products has been reported to be associated with many diseases such as cancers, neurological disorders and oral and heart diseases23. This can be attributed to the presence of harmful chemicals along with 28 known carcinogens45678910.
While there are regulations on the concentrations of chemicals in other commercially available products such as pesticides, medicines and food additives, no such policies are available for SLT products. Considering tobacco products, a lot of efforts have been employed for regulation of chemical contents of cigarette and cigarette smoke. One such effort includes mandating validation methods for testing of chemical constituents of cigarette11. International Organization for Standardization has been actively involved in the development of standards related to testing of cigarette smoke. Their technical committee has developed 64 international standards related to testing of tobacco and tobacco products, especially smoke11. However, majority of efforts are concentrated towards the regulation of cigarettes and cigarette smoke. In spite of many evidences on the hazardous effect of SLT products on humans, not much emphasis has been given on their regulation. Thus, there is a strong need for comprehensive identification and characterization of the toxic contents through analytical testing and extensive research about potential health hazards of these products. This type of analytical testing will help to establish a correlation between products, chemical constituents and their short-term and long-term toxicological effects on the organs and tissues.
Articles 9 and 10 of World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC) call for regulation of the contents and disclosures of tobacco products. According to Global Progress Report on SLT, 2016, from FCTC, the average implementation rate of Article 9 was around 50 per cent and that comprised mostly for smoking1213. Hence, the first step for regulation of SLT products has been taken up by the WHO FCTC focusing on implementation of Article 9 for regulation of SLT products14.
This review focuses on information on testing of SLT constituents, their regulation and challenges. It showcases the discrepancies and gaps in the regulation of toxic contents of SLT products highlighting country-wise and brand-wise differences in the previously tested samples. It also emphasizes the need for stringent policy regulations and their strong implementation for monitoring the chemical constituents of these products.
Carcinogens in smokeless tobacco products
SLT products contain a diversity of chemical compounds belonging to a variety of different classes such as organic tobacco-specific nitrosamines (TSNAs), polyaromatic hydrocarbons, inorganic metals and salts. TSNAs contain known potent carcinogens such as N-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N-nitrosoanabasine (NAB). Volatile N-nitrosamines contain N-nitrosodimethylamine, N-nitrosopyrrolidine, N-nitrosopiperidine, N-nitrosomorpholine and N-nitrosodiethanolamine. Table I includes a list of available chemical compounds identified from SLT products along with their classification as per International Agency for Research on Cancer (IARC) monographs15. TSNAs are formed from tobacco alkaloids in the presence of nitrates as explained below.
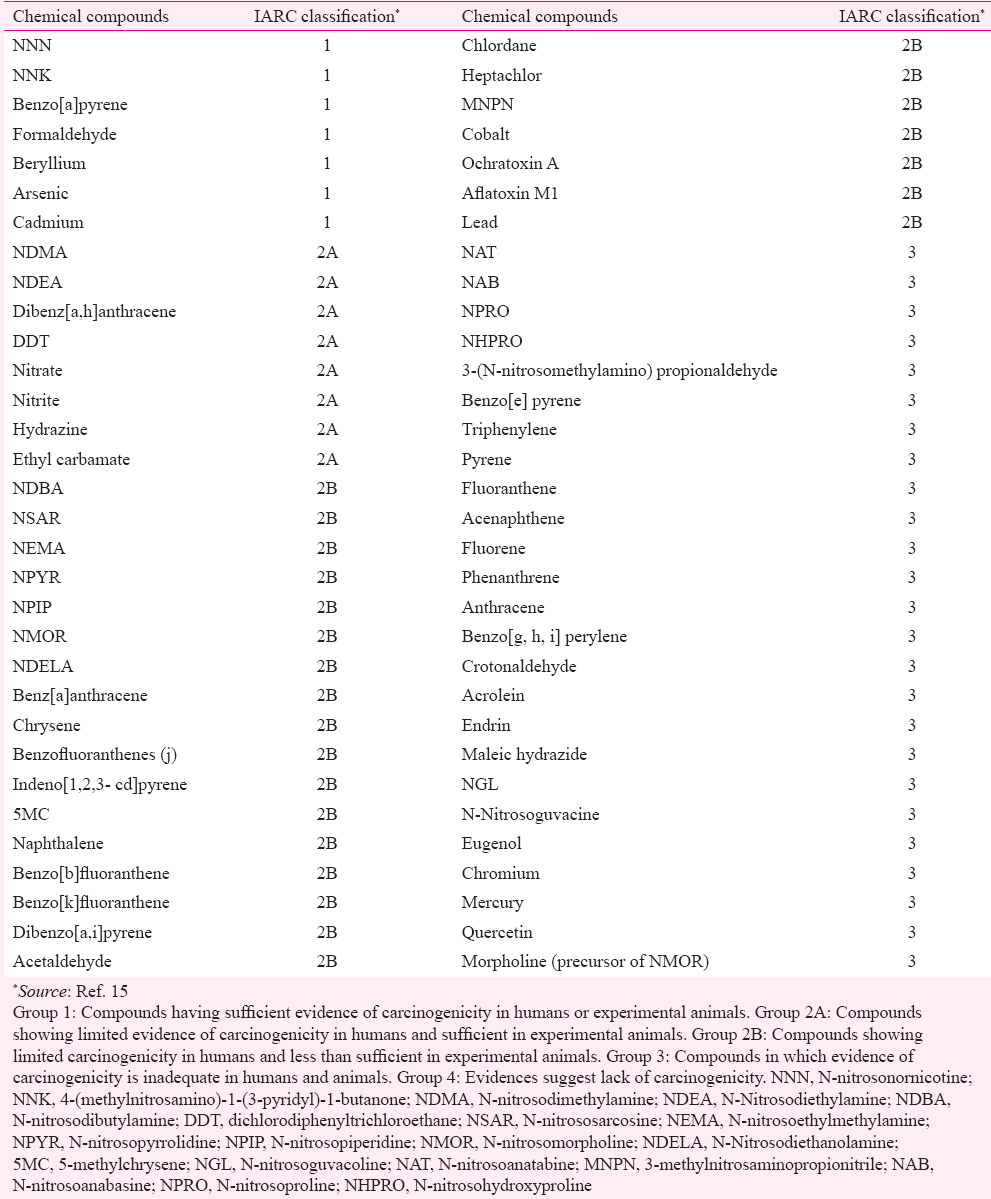
Role of nicotine and its conversion to tobacco-specific nitrosamines (TSNAs)
Tobacco plant contains four major alkaloids namely nicotine, nornicotine, anabasine and anatabine. Nicotine is the primary alkaloid and constitutes a major proportion (90-95%) of all alkaloid pools present in the commercially used tobacco plants16. It can undergo demethylation to form nornicotine, anabasine and anatabine. TSNAs (NNN, NNK, NAT and NAB) are formed by nitrosation of these alkaloids during curing and processing of tobacco products. NNN, NNK, NAT and NAB are known carcinogens and are found to be associated with oral, oesophageal and pancreatic cancers1718.
Variation in tobacco-specific nitrosamines (TSNAs) and benzo[a]pyrene (B[a]P)
Compiling all the published articles in Table II, there is a wide variation in the levels of TSNAs across different countries and across different brands within a country. There is a 60-fold variation (0.66-42.5 μg/g) in NNN content of moist snuff in USA, while for dry snuff, the variation is 100 folds (0.8-81.3 μg/g). Wide variations in the amount of other TSNAs are also observed. NNK content of most of the products in USA varies from 0.05 μg/g in dissolvables to 20.3 μg/g in dry snuff. In SLT products manufactured in India, NNN varies from 0.09 μg/g in gutka to 40 μg/g in khaini, while NNK varies from 0.04 μg/g in gutka to 24.1 μg/g in zarda.
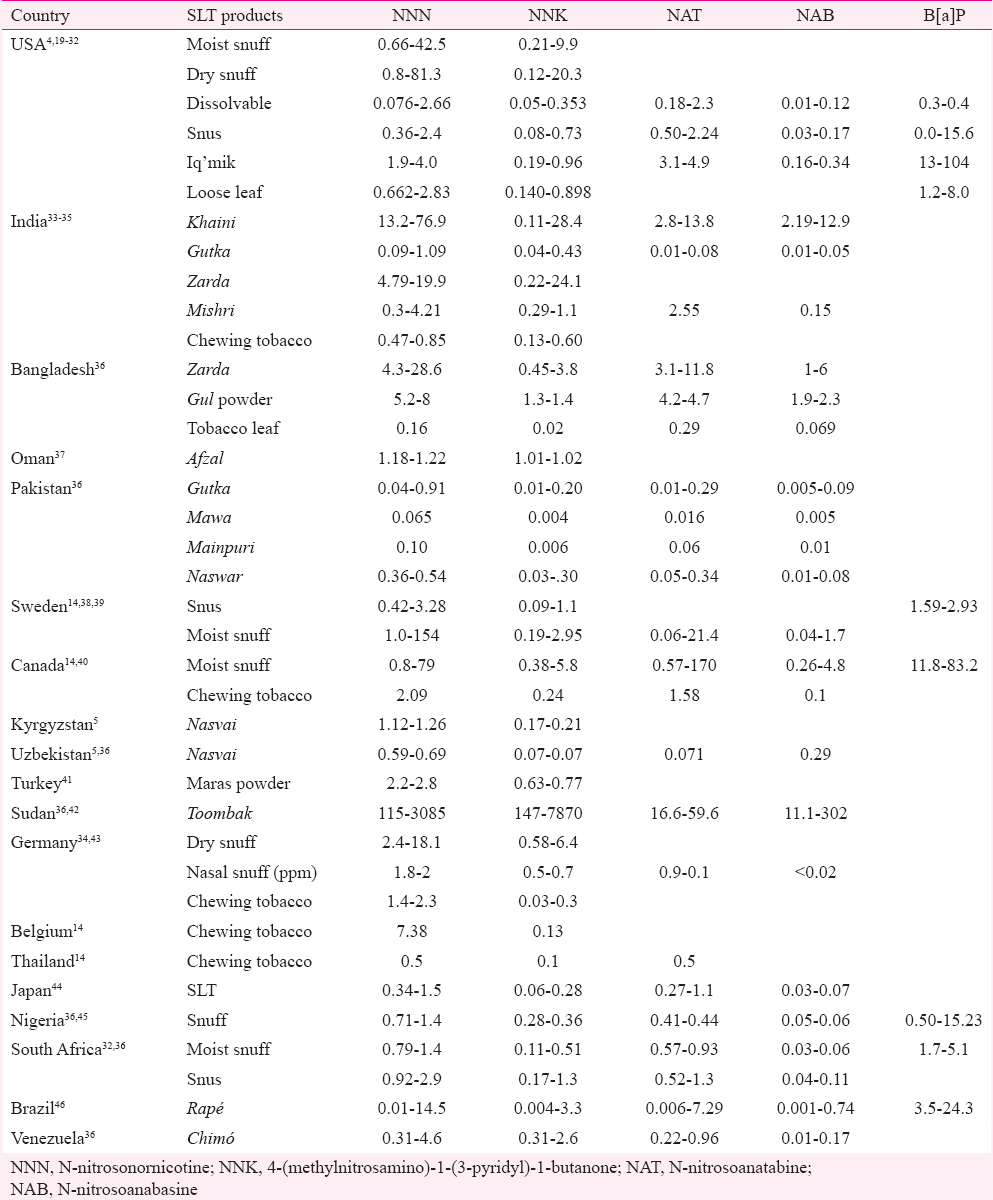
Globally, variations in NNN range from 0.01 μg/g (rapé from Brazil)) to 3085 μg/g (toombak from Sudan)47, NNK varies from 0.004 μg/g (rapé from Brazil and mawa from Pakistan) to 7870 μg/g (toombak from Sudan)47, NAT from 0.006 μg/g to 170 μg/g (moist snuff in Canada) and NAB from 0.001 μg/g (rapé in Brazil) to 4.8 μg/g (moist snuff in Canada). Variation of another potent Group 1 carcinogen, B[a]P ranges from 0 ng/g (snus in USA) to 104 ng/g in Iq’mik in USA (Table III). Such wide variations in the concentration of toxicants are influenced by various factors such as tobacco plant, tobacco type, nitrate and alkaloid content, method of cultivation, pesticides used, harvesting and processing techniques and storage conditions. As per a report, extremely high levels of TSNAs in Sudanese toombak have been attributed to high levels of tobacco alkaloids in Nicotiana rustica7.
Variation of heavy metals in smokeless tobacco products
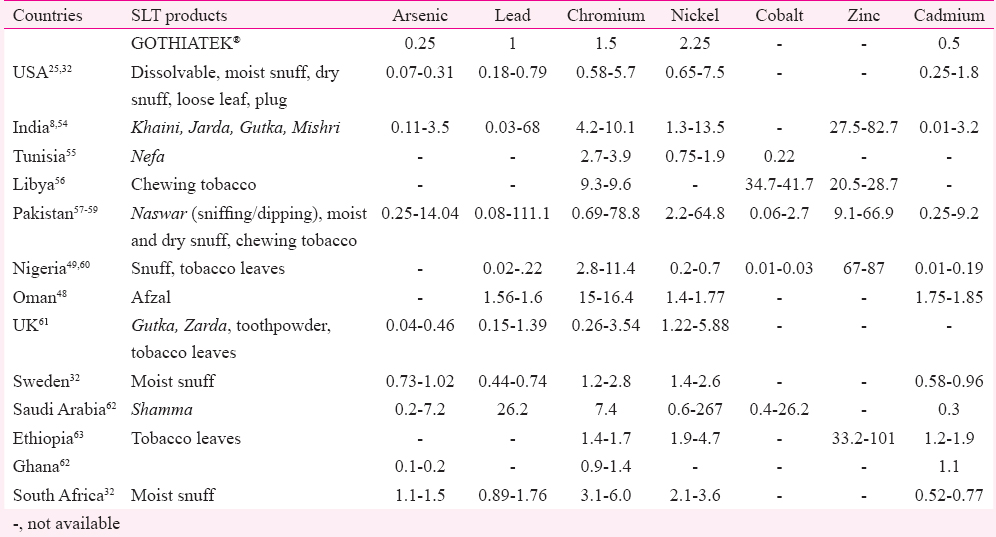
A variety of toxic metals such as arsenic, cadmium, chromium, lead and nickel have also been identified. These are either absorbed by the tobacco plant from soil or enter during the curing and processing of tobacco plants. Among these, arsenic and cadmium are classified as Group 1 carcinogens. Also, nickel and lead have been classified as Groups 2B carcinogens and chromium as Group 3 carcinogen, respectively48. It has been previously studied that toxicity of heavy metals has a direct correlation with the burden of metals in our body. Therefore, change in their concentration by consumption of SLT leads to severe toxicity49. Some other diseases include diseases of bone and kidney (excess of cadmium), neurological disorders (excess of lead)5051 and metabolic disorders (excess of zinc and copper)5253. Table III indicates the amounts of metals in different SLT products tested worldwide.
Only a few countries listed in Table III have tested their SLT products for estimation of metal contents. It is also important to note that all these reports are published one time by individual research groups following no standard operational procedures. Since there is no government agency involved, periodic testing and monitoring does not take place. For instance, Houas et al55 compared the mineral concentrations of SLT, water pipe tobacco and cigarettes from the Tunisian Market, while Brima62 estimated the concentration of metal level in Shamma, found in Saudi Arabia. Table IV indicates different standards for testing of SLT products.
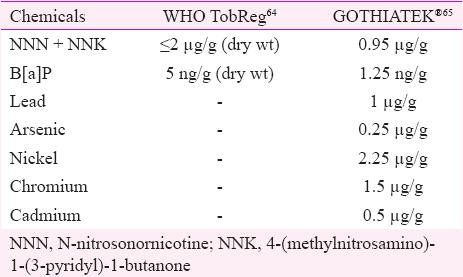
According to Tables III and IV, concentration of arsenic, lead and chromium in some of the SLT products from Pakistan (Naswar) was found to be 50 times more than GOTHIATEK® limits57. Nickel concentration in Shamma, a SLT product found in Saudi Arabia, was 118 times greater than GOTHIATEK® limits62. In India, lead content in one of the brands of gutka was found to be 68 times greater than the defined limit54. In general, there was a large variation in concentration in most of the metals, which was much more than the acceptable limits.
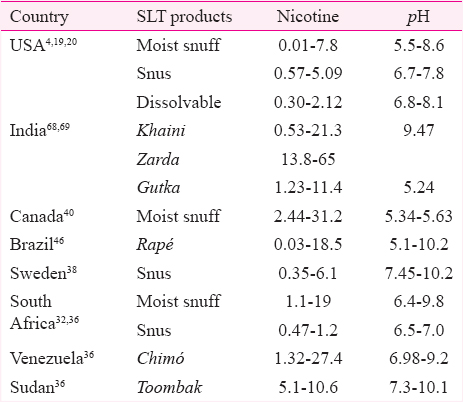
Variation in pH and its effect on nicotine absorption
Nicotine content of a SLT product is the primary determinant of cause of addiction among users. The unprotonated form of nicotine or ‘free nicotine’ is easily absorbed by the oral mucosa66. Absorption of nicotine at the buccal surface is governed by the pH of SLT product67. Higher pH facilitates more absorption and vice versa. At low pH, nicotine gets ionized and is thus unable to cross biological membranes67.
Table V depicts wide variations in the pH levels of various SLT products. As an example, the level of pH ranges from as low as is from 5.1 mg/g in Brazilian rapè to 10.2 mg/g in Swedish snus and Brazilian rapé. Variation in free nicotine content is from 0.01 mg/g in the USA moist snuff to 65 mg/g in Indian zarda.
Regulation of tobacco products
Given the huge variation in the toxic contents of currently available SLT products, it becomes extremely imperative to consider the acceptable levels of harm. According to Gray and Borland70, there are three major regulatory possibilities for tobacco: (i) regulation of carcinogens and toxins such as TNSAs, B[a]P and metals; (ii) regulation of nicotine for addictiveness, and (iii) regulation of additives. Considering the wide variations in chemical content and lack of monitoring agencies, there is an immediate need to develop validated methods for estimating the toxicity/carcinogenicity of SLT products70.
Two of the widely used standards are TobReg64 and GOTHIATEK®65. Tobacco Product Regulation (TobReg) group of WHO has set the maximum acceptable limit of NNN+NNK to ≤2 μg/g dry weight of tobacco and B[a]P to 5 ng/g dry weight of tobacco (Table IV). Swedish Match has published standards for maximum allowable levels of TSNAs, metals and trace elements, which are collectively known as GOTHIATEK® standard65 (Table IV). Rickert et al40 have stated in their report that some but not all experts on this topic have suggested that this standard is safe enough to be recommended by health authorities. GOTHIATEK® standard has been adopted by two big tobacco companies: British-American Tobacco and European Smokeless Tobacco Council (ESTOC)71.
Role of WHO FCTC
TobReg
The WHO established a tobacco-free initiative (TFI) in July 1998 to provide international attention to global tobacco epidemic. Its mandate is to reduce the global burden of disease and death caused by tobacco and thus working on a mission to protect the present and future generations from the consequences of tobacco consumption and exposure to tobacco smoke. TFI encompasses a Scientific Advisory Committee on Tobacco Product Regulation (SACTob)72 whose aim is to provide scientific information and recommendation on tobacco product regulation related to Articles 9, 10 and 11 of the WHO FCTC. Most of the efforts regarding tobacco regulation have been done for cigarettes. TobReg has defined three models for cigarettes, each mentioning mandatory limits for emissions of nine different smoke toxicants73.
Tobacco Laboratory Network (TobLabNet)
As a step towards regulation, the WHO has established Tobacco Laboratory Network (TobLabNet) with the aim to regulate and provide testing and research of contents and emissions of tobacco products. Its major goal is to establish testing and research capacity of tobacco products for regulatory compliance. The report by WHO FCTC at the Conference of Parties 7 at New Delhi in November 2016 states that the already available WHO TobLabNet methods for analysis of TSNAs, B[a]P and nicotine can be adapted or applied to other SLT74. Furthermore, owing to the wide range of SLT products, there is a need to perform product-specific analysis in South Asia which is not presently performed by the TobLabNet due to lack of relevant laboratory expertise and/or capacity. The analytic procedures for metals, humectants, aldehydes and many other toxicants present in SLT need to be standardized. It was also recommended that the Parties should consider asking SLT manufacturers to provide levels of pH and toxicants (TSNAs, B[a]P and nicotine) using WHO-recommended methods/ Standard Operating Procedures (SOPs), as recommended for cigarettes, from approved laboratories.
WHO Collaborating Centre on Tobacco Control
The WHO Collaborating Centre is a part of TFI whose aim is to form part of an international collaborative network carrying out activities on tobacco control and strengthen institutional capacity in countries and Regions. There are 16 WHO collaborating centres for tobacco control which work closely with TFI. Among these, six collaborating centres are working on tobacco testing and research (Table VI). However, the laboratories of these centres focus on technical training on testing compounds and emissions of smoking products, especially cigarettes.

Effort at country level
India
India is the largest consumer of SLT products by number. Prevalence of SLT use among men and women is 29.6 and 12.8 per cent75, respectively. Although the burden is highest in India, not much effort except a few studies76 towards testing of harmful contents of SLTs has been made. The Government of India in 2003 has established a law regarding tobacco known as Cigarettes and Other Tobacco Products Act which includes prohibition of advertisement and regulation of trade and commerce, production, supply and distribution. This also includes testing of nicotine and tar for all tobacco products77. To implement this law, the governments piloted National Tobacco Control Programme in 2007-2008; one of its components was to establish tobacco product testing laboratories for building regulatory capacity77. The Ministry of Health and Family Welfare, Government of India, has established National Tobacco Testing Laboratory at National Institute of Cancer Prevention and Research, Noida, Central Drug Testing Laboratory, Mumbai, and Regional Drug Testing Laboratory, Guwahati, with the sole purpose of providing scientific and analytical information to the Government of India and other regional countries and organizations such as the WHO [File No. T-20018/25/2016-NCD/FTS: 3059618/16, Ministry of Health and Family Welfare, National Tobacco Control Programme (Tobacco Control/NTTLs) dated 29th August 2017].while in some Asian countries like Thailand and South Korea, the responsibility of testing tobacco products has been given to the industry78.
USA
Most of the testing procedures of SLT products around the world have been done in various laboratories of the USA (although the USA is not the signatories to the WHO FCTC). The Food and Drug Administration has proposed a rule that mean level of NNN in any batch of finished SLT products should not exceed 1.0 μg/g of tobacco on a dry weight basis79.
Europe
GOTHIATEK® standards have been accepted by ESTOC (a pan European SLT lobby) members and have become a voluntary standard for most of the SLT products manufactured in Europe8081. The United Kingdom has enforced regulation regarding tobacco products, especially cigarettes, which states that a person cannot produce, supply or manufacture for export of any cigarettes with emission level greater than 10 mg of tar/cigarette, 1 mg nicotine/cigarette and 10 mg of carbon monoxide/cigarette82.
Gaps
The available data indicate that very few laboratories, which are not funded by industry, are working on the chemical composition of tobacco. Most of these efforts are primarily on cigarettes with only meagre focus on SLT. There is no centralized facility in almost all countries to perform these tests and produce results with certain regulatory standards. No global standards are provided for testing and measuring most of the compounds of SLT products. There is no regulation for additives and other flavouring agents in SLT products. Moreover, only partial guidelines have been proposed by the WHO FCTC for Articles 9 and 10.
Conclusion
Although the toxicological and clinical risks associated with many of the SLT products are known, little effort has been taken to regulate their constituents. Considering the hazardous impact of SLT products on human health and wide prevalence among different parts of the world, there is an urgent need to pay more attention towards research on SLT products, their ingredients and emissions. More emphasis should be given on the establishment of tobacco testing laboratories in every region, which will be precisely the driving force behind the successful implementation of Articles 9 and 10 of WHO FCTC. In addition, major initiatives are required that promote collaborations between academia, researchers, scientists and governments to ensure that reports from the laboratory are quickly interpreted and efficiently translated for implementation. It will provide better avenues for researchers to find out newer ways of reducing hazardous compounds from tobacco products. This will in turn help governments to fund better research and help eradicate the problems associated with SLT. Another important step is to develop SOPs for testing of each toxic chemical compound of SLT products. These steps will help in the establishment of permissible upper limits of all chemical ingredients of SLT. And finally, the regulation will also reduce the burden on medical system which is catering to the affected individuals and will also help in strengthening economy worldwide.
Financial support & sponsorship: This work was supported by two projects: Second Phase of Task Force Biomedical Informatics Centers of Indian Council of Medical Research (Project No. BIC/12(06)/2013) and WHO FCTC Global Knowledge Hub on Smokeless Tobacco (Reference No. 2016/643768-0).
Conflicts of Interest: None.
References
- Global burden of all-cause and cause-specific mortality due to smokeless tobacco use: Systematic review and meta-analysis. Tob Control. 2018;27:35-42.
- [Google Scholar]
- Smokeless tobacco-associated cancers: A systematic review and meta-analysis of Indian studies. Int J Cancer. 2016;138:1368-79.
- [Google Scholar]
- Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS One. 2014;9:e113385.
- [Google Scholar]
- Evidence supporting product standards for carcinogens in smokeless tobacco products. Cancer Prev Res (Phila). 2015;8:20-6.
- [Google Scholar]
- Variations of toxic and carcinogenic constituents in Nasvai: Call for systematic research and regulation. Tob Control. 2017;26:355-6.
- [Google Scholar]
- Constituent variations in smokeless tobacco purchased in Mumbai, India. Tob Regul Sci. 2017;3:305-14.
- [Google Scholar]
- Call to establish constituent standards for smokeless tobacco products. Tob Regul Sci. 2016;2:9-30.
- [Google Scholar]
- Trace metal concentration in different Indian tobacco products and related health implications. Food Chem Toxicol. 2010;48:2291-7.
- [Google Scholar]
- The ban on smokeless tobacco products is systematically violated in Chennai, India. Indian J Cancer. 2016;53:325-30.
- [Google Scholar]
- Standardized methods for the regulation of cigarette-smoke constituents. Trends Anal Chem. 2015;66:118-27.
- [Google Scholar]
- 2014. Global Progress Report on Implementation of the WHO Framework Convention on Tobacco Control. Geneva: World Health Organization; Available from: http://www.who.int/fctc/reporting/2014globalprogressreport.pdf?ua=1
- 2016. Global Progress Report on Implementation of the WHO Framework Convention on Tobacco Control. Geneva: World Health Organization; Available from: http://www.who.int/fctc/reporting/2016_global_progress_report.pdf?ua=1
- 2009. Report on the scientific basis of tobacco product regulation: Third report of a WHO study group 2009. Geneva: World Health Organization; Available from: http://apps.who.int/iris/bitstream/handle/10665/44213/9789241209557_eng.pdf;jsessionid=89F04EDF81FEDB289DDC735B12F376B1?sequence=1
- WHO International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Available from: https://monographs.iarc.fr/wpcontent/uploads/2018/07/List_of_Classifications.pdf
- Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. Proc Natl Acad Sci U S A. 2005;102:14919-24.
- [Google Scholar]
- Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers (Basel). 2014;6:1138-56.
- [Google Scholar]
- WHO International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In: Smokeless tobacco and some tobacco-specific n-nitrosamines. Vol 89. Lyon, France: IARC; 2007.
- [Google Scholar]
- Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: Findings from round 1 of the new product watch. Nicotine Tob Res. 2012;14:274-81.
- [Google Scholar]
- Surveillance of moist snuff: Total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine Tob Res. 2008;10:1645-52.
- [Google Scholar]
- Tobacco-specific N-nitrosamines and areca-derived N-nitrosamines: Chemistry, biochemistry, carcinogenicity, and relevance to humans. J Toxicol Environ Health. 1994;41:1-52.
- [Google Scholar]
- Chemical analysis of Alaskan Iq’mik smokeless tobacco. Nicotine Tob Res. 2013;15:1283-8.
- [Google Scholar]
- Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine Tob Res. 2003;5:885-9.
- [Google Scholar]
- Identification and analysis of a nicotine-derived N-nitrosamino acid and other nitrosamino acids in tobacco. Carcinogenesis. 1989;10:1725-31.
- [Google Scholar]
- The chemical composition of smokeless tobacco: A survey of products sold in the United States in 2006 and 2007. Regul Toxicol Pharmacol. 2012;64:367-87.
- [Google Scholar]
- Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res. 2006;8:309-13.
- [Google Scholar]
- Levels of (S)-N’-nitrosonornicotine in U.S. tobacco products. Nicotine Tob Res. 2013;15:1305-10.
- [Google Scholar]
- Monitoring tobacco-specific N-nitrosamines and nicotine in novel smokeless tobacco products: Findings from round II of the new product watch. Nicotine Tob Res. 2014;16:1070-8.
- [Google Scholar]
- A survey of N’-nitrosonornicotine (NNN) and total water content in select smokeless tobacco products purchased in the United States in 2015. J Agric Food Chem. 2016;64:4400-6.
- [Google Scholar]
- Chemical characterization of domestic oral tobacco products: Total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food Chem Toxicol. 2013;57:380-6.
- [Google Scholar]
- Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chem Res Toxicol. 2010;23:66-73.
- [Google Scholar]
- Chemical and toxicological characteristics of conventional and low-TSNA moist snuff tobacco products. Toxicol Lett. 2016;245:68-77.
- [Google Scholar]
- Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. Int J Cancer. 2005;116:16-9.
- [Google Scholar]
- Smokeless tobacco: Chemical composition and carcinogenicity. Int Res J Interdiscip Multidiscip Stud. 2016;7969:7-16.
- [Google Scholar]
- High levels of tobacco-specific N-nitrosamines and nicotine in Chaini Khaini, a product marketed as snus. Tob Control. 2015;24:e271-4.
- [Google Scholar]
- Global surveillance of oral tobacco products: Total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob Control. 2011;20:e2.
- [Google Scholar]
- Analysis of tobacco-specific nitrosamines in the common smokeless tobacco Afzal in Oman. Sultan Qaboos Univ Med J. 2016;16:e20-6.
- [Google Scholar]
- Review of the Scientific Literature on Snus (Swedish Moist Snuff). Project Number:24-18132C. 2013. Virginia: ENVIRON International Corporation Arlington, Virginia; Available from: https://www.accessdata.fda.gov/Static/widgets/tobacco/MRTP/18%20appendix-6a-environ-snus-monograph-2013.pdf
- [Google Scholar]
- Polycyclic aromatic hydrocarbons in US and Swedish smokeless tobacco products. Chem Cent J. 2013;7:151.
- [Google Scholar]
- Chemical and toxicological characterization of commercial smokeless tobacco products available on the Canadian market. Regul Toxicol Pharmacol. 2009;53:121-33.
- [Google Scholar]
- Tobacco specific nitrosamine levels of Maras powder (Turkısh Smokeless Tobacco) Indian J Med Res Pharm Sci. 2015;2:11-6.
- [Google Scholar]
- The Swedish Snus and the Sudanese Toombak: Are they different? Oral Oncol. 1998;34:558-66.
- [Google Scholar]
- Measurement of nicotine, tobacco-specific nitrosamines, and additives in the filler and gas from Japanese brand snuff. Nihon Eiseigaku Zasshi. 2016;71:76-83.
- [Google Scholar]
- Human health hazards of poly aromatic hydrocarbons in Nigerian smokeless tobacco. Toxicol Rep. 2015;2:1019-23.
- [Google Scholar]
- Comprehensive chemical characterization of Rapé tobacco products: Nicotine, un-ionized nicotine, tobacco-specific N’-nitrosamines, polycyclic aromatic hydrocarbons, and flavor constituents. Food Chem Toxicol. 2015;82:50-8.
- [Google Scholar]
- Unusually high levels of carcinogenic tobacco-specific nitrosamines in Sudan snuff (Toombak) Carcinogenesis. 1991;12:1115-8.
- [Google Scholar]
- Determination of heavy metals in the common smokeless tobacco Afzal in Oman. Sultan Qaboos Univ Med J. 2014;14:e349-55.
- [Google Scholar]
- Cadmium determination in cigarettes available in Nigeria. Afr J Biotechnol. 2005;4:1128-32.
- [Google Scholar]
- Determination of cadmium, zinc, nickel and cobalt in tobacco by reversed-phase high-performance liquid chromatography with 2-(8-quinolylazo)-4,5-diphenylimidazole as a chelating reagent. Anal Sci. 2005;21:1105-10.
- [Google Scholar]
- The level of nickel in smoker's blood and urine. Cent Eur J Public Health. 2004;12:187-9.
- [Google Scholar]
- Determination of toxic metals in Indian smokeless tobacco products. Scientific World Journal. 2009;9:1140-7.
- [Google Scholar]
- Comparison of mineral contents in three different tobacco formulations. Biomed Environ Sci. 2017;30:52-8.
- [Google Scholar]
- Determination of trace element concentrations of Libyan chewing and cigarette tobacco by instrumental neutron activation analysis. J Radioanal Nucl Chem Lett. 1989;135:273-9.
- [Google Scholar]
- Assessment of potential toxicity of a smokeless tobacco product (naswar) available on the Pakistani market. Tob Control. 2012;21:396-401.
- [Google Scholar]
- Analysis of cadmium, nickel, and lead in commercial moist and dry snuff used in Pakistan. Environ Monit Assess. 2013;185:5199-208.
- [Google Scholar]
- Quantitative analysis of some important metals and metalloids in tobacco products by inductively coupled plasma-mass spectrometry (ICP-MS) Chem Cent J. 2012;6:56.
- [Google Scholar]
- Copper, Iron and Zinc concentrations of tobacco leaves and ready-to-use snuff products on sale in Imo state Southeastern Nigeria. J Appl Sci Environ Manag. 2015;19:459-67.
- [Google Scholar]
- Determination of metal levels in Shamma (Smokeless tobacco) with inductively coupled plasma mass spectrometry (ICP-MS) in Najran, Saudi Arabia. Asian Pac J Cancer Prev. 2016;17:4761-7.
- [Google Scholar]
- Levels of heavy metals in the raw and processed Ethiopian tobacco leaves. Springerplus. 2016;5:232.
- [Google Scholar]
- WHO Tobacco Free Initiative. The scientific basis of tobacco product regulation: Report of a WHO study group. World Health Organ Tech Rep Ser No. 945 2007:1-112.
- Swedish Match Quality Standard. Available from: https://www.swedishmatch.com/Snus-and-health/GOTHIATEK/
- Characterisation of nicotine and cancer-enhancing anions in the common smokeless tobacco Afzal in oman. Sultan Qaboos Univ Med J. 2015;15:e469-76.
- [Google Scholar]
- Nicotine absorption from smokeless tobacco modified to adjust pH. J Addict Res Ther. 2014;5:1000184.
- [Google Scholar]
- Smokeless tobacco and public health in India. Ministry of Health and Family Welfare, Government of India. 2016. Available from: http://www.searo.who.int/india/tobacco/smokeless_tobacco_and_public_health_in_india.pdf?ua=1
- [Google Scholar]
- Comparison of nicotine concentration and pH of commercially available smokeless tobacco products. J Oral Res Rev. 2017;9:21.
- [Google Scholar]
- Research required for the effective implementation of the framework convention on tobacco control, articles 9 and 10. Nicotine Tob Res. 2013;15:777-88.
- [Google Scholar]
- Should the health community promote smokeless tobacco (snus): Comments from British American tobacco. PLoS Med. 2007;4:1703-4.
- [Google Scholar]
- WHO Scientific Advisory Committee on Tobacco Product Regulation WTFI. SACTob recommendation on tobacco product ingredients and emissions/scientific advisory committee on tobacco product regulation (SACTob) 2003. Geneva: World Health Organization; Available from: http://www.who.int/iris/handle/10665/42654
- [Google Scholar]
- Impact assessment of WHO TobReg proposals for mandated lowering of selected mainstream cigarette smoke toxicants. Regul Toxicol Pharmacol. 2017;86:332-48.
- [Google Scholar]
- Conference of the Parties to the WHO Framework Convention on Tobacco Control. Further Development of the Partial Guidelines for Implementation of Articles 9 and 10 of the WHO FCTC. 2016. FCTC/COP/7/9. Available from: http://www.who.int/fctc/cop/cop7/FCTC_COP_7_9_EN.pdf?ua=1
- [Google Scholar]
- 2010. Global Adult Tobacco Survey-2, Factsheet. New Delhi: Ministry of Health and Family Welfare, Government of India; Available from: http://www.searo.who.int/india/mediacentre/events/2017/gats2_india.pdf?ua=1
- An overview of the tobacco problem in India. Indian J Med Paediatr Oncol. 2012;33:139-45.
- [Google Scholar]
- Tobacco control policies in India: Implementation and challenges. Indian J Public Health. 2011;55:220-7.
- [Google Scholar]
- Regulating smokeless tobacco and processed areca nut in South-East Asia region: The journey so far and the road ahead. Indian J Public Health. 2017;61:S3-6.
- [Google Scholar]
- Tobacco product standard for n- nitrosonornicotine level in finished smokeless tobacco products. Fed Regist. 2017;82:8004-53.
- [Google Scholar]
- Transnational tobacco company interests in smokeless tobacco in Europe: Analysis of internal industry documents and contemporary industry materials. PLoS Med. 2013;10:e1001506.
- [Google Scholar]
- 2016. Tobacco and Related Products Regulations 2016. :2016. No. 507 Available from: http://www.legislation.gov.uk/uksi/2016/507/pdfs/uksi_20160507_en.pdf






