Translate this page into:
Red cell antigen phenotypes in blood donors & thalassaemia patients for creation of red cell antigen-matched inventory
For correspondence: Dr Swati Kulkarni, Department of Transfusion Medicine, National Institute of Immunohaematology, 13th Floor, New Multi-Storeyed Building, KEM Hospital Campus, Parel, Mumbai 400 012, Maharashtra, India e-mail: swatiskulkarni@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Patients with thalasssaemia are at a risk of alloimmunization and the presence of RBC alloantibodies further complicates transfusion therapy. Matching for the critical antigens of Rh, Kell, Kidd and Duffy blood group systems has been shown to minimize alloimmunization. The aim of the present study was to create a database of extensively typed donors for clinically significant and common blood group antigens of Rh, Kidd, Kell and Duffy systems for transfusion therapy of multitransfused thalassaemic patients.
Methods:
Five hundred O group regular blood donors were phenotyped for Rh, Kell, Duffy and Kidd blood group antigens using haemagglutination technique. Eighty four non-alloimmunized and 15 alloimmunized thalassaemia major patients with known antigenic profiles (determined by polymerase chain reaction with sequence-specific primers) were selected for this study.
Results:
By analyzing antigen profiles of 500 O group regular donors, a database of 193 donors matching perfectly for Rh, Duffy, Kell and Kidd antigens was prepared for 15 alloimmunized patients. For non-alloimmunized 84 thalassaemic patients, a database of 405 donors was created.
Interpretation & conclusions:
A database of 500 regular blood donors phenotyped for common antigens of Rh, Duffy, Kell and Kidd blood group systems was created, which would be useful in providing extended antigen-matched RBCs for thalassaemia patients. This will improve the quality and effectiveness of transfusion therapy.
Keywords
Alloimmunization
antigen-matched blood
blood groups
blood phenotypes database
inventory
multitransfused thalassaemics
Blood transfusion is a common practice in the treatment of patients with anaemia, thalassaemia, sickle cell anaemia and other haematological disorders and malignancies. Many such patients require repeated blood transfusions during their illness. In majority of the blood banks, only ABO and RhD blood group status of blood donors and recipients are matched when red blood cells (RBCs) are transfused. Although antibody screening and identification is performed in many blood banks, RBCs are not routinely tested for other minor blood group antigens unless the recipient has previously undergone immunization. The presence of RBC alloantibodies creates the potential for serologic incompatibility, makes the selection of appropriate units for future transfusion difficult, delays the use of a potential lifesaving therapy and presents risk of haemolytic transfusion reaction (HTR) and delayed HTR.
The rate of alloimmunization or the production of antibodies that may potentially destroy foreign or donor RBCs among multitransfused individuals is significantly higher (8-76%) in patients receiving multiple transfusions such as sickle cell disease and thalassaemia and it increases with repeated transfusions1. Hence, it is advocated that transfusions given to patients who are likely to become transfusion dependent over a period of time should also be matched for antigens other than ABO and RhD. Use of phenotyped matched RBC units for transfusion offers an advantage especially to patients with alloantibodies and prevents further alloimmunization to other antigens. It also helps reduce the incidence of alloimmunization in non-alloimmunized multitransfused patients2.
Available reports on antibody screening and identification in multitransfused patients and multiparous women have shown that most antibodies are directed against Rh, Duffy, Kell and Kidd blood group antigens and cause HTRs or haemolytic disease of the foetus and newborn (HDFN)345. The frequency and specificity of irregular red cell antibodies vary among different ethnic groups according to the genetic diversity of the population. Provision of antigen-matched blood is known to reduce alloimmunization among multitransfused patients6. Also, there was improved RBC survival and diminished frequency of transfusions7.
As multitransfused thalassaemic patients and blood donors are not phenotyped for minor blood group antigens in a pretransfusion setting, it is difficult to obtain compatible units due to lack of pre-typed donors. Hence, in the present study, 500 regular blood donors were typed for the common and clinically important blood group antigens of Rh, Duffy, Kell and Kidd blood group systems to determine their frequency and common phenotypes in the population and to create a database of donors to be used for provision of antigen-matched blood units to multitransfused thalassaemics.
Material & Methods
This study was carried out at the department of Transfusion Medicine, ICMR-National Institute of Immunohaematology, Mumbai, India (2013 to 2016). A total of 500 consecutive O group regular blood donors visiting KEM Hospital, Mumbai, and various blood donation camps, were enrolled for this study. The study was approved by the Institutional Ethics Committee and written informed consent was obtained from all the participants. Donors were typed for common blood group antigens of Rh (C, c, D, E, and e), Duffy (Fya and Fyb), Kell (K and k) and Kidd (Jka and Jkb) using commercially available antiserum using the manufacturer's instructions (IMMUCOR, Inc., USA) by conventional tube technique. The antigen profiles were analyzed for providing antigen-matched blood to 84 non-alloimmunized and 15 alloimmunized, multitransfused thalassaemia major patients from our previous study8. Antigen profiles of these patients were determined by PCR using sequence-specific primer (PCR-SSP) and haemagglutination. For PCR, DNA was prepared from ethylenediaminetetraacetic acid (EDTA) blood using commercially available DNA extraction kit (Qiagen, Germany). The common alleles of Rh, Duffy, Kell, Kidd and MNS antigens were genotyped using PCR-SSP along with known controls in Veriti® 96-well Thermal Cycler (Applied Biosystems, USA) as described earlier8. The amplified products were separated electrophoretically on two per cent agarose gel containing ethidium bromide and visualized under ultraviolet transilluminator, Gel Doc system (Bio-Rad, USA). For selecting antigen-matched donors, antigen profile of the patient determined by PCR-SSP was considered. Frequencies of blood group antigens in blood donors were calculated as percentage.
Results
Five hundred O group regular blood donors were phenotyped for common antigens of Rh, Duffy, Kell and Kidd blood group systems. Of these, 94 per cent (n=470) were RhD positive. The frequency of Rh antigens (C, c, D, E and e) in the donor population is shown in the Figure. The frequency of e antigen was the highest (99.4 %), followed by D and C antigens. The frequency of the antigens was in the order: e > D > C > c > E. Eight different most probable Rh phenotypes were identified, with the most common being R1R1 (CDe/CDe) having a frequency of 44 per cent and the most rare being r'r (Cde/cde) with a frequency of 0.4 per cent. Seventy five per cent (n=375) of donors belonged to the R1R1 and R1r phenotype. Rare Rh phenotypes R2R2 and r'r had frequency of <1 per cent. Seventy eight donors had Rh blood group phenotypes occurring with <10 per cent frequency. In the donor population, the apparent homozygosity for C, c, E and e antigens was found to be 44.0, 15.2, 0.6 and 82.4 per cent, respectively, and the heterozygosity for C/c and E/e was found to be 40.8 and 17.0 per cent, respectively.
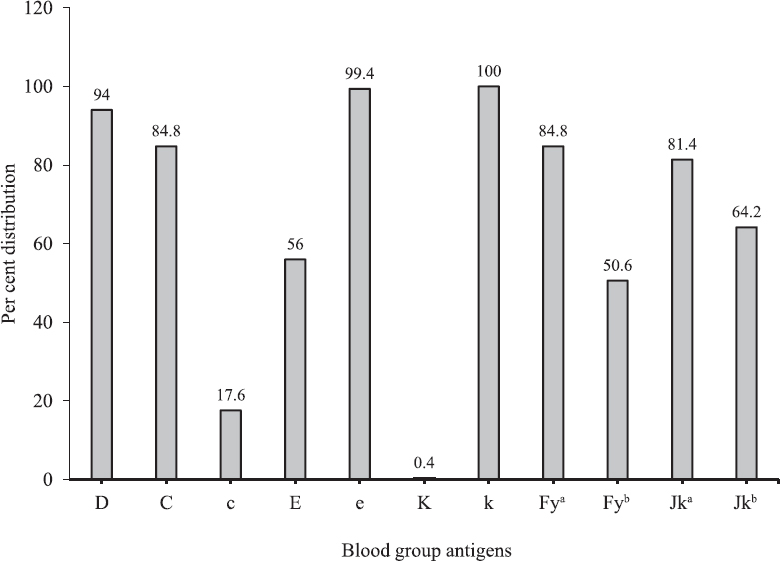
- Per cent distribution of Rh antigens (D, C, E, c and e), Duffy (Fya and Fyb), Kell (K and k) and Kidd (Jka and Jkb) antigens in O group regular blood donors enrolled in the present study.
Extended antigen matching between thalassaemic patients and blood donors: By analyzing antigen profiles of 500 O group regular donors, a database of 193 donors matching perfectly for Rh, Duffy, Kell and Kidd antigens was prepared for 15 alloimmunized thalassaemia major patients (Table I). Based on partial matching of donors and non-alloimmunized thalassaemic patients (n=84) for Rh antigens (C, c, D, E and e), 206 R1R1 donors were identified for 47 patients with thalassaemia, 131 R1r donors for 16 patients, 43 R1R2 donors for 13 patients, 10 R0r donors for four patients, three R2r donors for one patient and 12 rr donors for three patients. Approximately three donors matching for R1R1,R1R2, R2r and rr phenotypes were identified per patient. On an average, eight donors matching for R1r and only two donors for R0r phenotype were identified per patient.
| Patient number | Antigen profile (genotype) | Number of antigen-matched donors available | |||
|---|---|---|---|---|---|
| Rh | Duffy | Kidd | Kell | ||
| 1 | R1R1 | Fy(a+b+) | Jk(a+b+) | K−k+ | 17 |
| 2 | R1R1 | Fy(a+b−) | Jk(a+b+) | K−k+ | 24 |
| 3 | R1R1 | Fy(a+b−) | Jk(a−b+) | K−k+ | 8 |
| 4 | R1R1 | Fy(a+b+) | Jk(a+b−) | K−k+ | 8 |
| 5 | R1R1 | Fy(a−b+) | Jk(a+b−) | K−k+ | 2 |
| 6 | R1R1 | Fy(a+b−) | Jk(a−b+) | K−k+ | 8 |
| 7 | R1R2 | Fy(a+b+) | Jk(a+b+) | K−k+ | 5 |
| 8 | R0r | Fy(a+b−) | Jk(a+b+) | K−k+ | 1 |
| 9 | R1r | Fy(a+b+) | Jk(a+b+) | K−k+ | 11 |
| 10 | R1R1 | Fy(a+b−) | Jk(a+b−) | K−k+ | 16 |
| 11 | R1R1 | Fy(a+b−) | Jk(a+b−) | K−k+ | 16 |
| 12 | R1R1 | Fy(a+b+) | Jk(a+b+) | K−k+ | 35 |
| 13 | R2r | Fy(a−b−) | Jk(a+b−) | K−k+ | 3 |
| 14 | R1r | Fy(a+b+) | Jk(a+b−) | K−k+ | 11 |
| 15 | R1R1 | Fy(a+b−) | Jk(a+b−) | K−k+ | 28 |
For non-alloimmunized 84 thalassaemia patients, database of 405 donors perfectly matching for common and clinically important antigens of Rh, Duffy, Kell and Kidd blood group systems was prepared (Table II). On an average, five donors matching perfectly for Rh, Duffy, Kell and Kidd antigens per thalassaemia patient were identified. For some relatively rare Rh phenotypes, three donors per patient were identified.
| Rh phenotype | Duffy, Kidd and Kell phenotype | Number of thalassaemia patients | Number of antigen-matched donors | ||
|---|---|---|---|---|---|
| R1R1 | Fy(a+b+) | Jk(a+b−) | kk | 7 | 35 |
| Fy(a+b−) | Jk(a+b−) | kk | 7 | 28 | |
| Fy(a+b+) | Jk(a+b+) | kk | 7 | 35 | |
| Fy(a+b−) | Jk(a−b+) | kk | 7 | 36 | |
| Fy(a+b−) | Jk(a+b+) | kk | 17 | 56 | |
| Fy(a+b+) | Jk(a−b+) | kk | 1 | 8 | |
| Fy(a−b+) | Jk(a+b−) | kk | 1 | 8 | |
| R1r | Fy(a+b−) | Jk(a+b+) | kk | 3 | 29 |
| Fy(a−b+) | Jk(a+b−) | kk | 3 | 21 | |
| Fy(a+b−) | Jk (a−b+) | kk | 2 | 11 | |
| Fy(a+b−) | Jk(a+b−) | kk | 2 | 23 | |
| Fy(a+b+) | Jk(a+b+) | kk | 4 | 28 | |
| Fy(a+b+) | Jk(a−b+) | kk | 1 | 8 | |
| Fy(a+b+) | Jk(a+b−) | kk | 1 | 11 | |
| R1R2 | Fy(a+b+) | Jk(a+b+) | kk | 3 | 11 |
| Fy(a+b−) | Jk(a+b+) | kk | 3 | 10 | |
| Fy(a−b+) | Jk(a+b+) | kk | 2 | 6 | |
| Fy(a−b+) | Jk(a+b−) | kk | 2 | 7 | |
| Fy(a−b+) | Jk(a−b+) | kk | 1 | 3 | |
| Fy(a+b−) | Jk(a−b+) | kk | 2 | 6 | |
| Ror | Fy(a+b+) | Jk(a+b−) | kk | 1 | 3 |
| Fy(a+b+) | Jk(a+b+) | kk | 1 | 2 | |
| Fy(a−b+) | Jk(a+b+) | kk | 1 | 2 | |
| Fy(a+b−) | Jk(a+b+) | kk | 1 | 3 | |
| Fy(a+b−) | Jk(a+b+) | kk | 1 | 5 | |
| Fy(a−b+) | Jk(a−b+) | kk | 1 | 3 | |
| Fy(a+b+) | Jk(a+b+) | kk | 1 | 4 | |
| R2r | Fy(a−b+) | Jk(a+b−) | kk | 1 | 3 |
Discussion
In the present study, 500 regular O group blood donors were phenotyped for 11 common and clinically important antigens of Rh, Duffy, Kell and Kidd blood group systems. R1R1 was the most common Rh phenotype identified in the present study as compared to high prevalence of R1r and Rh-negative phenotype in Caucasians and R0r in Africans, (Table III)9101112131415. The frequency of Rh phenotypes were, however, comparable with other Indian studies except for the Rh-negative phenotype rr, which was reported to be highest (11.3%) in South Gujarat (Table III)9101112131415.
| Phenotype | North | South | East | West | Caucasians (Reid and Lomas-Francis 2004)14 | Africans (Reid and Lomas-Francis 2004)14 | Chinese (Lin-Chu et al, 1998)15 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Thakral et al, 20109 (Chandigarh) (n=1240) | Makroo et al, 201310(New Delhi) (n=3015) | Gundrajukuppam et al, 201611 (Tirupati) (n=1000) | Nag and Das 201212 (West Bengal) (n=3850) | Kahar and Patel 201413 (South Gujarat) (n=115) | Present study (Mumbai) (n=500) | |||||
| Weiner | Fisher-race | Percentage proportion | ||||||||
| R1R1 | CDe/CDe | 43.8 | 42.6 | 43.4 | 49.4 | 40.87 | 44.0 | 18.5 | 2.0 | 47.5 |
| R1r | CDe/cde | 30.0 | 32.2 | 31.2 | 27.0 | 23.48 | 30.2 | 34.9 | 21.0 | 8.1 |
| R2r | cDE/cde | 8.95 | 0.1 | 0.5 | 3.0 | 4.3 | 6.8 | 11.8 | 18.6 | 2.0 |
| R1R2 | CDe/cDE | 8.22 | 14.5 | 10.7 | 11.0 | 13.91 | 10.2 | 13.3 | 4.0 | 34.4 |
| R2R2 | cDE/cDE | 1.45 | 0.8 | 0.7 | 1.0 | - | 0.6 | 2.3 | 0.2 | 5.9 |
| R0r | cDe/cde | 0.97 | 1.3 | 1.2 | 2.7 | 0.87 | 2.2 | 2.1 | 45.8 | 0.3 |
| rr | cde/cde | 5.81 | 4.6 | 4.7 | 4.3 | 11.30 | 5.6 | 15.1 | 6.8 | 0.2 |
| r’r | Cde/cde | 0.56 | 0.3 | 0.6 | 0.6 | - | 0.4 | 0.8 | 0 | 0.4 |
Comparison of frequency of Duffy, Kell and Kidd phenotypes in different ethnic groups and Indian populations is illustrated in Table IV91013141516. The frequency of K+k+ phenotype in this study was lower than that of the Caucasians and Africans. K-k+ was the most common phenotype (99.6%). No K (K1) antigen homozygous donors were identified. The incidence of Duffy phenotypes varied among different ethnic groups with Fy(a+b−) as the most common (49.4%) phenotype in our study. Phenotypes Fy(a+b−) and Fy(a−b+) were reported to be higher in Chinese and Africans and as compared to our study. Fy(a−b−) phenotype was not identified in this study, but has high frequencies in Africans (68.0 %). In Kidd blood group system, Jk(a+b+) phenotype was the most common phenotype in the present study (45.4 %) and also in Caucasians and Chinese. In Africans, Jk(a+b−) was the most common phenotype. No Jk(a−b−) phenotype was identified in the present study (Table IV)91013141516.
| Phenotype | Indian population | Caucasians (Reid and Lomas- Francis 2004)14 | Africans (Reid and Lomas- Francis 2004)14 | Chinese (Lin Chu et al, 1998)15 | ||||
|---|---|---|---|---|---|---|---|---|
| Thakral et al, 20109 (Chandigarh) (n=1240) | Makroo et al, 201310 (New Delhi) (n=3015) | Agarwal et al, 201316 (New Delhi) (n=508) | Kahar and Patel 201413 (South Gujarat) (n=115) | Present Study (Mumbai) (n=500) | ||||
| Percentage proportion | ||||||||
| Duffy | ||||||||
| Fy(a+b−) | 43.85 | 42.1 | 36.22 | 37.39 | 49.4 | 17.0 | 9.0 | 91.0 |
| Fy(a−b+) | 13.25 | 12.3 | 15.36 | 4.35 | 15.2 | 34.0 | 22.0 | 0.3 |
| Fy(a+b+) | 42.90 | 45 | 48.03 | 9.57 | 35.4 | 49.0 | 1.0 | 8.9 |
| Fy(a−b−) | 0 | 0.3 | 0.39 | 48.69 | 0 | Rare | 68.0 | 0 |
| Kidd | ||||||||
| Jk(a+b−) | 33.44 | 32.5 | 30.71 | 28.69 | 35.8 | 28.0 | 57.0 | 23.2 |
| Jk(a−b+) | 17.35 | 18.5 | 22.83 | 19.13 | 18.6 | 23.0 | 9.0 | 26.8 |
| Jk(a+b+) | 49.21 | 48.9 | 46.06 | 52.17 | 45.6 | 49.0 | 34.0 | 49.1 |
| Jk(a−b−) | 0 | Rare | 0.39 | 0 | 0 | Rare | Rare | 0.9 |
| Kell | ||||||||
| K+k+ | 5.68 | 3.5 | 1.97 | 6.09 | 0.4 | 8.8 | 2.0 | 0 |
| K−k+ | 94.32 | 96.5 | 98.03 | 93.91 | 99.6 | 91.0 | 98.0 | 100 |
| K+k− | 0 | Rare | 0 | 0 | 0 | 0.2 | Rare | Rare |
The frequency of Fy(a+b−) was higher (49.4 %) in the present study as compared to other Indian studies91016. Frequencies of Duffy phenotypes were similar to other Indian studies except in a report from South Gujarat where the frequency null phenotype of Fy(a−b−) was significantly high (48.69%) and of Fy(a+b+) was lower13. In Kell blood group system, K−k+ was the frequent phenotype among all the studies. The frequency of K+k+ was significantly low (0.4%) as compared to South Gujarat and Chandigarh913.
The information on different blood group antigens in any given population is necessary to predict the availability of blood units that lack the corresponding antigen(s)10. Our study showed some variation between blood group antigen and phenotype frequency in different parts from India. Such a variation in antigenic frequencies was expected as the donors in the present study were from cosmopolitan city of Mumbai.
Singer et al17 has shown a decrease in alloimmunization rates from 33 to 2.8 per cent by providing phenotype-matched blood for Rh and Kell antigens. Patients who were given antigen-matched RBC units had improved RBC survival and diminished frequency of transfusions1819. As most of the antibodies are produced against common Rh, Kell, Duffy and Kidd blood group system antigens, genotyping only for these antigens and giving a partial matched blood is the need of the hour and will substantially minimize the incidence of alloimmunization.
To provide antigen-negative and antigen-matched blood to these multitransfused patients, large-scale screening of regular donors for clinically important antigens is essential. In the present study, a database of 500 regular O group donors phenotyped for common Rh, Duffy, Kell and Kidd blood group antigens was prepared. Of these, 193 donors were identified for 15 alloimmunized patients matching perfectly for above mentioned blood group antigens for prophylactic transfusion therapy. As 60-70 per cent of antibodies are produced against Rh antigens, at least partial matching of these antigens in thalassaemia patients will reduce the alloimmunization to a large extent20. Based on partial matching for Rh antigens (C, c, D, E and e), donors matching for all common phenotypes in patients were identified. We also analyzed data for extended antigen matching between donors and 405 O blood group regular donors matching perfectly for D, C, c, E, e, Fya, Fyb, Jka, Jkb, K and k antigens were identified in 84 thalassaemia patients. This was an initial attempt to identify antigen-matched donors for multitransfused patients. The matched donors used for transfusions will drastically reduce the incidence of alloimmunization in these patients.
Financial support & sponsorship: Authors thank the Indian Council of Medical Research, New Delhi, for providing financial aid to carry out this study.
Conflicts of Interest: None.
References
- Challenges of alloimmunization in patients with haemoglobinopathies. Br J Haematol. 2012;159:394-404.
- [Google Scholar]
- Red cell alloimmunization in repeatedly transfused patients. Asian J Transfus Sci. 2017;11:115-20.
- [Google Scholar]
- Alloimmunization to red cells in thalassemics: Emerging problem and future strategies. Transfus Apher Sci. 2011;45:167-70.
- [Google Scholar]
- Alloimmunization and red cell autoimmunization in multitransfused thalassemics of Indian origin. Hematology. 2010;15:174-7.
- [Google Scholar]
- High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062-71.
- [Google Scholar]
- Importance of extended blood group genotyping in multiply transfused patients. Transfus Apher Sci. 2017;56:410-6.
- [Google Scholar]
- Prospective RBC phenotype matching in a stroke-prevention trial in sickle cell anemia: A multicenter transfusion trial. Transfusion. 2001;41:1086-92.
- [Google Scholar]
- Molecular genotyping of clinically important blood group antigens in patients with thalassaemia. Indian J Med Res. 2018;148:713-20.
- [Google Scholar]
- Phenotype frequencies of blood group systems (Rh, Kell, Kidd, Duffy, MNS, P, Lewis, and Lutheran) in North Indian blood donors. Transfus Apher Sci. 2010;43:17-22.
- [Google Scholar]
- Prevalence of Rh, Duffy, Kell, Kidd MNSs blood group antigens in the Indian blood donor population. Indian J Med Res. 2013;137:521-6.
- [Google Scholar]
- Prevalence of principal Rh blood group antigens in blood donors at the blood bank of a tertiary care hospital in Southern India. J Clin Diagn Res. 2016;10:EC07-10.
- [Google Scholar]
- ABO and rhesus blood groups in potential blood donors at Durgapur Steel city of the District of Burdwan, West Bengal. Asian J Transfus Sci. 2012;6:54-5.
- [Google Scholar]
- Phenotype frequencies of blood group systems (Rh, Kell, Kidd, Duffy, MNS, P, Lewis, and Lutheran) in blood donors of south Gujarat, India. Asian JTransfus Sci. 2014;8:51-5.
- [Google Scholar]
- The Blood Group Antigen FactsBook (2nd ed). San Diego, CA: Academic Press; 2004.
- The distribution of blood group antigens and alloantibodies among Chinese in Taiwan. Transfusion. 1988;28:350-2.
- [Google Scholar]
- Blood group phenotype frequencies in blood donors from a tertiary care hospital in North India. Blood Res. 2013;48:51-4.
- [Google Scholar]
- Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96:3369-73.
- [Google Scholar]
- DNA-based typing of blood groups for the management of multiply-transfused sickle cell disease patients. Transfusion. 2002;42:232-8.
- [Google Scholar]
- Blood group genotyping in multi-transfused patients. Transfus Apher Sci. 2013;48:257-61.
- [Google Scholar]
- Partial matching of blood group antigens to reduce alloimmunization in Western India. Transfus Apher Sci. 2016;54:390-5.
- [Google Scholar]






