Translate this page into:
Recent HCMV infection in early pregnancy associates with congenital transmission & adverse pregnancy outcome: A prospective cohort study
#Equal contribution
For correspondence: Dr Vainav Patel, Department of Viral Immunopathogenesis Lab, ICMR – National Institute for Research in Reproductive and Child Health, Mumbai 400 012, Maharashtra, India e-mail: patelv@nirrch.res.in
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Human Cytomegalovirus (HCMV) infection, leading to >90 per cent seropositivity in women of reproductive age from India, is the largest cause of congenital infections worldwide. HCMV infection status was prospectively monitored together with congenital transmission (cCMV) and adverse pregnancy outcomes (APO) in a public health setting where maternal or neonatal screening was not in practice.
Methods
Eighty three pregnant women, with (n=45) and without (n=38) bad obstetric history (BOH), were monitored for HCMV infection by ELISA-(IgM, IgG, IgG avidity) for all TORCH (Toxoplasma, Rubella, HCMV, HSV 1 & 2) pathogens along with HCMV-specific chemiluminescent microparticle immunoassay (CMIA) and nested polymerase chain reaction (PCR). Descriptive statistics were applied on data sets to determine associations between maternal infection status, pregnancy outcome and cCMV in 52 mother-neonate dyads.
Results
Combined avidity, PCR-based and HCMV IgM screening, compared to the latter alone, was successful in identifying incident infection during early pregnancy. Pregnancy loss was associated strongly with BOH and concurrent HCMV infection. Features associated with APO and cCMV, were high PCR positivity (first trimester) and high rates of HCMV-specific IgM and intermediate IgG avidity (P=0.0211, 0.0455). Also, recent HCMV infection (intermediate IgG avidity), observed mainly in the BOH group, but not recurrent infection (IgM positivity), in first and second trimesters, was associated with neonatal saliva positivity and adverse outcomes, including neonatal death (P=0.0762). Exposure to other TORCH pathogens, while detected, did not include IgM positivity or low/intermediate IgG.
Conclusion
This study highlights the significance of conducting early, multi-pronged screening for maternal HCMV infection during pregnancy, especially in public health settings with high HCMV seroprevalence.
Keywords
Adverse pregnancy outcome
avidity
bad obstetric history
congenital transmission
HCMV
nested PCR
prospective
TORCH
Human cytomegalovirus (HCMV), is omnipresent, and infection is very common1. Following primary infection, the virus establishes latent infection within the host and may later reactivate in immunocompromised individuals and during pregnancy, where immunity is also altered, leading to an array of possibly severe clinical outcomes for the fetus1,2. Congenital HCMV infection (cCMV) is caused by in-utero mother-to-foetus transmission via the placenta or postnatally through breastfeeding3. The incidence of cCMV reported is 0.2–2.2 per cent of all live births worldwide, where India bears the highest burden of cCMV births (>1.5%)4,5. Sequalae reported in 15 per cent cCMV cases at birth include low birth weight, hepatosplenomegaly, microcephaly, jaundice, sensorineural hearing loss and later onset conditions such as developmental disability and impaired vision6. Israel and eight European countries routinely screen pregnant women for HCMV7,8. Whereas India, despite having the highest HCMV seroprevalence among women of reproductive age and the highest birth prevalence of cCMV, does not yet have a national maternal or neonatal screening programme5. Recent evidence has emerged to show that the dynamics of maternal HCMV infection could have an impact on pregnancy outcome and cCMV with accompanying sequalae9,10. Thus, screening for maternal infection could facilitate better management of pregnancy outcomes as well as neonatal interventions. We previously reported the importance of integrating both serology and PCR for HCMV screening in high-risk pregnancies11. This longitudinal study aimed to determine HCMV infection status across trimesters in women with and without BOH and the consequent impact on cCMV as well as pregnancy outcome.
Materials & Methods
The present prospective cohort study was conducted at ICMR-National Institute for Research in Reproductive and Child Health (NIRRCH), Mumbai in collaboration with Nowrosjee Wadia Maternity Hospital (NWMH) and Bai Jerbai Wadia Hospital for Children (BJWHC), Mumbai, Maharashtra. This study was approved by the Institutional Ethics Committee of all participating sites (NIRRCH, NWMH, BJWHC). A signed informed consent was obtained from all the study participants, and assent was taken from parents of the newborns.
Study participants
Eighty-three pregnant women aged 18–45 yr were recruited (2021-2023), either in their first (<12 wk) or second trimester (13-20 wk) from NWMH (Supplementary Table I); 38 had no history of previous or current pregnancy complications [without BOH (bad obstetric history) group] and 45 had either pregnancy complications diagnosed at recruitment or bad obstetric history (BOH group)11. Participants were followed until delivery, and newborns were recruited, and their birth weight, APGAR score, NICU (neonatal intensive care unit) admissions, and symptoms shown (if any) were noted. Pregnant women with gestational diabetes, autoimmune disorders, HIV, Hepatitis B, reproductive tract infections/sexually transmitted infections (RTIs/STIs) were excluded.
Sample collection
Peripheral blood and saliva were collected at the time of recruitment and once during each trimester: first or second trimester upto third trimester (>28 wk). Cord blood12 at delivery and saliva (<72 h of birth) of newborns were collected before or post-1 h of breastfeeding4,13 to detect cCMV.
TORCH serology
Peripheral and cord blood plasma was used for TORCH (Toxoplasma, Rubella, HCMV, HSV 1 & 2) IgM, IgG ELISAs and IgG avidity was measured as described previously11.
HCMV chemiluminescent microparticle immunoassay (CMIA)
IgG antibody titers against HCMV were determined in plasma samples by CMIA using ARCHITECT™ Abbott™ kits (Abbott Diagnostics, USA) on Architect i1000SR immunoassay analyzer by Abbott Diagnostics as described previously14. Specimens with concentration values <6.0AU/ml are considered nonreactive and ≥6.0AU/ml are considered reactive to IgG antibodies and indicate past or acute infection.
HCMV nested-PCR
HCMV nested-PCR was performed on saliva of participants and of their newborns and DNA extracted from PBMCs, plasma and whole blood to detect HCMV mtrII region as described previously11,15. Individuals having positivity in any two compartments, i.e. whole blood, plasma or saliva were considered HCMV infected and positivity only in PBMCs was considered as latent HCMV infection16.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 10.0.1 (GraphPad Software, San Diego, California, USA). Data sets were compared using Fisher’s exact test, One-way ANOVA and P values <0.05 were considered significant.
Results
Participant characteristics
Eighty-three pregnant women, with or without BOH were recruited and followed up as shown in supplementary figure 1, and supplementary table I. Of the 45 BOH participants, majority had a history of spontaneous abortion (SA; n=30), followed by missed abortion (MA; n=17), neonatal death (NND; n=10), intrauterine foetal death (IUFD; n=10) and foetal growth restriction (FGR; n=2; Supplementary Fig. 2).
Integrated HCMV screening
HCMV-infected participants by serology were 34 per cent with 13 per cent having recent HCMV infection, 21 per cent recurrent HCMV infection and 66 per cent past exposure to HCMV (Fig. 1 and Supplementary Table II). Distribution of IgM positivity, high avidity and intermediate avidity was similar in both groups. HCMV PCR positivity revealed a much higher prevalence of infection overall (71%) with significantly higher prevalence (84%) in ‘without BOH’ group compared to the BOH group (60%; P=0.0171). Serological titers of anti-HCMV IgG were also estimated prospectively using CMIA which showed high concordance (95.65%) with IgG positivity as expected (Supplementary Table III). Integrated screening revealed a total prevalence of 82 per cent HCMV infection in the cohort.
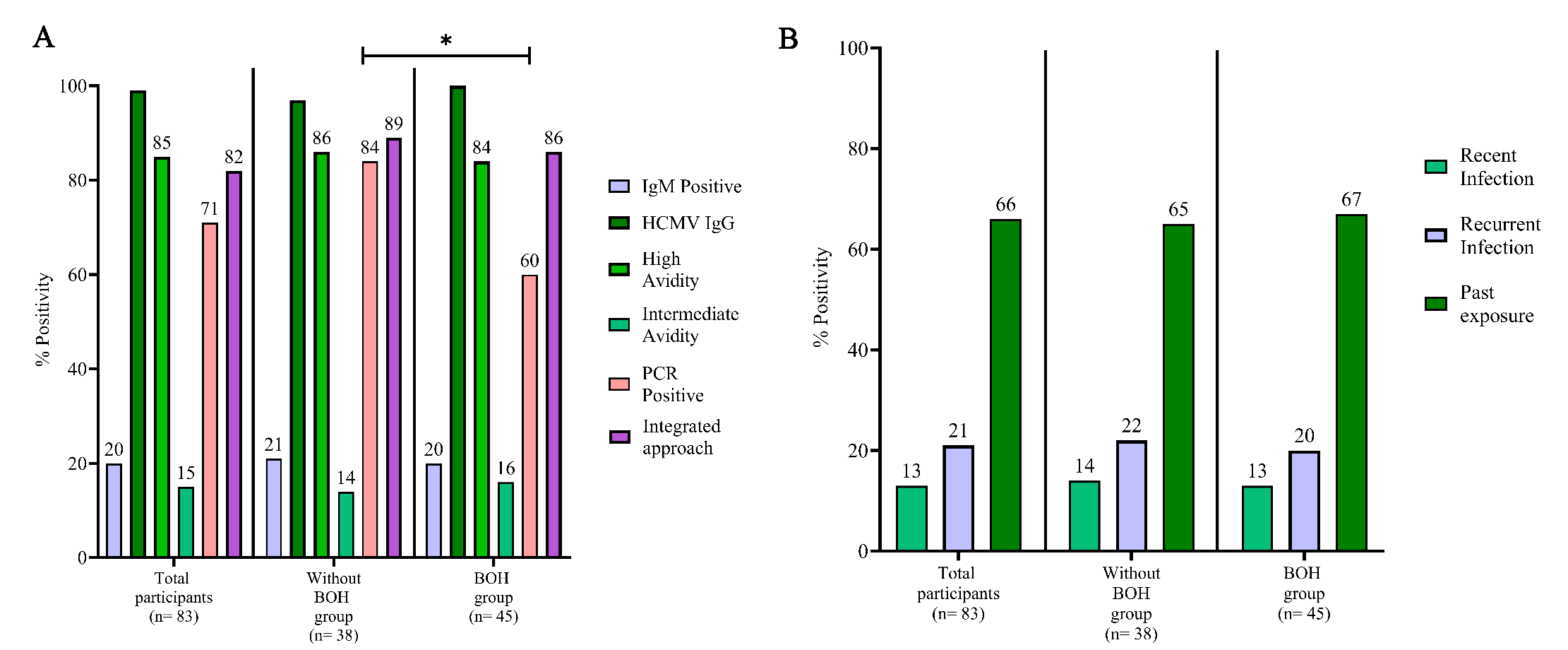
- HCMV infection screening using different approaches. (A) HCMV infection status during pregnancy by serology (IgM and IgG avidity), nested HCMV PCR and an integrated screening approach (IgM, IgG avidity or HCMV nested PCR). (B) HCMV infection status as per serological findings. Recent infection: IgM-, IgG+, low/intermediate avidity, recurrent infection: IgM+, IgG+, high avidity and past exposure: IgM-, IgG+, high avidity. Comparisons between groups was evaluated by Fisher’s exact test.
Prevalence and incidence across trimesters
Trimester wise analysis (Fig. 2A-C) demonstrated that HCMV IgM positivity (15%) and intermediate avidity (10%) based prevalence was lower than that obtained by PCR (58%) and showed a gradual decline from first to third trimester. Increased prevalence of HCMV intermediate avidity IgG was observed in both the first and second trimesters for BOH group. HCMV PCR positivity also demonstrated a consistent dip in 2nd trimester, which was more pronounced in ‘without BOH’ group (Supplementary Table IV).
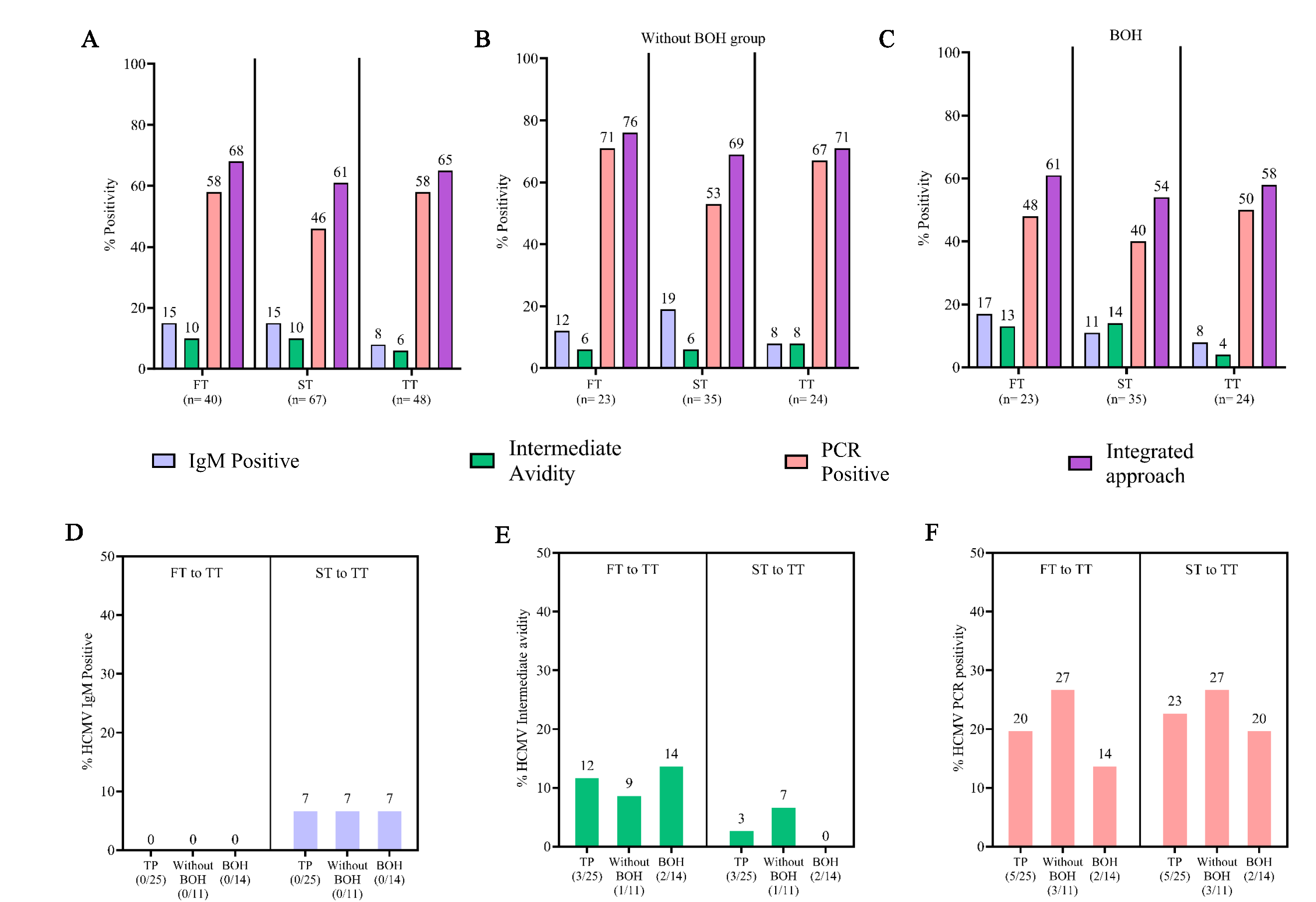
- Prevalence and incidence of HCMV infection during pregnancy. Incidence of HCMV infection was determined, where an individual initially negative for HCMV parameters and turned positive during pregnancy. (A) Prevalence of HCMV infection during 1st, 2nd and 3rd trimester by IgM, intermediate avidity, HCMV PCR and HCMV infection status by integrated approach in total participants (n=83), (B) in without BOH group (n=38), and (C) in BOH group (n=45). (D) Incidence of HCMV infection during pregnancy from 1st to 3rd and 2nd to 3rd trimester by determining IgM, (E) intermediate avidity, and (F) HCMV PCR. Integrated approach: Positivity either by IgM, intermediate avidity or PCR. FT, first trimester; ST, second trimester; TT, third trimester; TP, total participants.
As depicted in figure 2D-F, IgM screening alone detected a very limited incidence of infection, only from the second trimester onwards (7%). In the same cohort, however, concurrent avidity and PCR-based screening clearly highlighted higher incidence, 12 per cent and 20 per cent, respectively.
Adverse pregnancy outcomes: Pregnancy loss and association with HCMV infection
In our cohort (Table IA), 14 per cent (n=10) were cases of pregnancy loss (PL), including IUFD (1.4%) and miscarriage (12.6%). Of these, a greater proportion, although not statistically significantly associated (P=0.1887), belonged to BOH group. Distribution of PL was marginally higher in first trimester compared to that in the second. In PL cases, the prevalence of BOH and concurrent HCMV infection was almost twice that observed in women with normal pregnancy outcomes (NPO; Table IB). Nine out of 10 cases with PL showed evidence of active HCMV infection, with only two scoring positives for HCMV IgM (Table IC). None of these individuals were IgM positive and showed only past exposure for other TORCH pathogens revealed through IgG positivity and high avidity (Table ID).
| Total participants (n=83) | Without BOH group (n= 38) | BOH group (n= 45) | P value |
|---|---|---|---|
| Recorded pregnancy outcome (n= 71) | 36 | 35 | |
|
Pregnancy loss (PL): 14% (10/71) IUFD: 1.4% (1/71) Miscarriage: 12.6% (9/71) |
8% (3/36) | 20% (7/35) |
0.1887 RR: 2.4 |
| SA | 8.4% (6/71) | ||
| MA | 4% (3/71) | ||
| First trimester | 60% (6/10) | ||
| Second trimester | 40% (4/10) | ||
Comparisons between groups was evaluated by Fisher’s exact test. SA, spontaneous abortion; MA, missed abortion; IUFD, intrauterine fetal death; RR, relative risk; BOH, bad obstetric history
| Pregnancy loss | *Normal pregnancy outcome | |
|---|---|---|
| BOH + HCMV | 60% (6/10) | 35% (11/31) |
| HCMV | 30% (3/10) | 48% (15/31) |
| BOH | 10% (1/10) | 9.6% (3/31) |
| No BOH + No HCMV | (0/10) | 6 (2/31) |
| Parameters | HCMV infection |
|---|---|
| PCR positive | 60% (6/10) |
| IA | 30% (3/10) |
| HCMV IgM positive | 20% (2/10) |
| HCMV exposed | 10% (1/10) |
IA, intermediate avidity; PL, pregnancy loss
| Groups | HCMV serology | IgM/IA/PCR status | Pregnancy outcome | TRH IgM/IgG/Avidity |
|---|---|---|---|---|
| Without BOH group | RR | IgM, P | MA | TRH, IgG, High |
| PE | P | MA | ||
| RI | IA, P | SA | ||
| BOH | PE | P | IUFD | |
| PE | N | SA | ||
| PE | P | SA | ||
| PE | P | MA | ||
| RI | IA | SA | ||
| RI | IA | SA | ||
| RR | IgM | SA |
RI, recent infection (IgM-, IgG+, low/intermediate avidity); RR, recurrent HCMV infection (IgM+, IgG+, high avidity); PE, past exposure (IgM-, IgG+, high avidity); P, PCR Positive; IA, intermediate avidity; T, toxoplasma; R, rubella; H, HSV; IUFD, intrauterine fetal death
Adverse pregnancy outcomes: Symptomatic births and association with maternal HCMV infection
We monitored 66 live births including two cases of twin births, 35 in ‘without BOH’ group and 31 in BOH group (Table II). In 14 cases of lost to follow up, pregnancy outcomes were recorded. HCMV infection associated symptoms were seen in 50 per cent of newborns with similar distribution in both groups (Table II). These included preterm birth (PT): 24 per cent, low birth weight (LBW) <2.5 kg: 32 per cent, very low birth weight (VLBW) <1.5 kg: 4.5 per cent and jaundice 17 per cent. BOH did not seem to be associated with these outcomes. When maternal HCMV infection status was analysed for these 33 neonates, 91 per cent of mothers were HCMV positive during first trimester, which declined to 48 per cent during second trimester and increased to 61 per cent in third trimester (Fig. 3A). The dynamics of infection in NPO group, remained unchanged across trimesters; 57 per cent, 60 per cent, and 60 per cent during first, second and third trimester, respectively. Co-occurrence of PT and LBW was high (30%), as expected (Fig. 3B; Supplementary Fig. 3).
| Total participants (n=83) | Without BOH group (n= 38) | BOH group (n= 45) |
|---|---|---|
| Recorded pregnancy outcome (n=74) | 36 | 38 |
| Live births* (n=66) | 35 | 31 |
| Longitudinal sampling not available (n=14) | 8 | 6 |
| Complete sampling* (n=52) | 27 | 25 |
| APGAR score (1 min/5 min) | 7-10 | 7-10 |
| NICU admissions | 5 | 4 |
| a. Symptomatic births: 50% (33/66) | 51% (18/35) | 48% (15/31) |
| Preterm births: 24% (16/66) | 26% (9/35) | 23% (7/31) |
| Low birth weight (<2.5 kg): 32% (21/66) | 37% (13/35) | 26% (8/31) |
| Very low birth weight (<1.5 kg): 4.5% (3/66) | 3% (1/35) | 6% (2/31) |
| Jaundice: 17% (11/66) | 17% (6/35) | 16% (5/31) |
| b. Congenital HCMV transmission (cCMV) | ||
| Cord blood (n=32) | 17 | 15 |
| Neonatal Saliva (n=52) | 27 | 25 |
| 1. Cord blood positive#: 34% (11/32) | 47% (8/17) | 20% (3/15) |
| 2. Neonate saliva PCR positive: 23% (12/52) | 26% (7/27) | 20% (5/25) |
| (1 or 2) cCMV births: 35% (18/52) | 44% (12/27) | 24% (6/25) |
| Symptomatic births (SB) of complete sampling: 48% (25/52) | 52% (14/27) | 44% (11/25) |
| SB within cCMV: 61% (11/18) | 50% (6/12) | 83% (5/6) |
| c. Association of maternal HCMV infection status in second trimester with symptomatic births | ||
| Recurrent HCMV infection and SB$ | 33% (2/6) | |
| Recent HCMV infection and SB$ | 100% (4/4) | |
$Comparisons between groups (recurrent HCMV infection along with SB and recent HCMV infection along with SB) was evaluated by Fisher’s exact test (P= 0.0762). *includes 2 cases of twin births. #Positive either by IgM, Intermediate avidity or HCMV PCR, recent infection: IgM-, IgG+, low/intermediate avidity, recurrent HCMV infection: IgM+, IgG+, high avidity. cCMV, congenital HCMV infection; NICU, neonatal intensive care unit; SB, symptomatic birth; APGAR, appearance, pulse, grimace, activity, and respiration; PCR, polymerase chain reaction
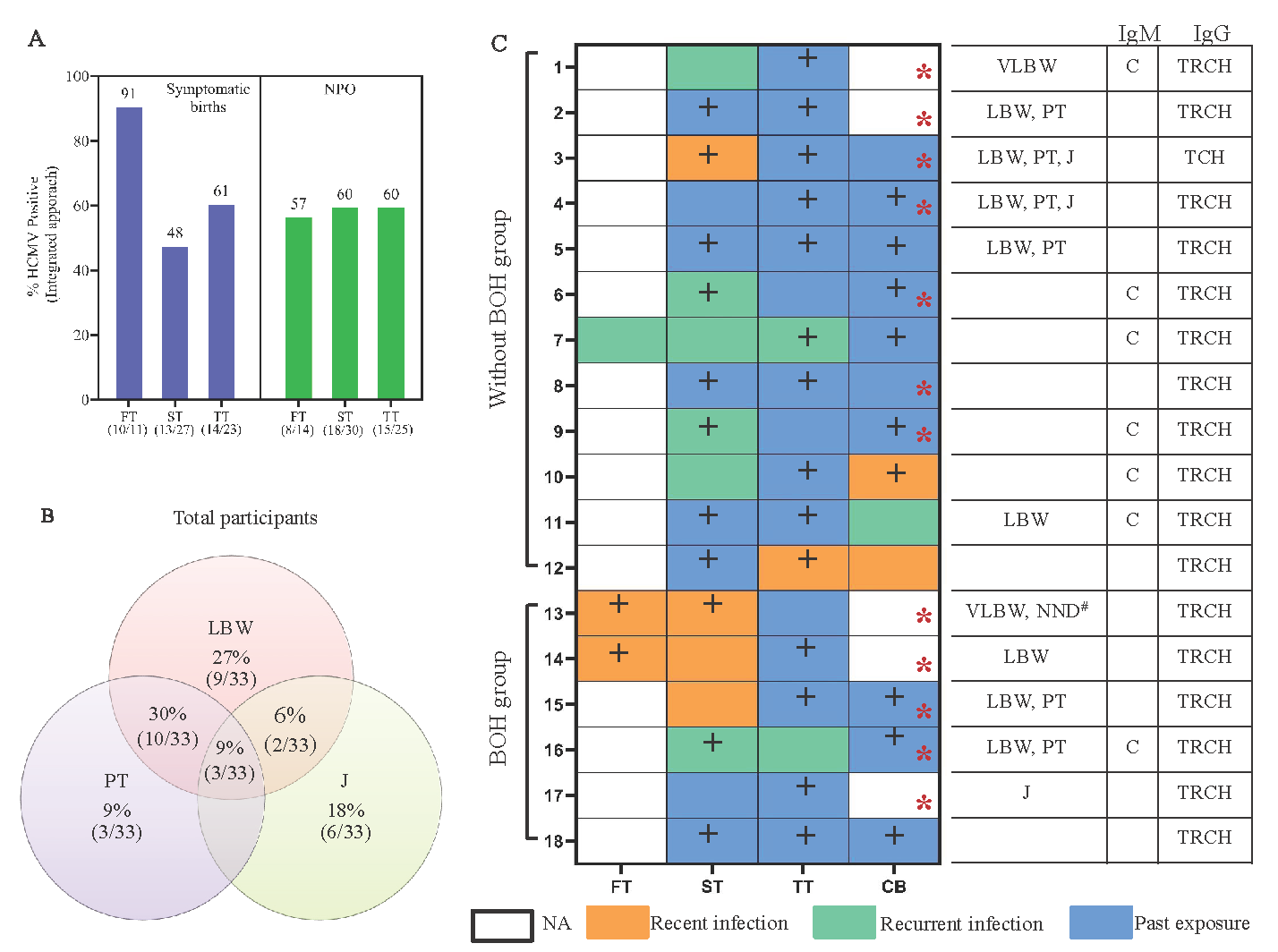
- Maternal HCMV infection status and association with pregnancy outcome. (A) Maternal HCMV infection status across trimesters in symptomatic birth and normal pregnancy outcome group. (B) Coincidence of HCMV infection associated symptoms in newborns. (C) Maternal HCMV infection status of newborns with cCMV. Recent infection: IgM-, IgG+, low/intermediate avidity, recurrent HCMV infection: IgM+, IgG+, high avidity and past exposure: IgM-, IgG+, high avidity. Note: ultrasound finding of the # case of Neonatal death also showed fetal growth restriction and the neonate was reported to have VACTREL syndrome post birth. NA, data not available; CB, cord blood; +, HCMV PCR+; Red*, neonate saliva HCMV PCR+; LBW, low birth weight; VLBW, very low birth weight; PT, pre term; J, jaundice; NND, neonatal death; T, toxoplasma; R, rubella; C, HCMV; H, HSV.
Congenital HCMV infection: Association of maternal infection status with symptomatic birth
Maternal and cognate neonate saliva screening was performed to assess cCMV in 52 mother-neonate dyads. Thirty-two cord blood samples were received (Table II). Overall rate of cCMV, detected either through cord blood (34%) or neonatal saliva positivity (23%) was 35 per cent with more frequent detection (44%) in ‘without BOH’ compared to the BOH group (24%), probably reflecting higher prevalence of HCMV PCR positivity observed overall in ‘without BOH’ group (Fig. 1A). When maternal infection status was assessed in 18 cCMV neonates (transmitter group; Fig. 3C), trimester-wise stratification of infection status showed, in BOH group, a greater proportion of recent infection (3/6) during second trimester compared to that in ‘without BOH’ group (1/12), a trend also apparent in limited data available from the first trimester. Conversely, without BOH group showed a preponderance of recurrent infection.
We evaluated the impact of these apparently distinct infection signatures on birth outcomes by integrating symptomatic births (SB) data with analysis of cCMV (Table II and Supplementary Fig. 4). SB occurred with a 48 per cent prevalence within the cCMV screening cohort. When distributed into cCMV cohort (n=18), the prevalence of SB was higher at 61 per cent. Groupwise distribution within transmission cohort showed much greater proportion of SB including the only case of NND in BOH (83%) compared to without BOH group (50%) that was associated with aforementioned unique signature of recent infection observed in BOH group. Furthermore (Fig. 3C and Table II), we observed higher prevalence of SB in women with recent infection (4/4) compared to recurrent infection (2/6; P=0.0762).
Distinct maternal HCMV infection signatures associated with pregnancy outcomes
Further, we wanted to ascertain if particular infection signatures (serological or molecular) were differentially associated with varying pregnancy outcomes (Supplementary Table V). For PL and SB+cCMV, significantly and incrementally higher frequency of detection by IgM and intermediate avidity IgG (Fig. 4A), but not PCR (Fig. 4B) was observed compared to women with NPO (P=0.0211, 0.0455). Also, within the ‘without BOH’ group, participants with PL showed the highest frequency of detection followed by those with SB+cCMV (P=0.0813) compared to women with NPO. However, in the BOH group, participants with either PL or SB+cCMV had a similar frequency of detection, which was higher than in women with NPO. Interestingly, analysis of anti-HCMV IgG titers by CMIA in these samples showed similar titers in PL and NPO which trended lower in the SB group (Fig. 4C). Further, prospective analysis of matched samples across trimesters showed a significant decline in IgG titers for SB group but not in NPO group (P=0.0801, 0.0455; Fig. 4D and E).
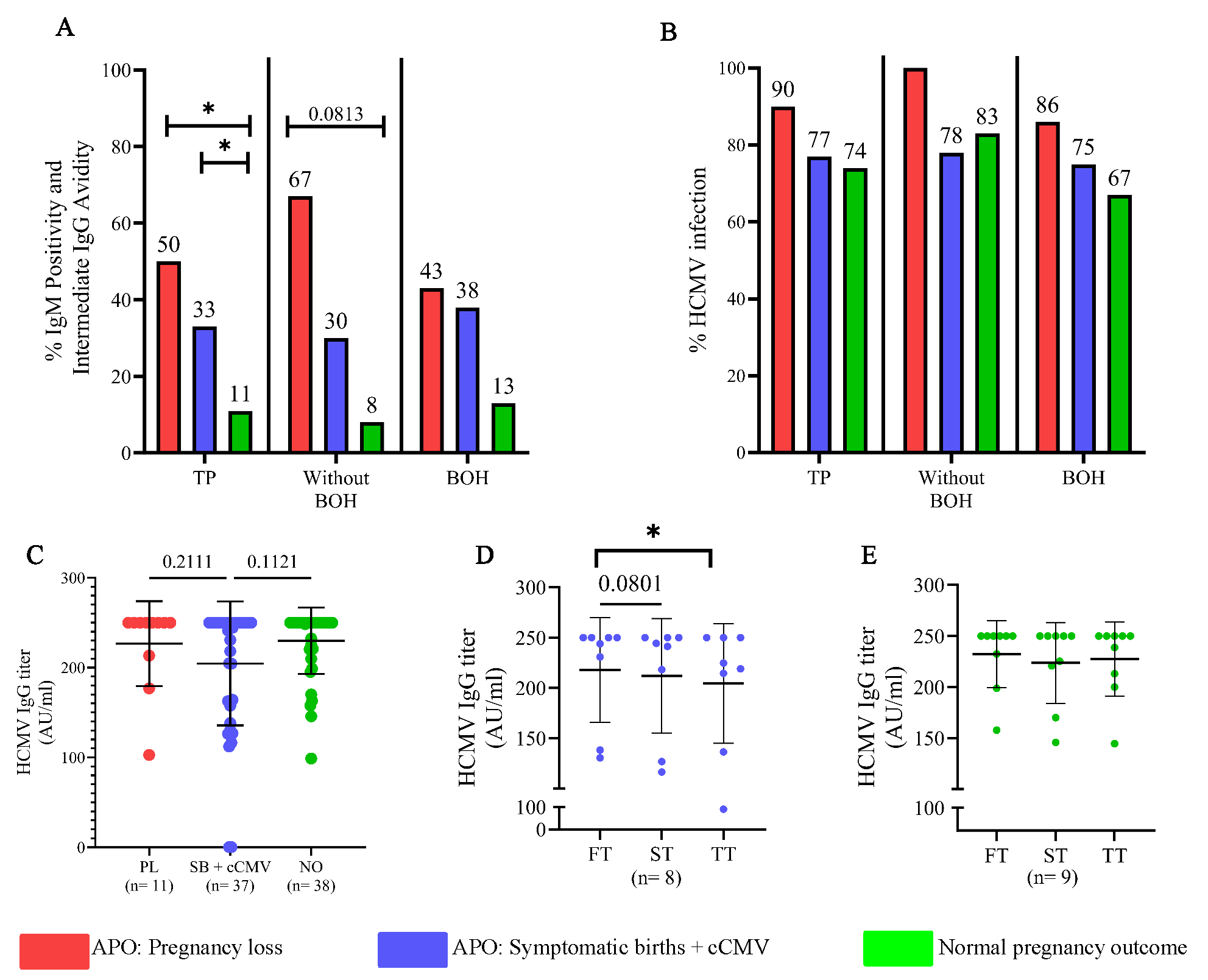
- Distinct HCMV infection signatures associated with pregnancy outcomes. (A) Integration of occurrence of IgM positivity or intermediate avidity during pregnancy in participants with pregnancy loss, symptomatic births and normal pregnancy outcome. (B) HCMV infection status (integrated approach) by IgM+ or intermediate avidity and HCMV PCR+. (C) Comparison of HCMV IgG titer during pregnancy in participants with pregnancy loss, symptomatic births and normal pregnancy outcome. (D) Comparison of HCMV IgG titer in participants with symptomatic births and cCMV across trimesters and (E) Comparison of HCMV IgG titer in participants with normal pregnancy outcome across trimesters. Comparisons between groups (A, B) was evaluated by Fisher’s exact test. Comparison between groups (C) was evaluated by One-way ANOVA Kruskal Wallis test and (D, E) Friedman test. P< 0.05 was considered significant. TP, total participants; APO, adverse pregnancy outcome.
Adverse pregnancy outcomes: Symptomatic births and neonatal saliva HCMV infection status
cCMV was determined through screening of both cord blood and neonatal saliva detected in 18 neonates (Fig. 3C). We analysed the contribution of each screened compartment to the occurrence of SB (Fig. 5A). SB was much more frequent in saliva positive neonates (transmitters; 75%) compared to saliva negative (non-transmitters; 40%; P=0.0490). However, this discriminant signature was not observed in cord blood. Also, when positivity for both compartments was combined (Fig. 5B, Supplementary Table VI) a higher percentage of SB was observed in positive samples, mainly in BOH group (P=0.0561). Considering the aforementioned association of neonatal saliva positivity for HCMV with SB, we assessed maternal infection signatures (n=24) in the neonatal screening group (Table II) who had SB (Fig. 5C and D). A clearly disparate infection profile, comprising of early infection: intermediate HCMV IgG avidity in the first two trimesters, was apparent in transmitter compared to non-transmitters. Conversely, when the 27 mothers with NPO in the neonatal screening group were analysed, only three had neonates positive in saliva and did not show evidence of recent infection during pregnancy (Supplementary Fig. 5). In 24 non-transmitting mothers, only one in both groups showed evidence of recent infection. These results highlighted association of recent infection in early pregnancy with neonatal saliva positivity and SB. Consistent with our earlier observations, exposure to other TORCH pathogen was prevalent but IgM or intermediate avidity, when detected, was only for HCMV.
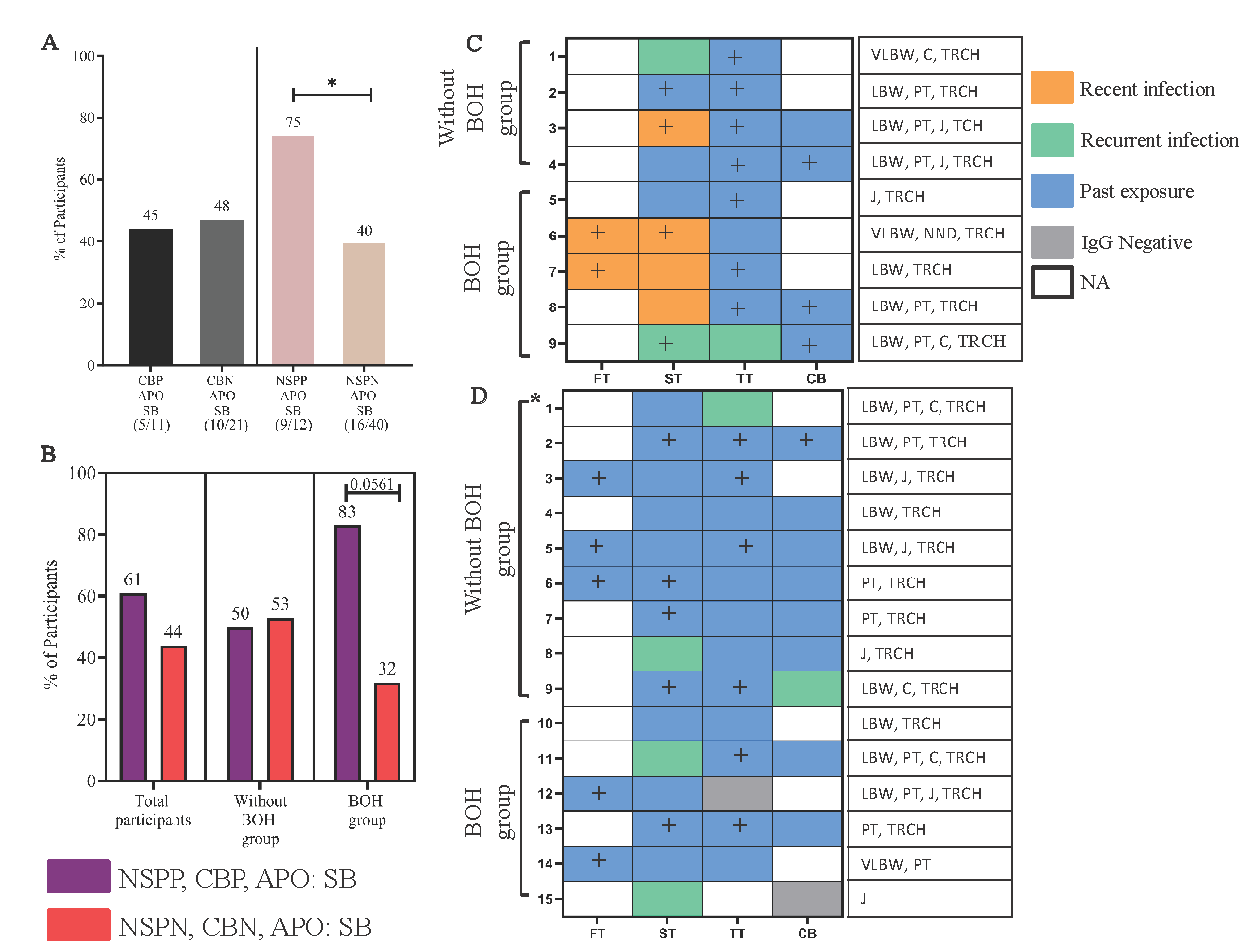
- Association of positivity screened in cord blood and neonate saliva and maternal HCMV infection status during pregnancy with adverse pregnancy outcome: symptomatic births (APO: SB). (A) Comparison of positivity in cord blood and neonate saliva with APO: SB in 52 mother-neonate dyads. (B) Comparison of transmitters and non-transmitters having APO: SB. Maternal HCMV infection status across trimesters of newborns who were (C) transmitter group: HCMV saliva PCR positive and had APO: SB; (D) non-transmitter group: HCMV saliva PCR negative and had APO: SB. Recent infection: IgM-, IgG+, low/intermediate avidity, recurrent HCMV infection: IgM+, IgG+, high avidity and past exposure: IgM-, IgG+, high avidity. Comparisons between groups was evaluated by Fisher’s exact test. *: twin birth. CBP, cord blood positive; CBN, cord blood negative; NSPP, neonate saliva PCR positive; NSPN, neonate saliva PCR negative; APO, adverse pregnancy outcome i.e. SB, symptomatic births, includes low birth weight (LBW), pre term (PT) and jaundice (J). +: HCMV PCR positive.
Discussion
Despite India’s highest maternal HCMV seroprevalence and congenital transmission (cCMV) rates, basic prevalence data and incidence data on HCMV infection dynamics during pregnancy and cCMV-associated sequelae are lacking17,18. In our study, using an integrated approach: serology and PCR11, we longitudinally assessed maternal HCMV infection during pregnancy and cCMV with accompanying adverse pregnancy outcomes (APO) in an India setting. We clearly demonstrated the impact of BOH and concurrent HCMV infection on pregnancy loss (PL) and provided evidence for recent HCMV infection at an early stage of pregnancy leading to cCMV and APO.
While serology (IgM/IgG) is generally preferred for screening and diagnosis of HCMV infection, detection of low/intermediate avidity IgG antibodies indicative of early, class-switched and infection associated responses or PCR based screening is not generally performed19. Our results, incorporating serology, IgG avidity and PCR, greatly increased the sensitivity for detecting HCMV infection. We demonstrate utility and superiority of this approach, compared to IgM screening alone, in detecting the incidence of infection as early as in 2nd trimester. The study by Ebina et al20, which did not incorporate molecular screening, demonstrated that HCMV IgG avidity-based screening within 28 weeks of gestation, is useful in predicting cCMV20. Our incorporation of molecular screening highlights significant prevalence and incidence of early HCMV infection during pregnancy, including those without any previously reported BOH from India11,21.
Early HCMV infection/reactivation during pregnancy can have grave consequences on the foetus, resulting in PL22, including miscarriage and IUFD, significantly observed in our cohort. This prevalence was similar to that reported in 2019-2021 National Family Health Survey-5 for India23,24. This study also highlighted BOH as a risk factor for PL, which supports earlier cross-sectional data reported from India11. Interestingly, in our cohort, participants with PL majorly had BOH with concurrent HCMV infection compared to either BOH or HCMV infection alone. These results reveal a novel role for HCMV pathology in background of BOH and needs to be explored to evaluate possibly higher reactivation and re-infection occurring in these women.
Our use of sensitive nested-PCR delineated a unique signature of high positivity during the first trimester in women who had symptomatic births (SB), highlighting the importance of this approach in early pregnancy screening. Interestingly, when all adverse outcomes were evaluated till <20 wk, serological (including IgG avidity), but not PCR-based screening incrementally segregated participants in terms of outcome. Périllaud-Dubois et al19 and Faure-Bardon et al25, also underscored the importance of independently screening for HCMV IgG avidity in pregnancy19,25. Indeed, in our cohort, individuals with PL had high (similar to NPO group) HCMV IgG titers, which were mainly of intermediate avidity, thus underscoring the importance of viral IgG avidity screening in early pregnancy. Further, prospective CMIA analysis delineated a distinct profile of decreasing HCMV IgG titers observed in the SB, but not in NPO group, supporting an etiological role of cCMV. The apparent lack of resolution by integrating PCR for both trimesters may reflect sporadic replication of HCMV throughout pregnancy, which may be clinically relevant only when the immunogenic threshold is crossed, leading to the production of IgM (primary/recurrent infection) and low-intermediate avidity IgG (recent infection).
Our results correlating maternal HCMV infection signatures with cCMV, extended previously reported data supporting asymptomatic birth outcomes, known to be associated with recurrent maternal infection26,27. We also observed a unique maternal signature, mainly in the BOH group, of recent infection during the first and second trimesters that was associated strongly with SB including NND. Further, our results associating cCMV with SB underscored the importance of non-invasive screening of neonatal saliva4,13 using a sensitive, nested-PCR approach targeting HCMV mtrII region for the first time in a setting of cCMV28,29. Limitations of our study include limited sample size and further follow up of cCMV cases to evaluate post-natal sequelae such as sensory neural hearing loss.
The goal of maternal screening for HCMV infection is to mitigate possible APO and two recent, effective interventions are: passive immunization by administering anti-HCMV hyperimmunoglobulin and Valacyclovir as an effective treatment which can prevent cCMV, if infection is detected early in pregnancy30–33.
Our study highlights the importance of early maternal and neonatal HCMV screening in a high prevalence, resource-limited, public health setting, with potential to improve child health and limit resource intensive interventions required for many serious sequalae associated with cCMV.
Acknowledgment
Author acknowledges all the study participants for their participation. Authors also acknowledge all the staff of Nowrosjee Wadia Maternity Hospital, Mumbai, for their help in enrolment of study participants.
Declaration
Authors declare that a part of this study was presented as a poster at American Society of Reproductive Immunology (ASRI), conference held from May 18-22, 2024, and abstract is published in American Journal of Reproductive Immunology.
Data availability
All experimental data, except personally identifiable information, is available upon request.
Financial support & sponsorship
This study received fund from India Alliance DBT Wellcome (Team Science Grant: IA/TSG/19/1/600019), Department of Biotechnology (senior research fellowship (DBT/2018/NIRRH/1090) awarded to first author (HP), and ICMR-NIRRCH (Institutional funds).
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Cytomegalovirus seroprevalence and immunity in Indian perinatal women: Experience of a cord blood bank. J Clin Diagnostic Res. 2019;13:11-4.
- [CrossRef] [Google Scholar]
- An update on current antiviral strategies to combat human cytomegalovirus infection. Viruses. 2023;15:1358.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cytomegalovirus infection in pregnancy. Birth Defects Res. 2017;109:336-346.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Congenital cytomegalovirus infection burden and epidemiologic risk factors in countries with universal screening: A systematic review and meta-analysis. JAMA Netw Open. 2021;4:e2120736.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An overview of cytomegalovirus infection in pregnancy. Diagnostics (Basel). 2022;12:2429.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Screening for cytomegalovirus during pregnancy. Infect Dis Obstet Gynecol. 2011;2011:1-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Universal screening programme for cytomegalovirus infection in the first trimester of pregnancy: Study protocol for an observational multicentre study in the area of Barcelona (CITEMB study) BMJ Open. 2023;13:e071997.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Universal screening with use of immunoglobulin G avidity for congenital cytomegalovirus infection. Clin Infect Dis. 2017;65:1652-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical factor associated with congenital cytomegalovirus infection in pregnant women with non-primary infection. J Infect Chemother. 2018;24:702-6.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated immune monitoring of HCMV infection in pregnant women with complications and its association with adverse pregnancy outcomes. Microb Pathog. 2023;179:106109.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Umbilical cord blood screening for cytomegalovirus DNA by quantitative PCR. J Clin Virol. 2006;37:313-6.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17:e177-e188.
- [CrossRef] [PubMed] [Google Scholar]
- sMAdCAM: IL-6 Ratio influences disease progression and anti-viral responses in SARS-CoV-2 infection. Front Immunol. 2021;12:619906.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Detection of Cytomegalovirus in Liver Tissue by Polymerase Chain Reaction in Infants With Neonatal Cholestasis. Pediatr Infect Dis J. 2018;37:632-6.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of major leukocyte subpopulations for monitoring secondary cytomegalovirus infections in renal-allograft recipients by PCR. J Clin Microbiol. 1998;36:1008-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health. 2022;22:1659.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cytomegalovirus infection: An Indian perspective. Indian J Med Microbiol. 2009;27:3-11.
- [CrossRef] [PubMed] [Google Scholar]
- Positive predictive values of CMV-IgM and importance of CMV-IgG avidity testing in detecting primary infection in three different clinical settings. A French retrospective cohort study. J Clin Virol. 2020;132:104641.
- [CrossRef] [PubMed] [Google Scholar]
- The IgG avidity value for the prediction of congenital cytomegalovirus infection in a prospective cohort study. J Perinat Med. 2014;42:755-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomegalovirus infection in pregnant women and its association with bad obstetric outcomes in Northern India. Microb Pathog. 2017;113:282-5.
- [CrossRef] [PubMed] [Google Scholar]
- The risk of herpes simplex virus and human cytomegalovirus infection during pregnancy upon adverse pregnancy outcomes: A meta-analysis. J Clin Virol. 2018;104:48-55.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy outcomes among Indian women: Increased prevalence of miscarriage and stillbirth during 2015-2021. BMC Pregnancy Childbirth. 2023;23:150.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Stillbirth undercount in the sample registration system and national family health survey, India. Bull World Health Organ. 2023;101:191-201.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-32.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomegalovirus shedding in seropositive healthy women of reproductive age in Tianjin, China. Epidemiol Infect. 2020;148:e34.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod Toxicol. 2006;21:399-409.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of three polymerase chain reaction tests targeting morphological transforming region II, UL-83 gene and glycoprotein O gene for the detection of human cytomegalovirus genome in clinical specimens of immunocompromised patients in Chennai, India. Virol J. 2006;3:20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Detection of cytomegalovirus in liver tissue by polymerase chain reaction in infants with neonatal cholestasis. Pediatr Infect Dis J. 2018;37:632-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of congenital cytomegalovirus infection: Review and case series of valaciclovir versus hyperimmune globulin therapy. Viruses. 2023;15:1376.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Outcome of pregnancies with recent primary cytomegalovirus infection in first trimester treated with hyperimmunoglobulin: Observational study. Ultrasound Obstet Gynecol. 2021;57:560-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with valacyclovir during pregnancy for prevention of congenital cytomegalovirus infection: A real-life multicenter Italian observational study. Am J Obstet Gynecol MFM. 2023;5:101101.
- [CrossRef] [PubMed] [Google Scholar]
- Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: A randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:779-85.
- [CrossRef] [PubMed] [Google Scholar]






