Translate this page into:
Reactivation of latent human cytomegaloviral infection in critically ill patients
For correspondence: Harbachou Viktar Uladzimiravich, 210009, Frunze Av., 27, Vitebsk, Republic of Belarus, Europe e-mail: gorvik_1994@mail.ru
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Human cytomegalovirus (HCMV) is a frequent participant in the infectious process in critically ill patients. This study aimed to determine the incidence of HCMV reactivation in critically ill patients and estimate the clinical effect of reactivation on the course of the pathological process.
Methods:
To determine the incidence of HCMV reactivation, plasma and sputum samples were collected from 82 critically ill patients. HCMV reactivation was determined by quantitative PCR together with the presence of circulating HCMV IgG in the plasma. The statistical analysis of clinical data employed methods of descriptive (median with 95% confidence interval; minimum and maximum values, interquartile range) and nonparametric statistics [Mann-Whitney U test, odds ratio (OR), Kaplan–Meier survival analysis].
Results:
HCMV reactivation was found in 36.6 per cent of cases. An association between the presence of sepsis and the development of HCMV reactivation (P<0.001), as well as higher HCMV viral loads in septic patients, was found. There was also an association between the presence of HCMV DNA and the subsequent development of sepsis (OR=1.504). The involvement of HCMV in the emerging immunological shifts manifested by a decrease in CD8+ T-lymphocytes (P=0.01) and an increase in the immunoregulatory index (P=0.03) was found.
Interpretation & conclusions:
HCMV reactivation can influence the course of bacterial pathology with a deteriorating effect on such groups of patients. Monitoring the viral load of latent HCMV can be helpful in the assessment of the host immune status, the course of the pathological process, and its clinical prognosis.
Keywords
Cytomegalovirus
immunosuppression
reactivation infection
sepsis
Despite improvements in medical care over time, the proportion of immunocompromised patients in intensive care units (ICUs) remains high. In turn, such conditions typical for critically ill patients can cause reactivation of latent cytomegalovirus infection1. It is expected primarily in cases of severe bacterial infections in patients in ICUs2,3.
Human cytomegalovirus (HCMV) reactivation can directly or indirectly influence the course of bacterial pathology with a deteriorating effect4. Thus, some studies have found that the course of the underlying disease in patients with reactivation of HCMV may be accompanied by more frequent development of bacterial complications, which cannot be ignored by clinicians5-7. Thus, the study was aimed to determine the frequency of HCMV reactivation in critically ill patients, and to evaluate the clinical impact of reactivation on the course of the underlying pathology.
Material & Methods
The study was conducted in the department of Infectious Diseases, Vitebsk State Medical University, Vitebsk, Belarus, after approval by the Institutional Ethics Committee. All patients gave informed written consent to participate in the study (if it was impossible to obtain it due to the severity of the condition, consent was obtained from an authorized representative).
Study design and patient inclusion/exclusion criteria: The study cohort included prospectively recruited septic and non-septic critically ill patients. With a two-tailed test, an alpha level of 0.05, a power of 90 per cent and consideration of the weighted mean occurrence of HCMV reactivation, 41 patients per group were required for analysis7. Thus, 82 adult patients (aged 18-90 yr) with severe bacterial infections and subsequent development of multiple organ dysfunction syndrome (as defined by SOFA score)8 and length of hospital stay more than five days were included in the study. Following definition was used to refer to severe bacterial infection “an infection which can lead to tissue damage, organ failure and death or trigger an exaggerated immune response; this infection leads to the need for oxygen, vasopressors or fluids to support blood pressure or intubation”.
Participants were hospitalized in the Intensive Care Units (ICUs) of the three hospitals (Vitebsk Regional Clinical Infectious Hospital; Vitebsk Regional Clinical Hospital; Vitebsk City Clinical Hospital of Emergency Care) and thoracic surgery department of Vitebsk Regional Clinical Hospital, from 2016 to 2020. Patients on immunosuppressive agents, high dose corticosteroid pulse therapy, or presenting with primary immunodeficiencies and HIV infection were excluded from the study. Organ or stem cell recipients, as well as cancer patients who underwent hormonal and/or radiation therapy in the previous 12 months, were also excluded from the study. The study design is shown in Figure 1.
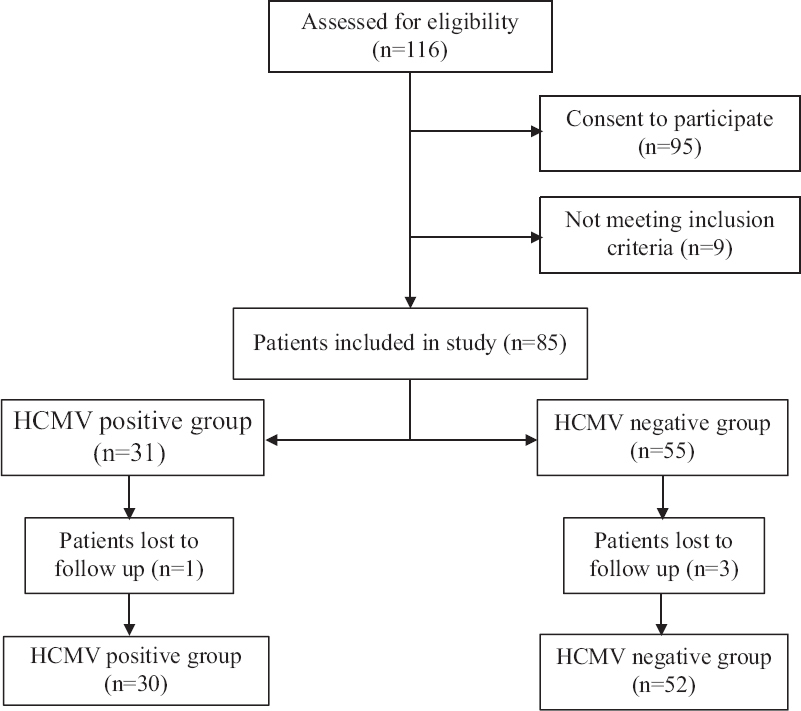
- Study design.
Human cytomegalovirus (HCMV) detection: The presence of HCMV DNA in the plasma and sputum and viral load in a clinical specimen was assessed by quantitative PCR (qPCR) initially at the time of development of the critical condition and then every 5-10 days until discharge or death. This study was not randomized, and therefore, no active interventions in the course of the disease were planned and the samples were collected simultaneously with the routine diagnostic blood or sputum sampling within the periods prescribed in the study design. Sputum was not collected if the patient was not on a ventilator or did not have sputum production, so the number of blood and sputum samples collected was different.
Latent HCMV was additionally confirmed by the detection of HCMV IgG in the plasma by enzyme-linked immunosorbent assay (Vector-Best, Novosibirsk, Russia) with the absence of clinical manifestations of active HCMV infection, which excluded the primary infection. The criteria for HCMV reactivation were the detection of HCMV DNA together with the prior presence of HCMV IgG in the plasma. Patient follow up continued until discharge or death.
To determine the incidence of HCMV reactivation, blood plasma and/or sputum samples were collected from 82 critically ill patients who met the inclusion criteria (Table I). To evaluate the contribution of the reactivation to the clinical outcome, the results of laboratory and instrumental examinations of the patients were analyzed. In addition, we also evaluated clinically important aspects that potentially influence the likelihood of reactivation such as the presence of nosocomial infections [as defined by Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network (NHSN) surveillance definition]9, duration of mechanical ventilation of lungs (MVL) and transfusion of blood substitutes. The development of secondary bacterial infections was confirmed by microbiological cultures; coinfection was determined according to the CDC definition.
| Clinical characteristics | Values |
|---|---|
| Number of patients, n | |
| Total | 82 |
| Men | 56 |
| Women | 26 |
| Age (yr), median (range) | |
| Total | 56.5 (29-89) |
| Men | 55.5 (33-84) |
| Women | 58.5 (29-89) |
| Nosocomial infection, n (%) | 72 (87.8) |
| Mechanical ventilation >3 days, n (%)* | 34 (41.5) |
| Transfusion of blood substitutes, n (%) | 71 (86.6) |
| Primary condition, n (%) | |
| Pneumonia | 22 (26.8) |
| Surgery | 18 (22.0) |
| Purulent | 15 (18.3) |
| Sepsis | 9 (11.0) |
| Neurology | 7 (8.5) |
| Trauma | 4 (4.9) |
| Burn | 2 (2.4) |
| Other | 5 (6.1) |
| Concurrent condition | 82 (100) |
*Short-term intraoperative replacement of the respiration function as well as MVL in the early post-operative period were not included in the analysis. MVL, mechanical ventilation of lungs
The primary endpoint was the patient’s discharge from the hospital or death. The secondary endpoints were the length of hospital stay, duration of MVL, duration of vasopressor support, and development of secondary bacterial infections.
Peripheral blood (5 ml) was collected in tubes coated with ethylene diamine tetraacetic acid. To obtain plasma, blood was centrifuged at 1200 g at room temperature for 15 min and stored in DNase/RNase-free tubes. Sputum was collected using bronchial aspirate sampling catheters. Plasma and sputum samples were stored at −80°C; repeated freeze-thaw cycles were excluded.
For homogenization, sputum aliquots were previously treated 1:1 with dithiothreitol 10 per cent solution, vortexed, and left at room temperature for 30 min. After that, DNA was extracted using ReliaPrep Viral Total Nucleic Acid Purification Kit (Promega, Madison, USA) according to the manufacturer’s instructions.
qPCR tests were performed in the molecular-genetic laboratory of Vitebsk State Medical University. Amplification was performed using Sivital HCMV assay (Sivital, Vitebsk, Belarus) according to the manufacturer’s instructions on Biorad CFX 96 thermocycler (Bio-Rad Laboratories, Hercules, USA). Amplification was performed in a 25 μl reaction mixture containing 5 μl of DNA extract. The reaction consisted of one cycle of five minutes at 95°C, followed by 45 cycles of 20 sec at 95°C and 60 sec at 60°C. HCMV DNA concentration in samples was expressed as the IU per millilitre.
Statistical analysis: Data processing was performed using Statistica 10.0 software package (Statsoft, Tulsa, United States) and GraphPad Prism 8.4.2 software (GraphPad Software, San Diego, USA). The analysis used methods of descriptive (median with 95% confidence interval (CI); minimum and maximum values, interquartile range) and nonparametric statistics (Mann-Whitney U test, odds ratio (OR), Kaplan-Meier survival analysis). The significance threshold was set at P=0.05.
Results
None of the participants included in this study had any signs of active CMV infection and were HCMV IgG-positive, which made it possible to detect latent HCMV. Participants were divided into two groups depending on their HCMV status (Table II). The results of amplification of plasma and sputum samples were also analyzed separately. The clinical and laboratory parameters of the study participants are shown in Table III.
| Characteristics | HCMV DNA− | HCMV DNA+ |
|---|---|---|
| Number patients, n (%) | ||
| Total | 52 of 82 (63.4) | 30 of 82 (36.6) |
| Men | 34 of 52 (65.4) | 22 of 30 (73.3) |
| Women | 18 of 52 (34.6) | 8 of 30 (26.7) |
| Age (yr), median (range) | ||
| Total | 56.1 (29-84) | 58.2 (33-89) |
| Men | 53.5 (37-84) | 58 (33-80) |
| Women | 55 (29-81) | 67.5 (37-89) |
| Nosocomial infection, n (%) | 45 of 52 (86.5) | 27/30 (90) |
| Mechanical ventilation >3 days, n (%)* | 24 of 52 (46.2) | 10/30 (33.3) |
| Transfusion of blood substitutes, n (%) | 45 of 52 (86.5) | 26/30 (86.7) |
| Concurrent condition, n (%) | 52 of 52 (100) | 30/30 (100) |
| Neurology, n (%) | 5 of 7 (71.4) | 2/7 (28.6) |
| Pneumonia, n (%) | 13 of 22 (59) | 9/22 (41) |
| Sepsis, n (%) | 4 of 9 (44.4) | 5/9 (55.6) |
| Trauma, n (%) | 1 of 4 (25) | 3/4 (75) |
| Purulent, n (%) | 11 of 15 (73.3) | 4/15 (26.7) |
| Surgery, n (%) | 13 of 18 (72.2) | 5/18 (17.8) |
| Burns, n (%) | 2 of 2 (100) | 0/2 (0) |
| Other, n (%) | 3 of 5 (60) | 2/5 (40) |
*Short-term intraoperative replacement of the respiration function as well as mechanical ventilation in the early post-operative period were not included in the analysis. HCMV, human cytomegalovirus
| Parameter | Total (n=82) | HCMV DNA+ (n=30) | HCMV DNA− (n=52) | Mann-Whitney U test, P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Me | IQR | 95% CI | Me | IQR | 95% CI | Me | IQR | 95% CI | ||
| Hospitalization (days) | 42.5 | 22.0-58.0 | 36.0-49.0 | 49.0 | 38.0-67.0 | 41.0-63.6 | 35.5 | 19.5-55.0 | 23.5-44.1 | 0.03 |
| Antibiotic therapy (days) | 34.5 | 20.0-49.0 | 27.0-41.0 | 41.5 | 30.0-52.0 | 32.9-48.5 | 27.0 | 18.0-43.0 | 21.0-38.2 | 0.02 |
| Changes in antibiotic therapy regimens (n) | 2.5 | 2.0-4.0 | 2.0-3.0 | 3.0 | 2.0-5.0 | 2.0-4.0 | 2.0 | 2.0-4.0 | 2.0-3.0 | 0.07 |
| Vasopressor therapy (days) | 0.0 | 0.0-5.0 | 0.0-2.0 | 0.0 | 0.0-6.0 | 0.0-2.0 | 2.0 | 0.0-5.0 | 0.0-2.5 | 0.68 |
| C-reactive protein level, (mg/l) | 136.5 | 79.6-222.6 | 106.9-191.1 | 125.2 | 79.9-222.1 | 81.9-202.3 | 137.5 | 73.1-223.0 | 109.3-199.3 | 0.93 |
| Transfusions of blood substitutes (n) | 4.0 | 2.0-9.0 | 3.0-5.3 | 4.5 | 2.0-11.0 | 2.0-7.8 | 4.0 | 2.0-8.0 | 2.0-5.0 | 0.67 |
| Mechanical ventilation (days) | 0.0 | 0.0-11.0 | 0.0-4.3 | 0.0 | 0.0-10.0 | 0.0-5.5 | 0.0 | 0.0-11.5 | 0.0-6.5 | 0.74 |
| Mortality, n (%) | 28 (34.1) | 10 (33.3) | 18 (34.6) | 0.91 | ||||||
HCMV, human cytomegalovirus; IQR, interquartile range; CI, confidence interval
HCMV DNA was detected in 30 out of 82 patients (36.6%). Of these, in 17 out of 55 patients (30.9%), HCMV DNA was detected in the blood, in 12 out of 38 patients (31.6%) in sputum and in two out of 11 patients (18.2%) in both blood and sputum.
Mortality in case of HCMV reactivation did not statistically vary (Mann-Whitney U test, P=0.91; Kaplan–Meier survival analysis, P=0.75) (Fig. 2) and also did not depend on the viral load of HCMV DNA (Kaplan–Meier survival analysis, P=0.88) (Fig. 3). There were also no difference in mortality between the two groups either during the 28 day hospital stay or during the entire period of hospitalization.
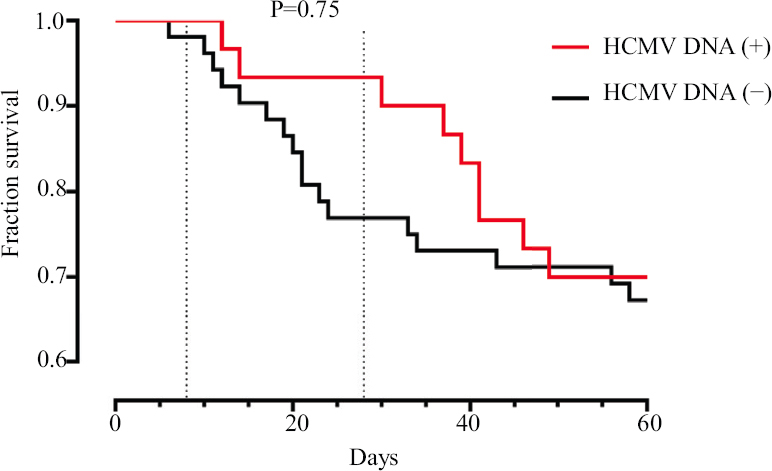
- Kaplan-Meier survival analysis in patients with and without HCMV DNA in biological fluids. HCMV, human cytomegalovirus.
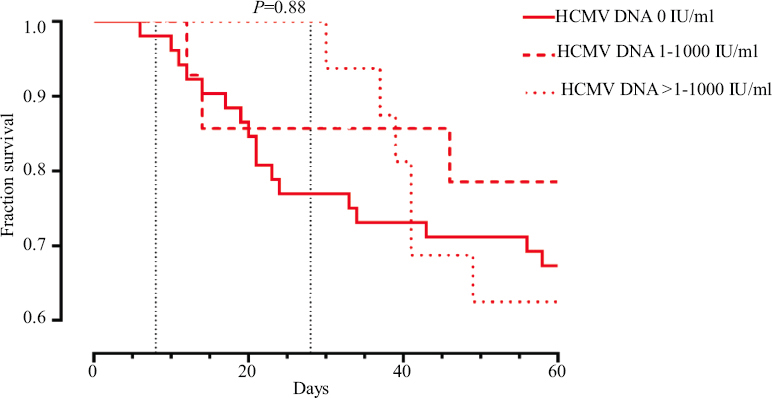
- Kaplan–Meier survival analysis depending on HCMV viral load.
The HCMV-positive group was characterized by longer hospitalization (median 49.0 vs. 35.5; P=0.03) and antibiotic therapy (median 41.5 vs. 27.0; P=0.02). In addition, an association was found between a higher incidence of bacterial nosocomial infections from the ESKAPE group in HCMV-positive patients [27 of 30 (90.0%) vs. 45 of 52 (86.5%); OR = 1.4, 95% CI 0.33-5.88], although not significant (P=0.64). At the same time, in the HCMV-positive group, 12 (40.0%) patients were found to have three or more nosocomial pathogens, which was registered in only 10 (19.2%) patients in the HCMV-negative group (P=0.01, Table IV). Furthermore, the presence of HCMV DNA in the sputum was accompanied by the detection of two or more bacterial pathogens in sputum in all cases. The hypothesis of increased risk of nosocomial infections in patients with HCMV-induced modulation is supported by some other studies10,11. The main clinical and laboratory parameters are presented in Figure 4A-F.
| Pathogens | HCMV DNA in blood (+) | HCMV DNA in sputum (+) | HCMV DNA in blood and sputum (+) | HCMV DNA (−) |
|---|---|---|---|---|
| Klebsiella pneumoniae, n | ||||
| Blood | 0 | 0 | 0 | 1 |
| Sputum | 4 | 0 | 0 | 6 |
| Pseudomonas aeruginosa, n | ||||
| Blood | 0 | 0 | 0 | 0 |
| Sputum | 1 | 0 | 0 | 5 |
| Acinetobacter baumannii, n | ||||
| Blood | 0 | 0 | 0 | 0 |
| Sputum | 2 | 0 | 0 | 4 |
| Other, n* | ||||
| Blood | 0 | 0 | 0 | 1 |
| Sputum | 1 | 0 | 0 | 1 |
| 2 pathogens (n=23) | 4 | 3 | 0 | 17 |
| 3 and more pathogens (n=23) | 2 | 9 | 1 | 10 |
| Absence of pathogens (n=10) | 3 | 0 | 0 | 7 |
*Other pathogens included Staphylococcus aureus, Escherichia coli, Proteus mirabilis, Enterococcus faecium, Salmonella panama, Candida albicans, Citrobacter spp. HCMV, human cytomegalovirus
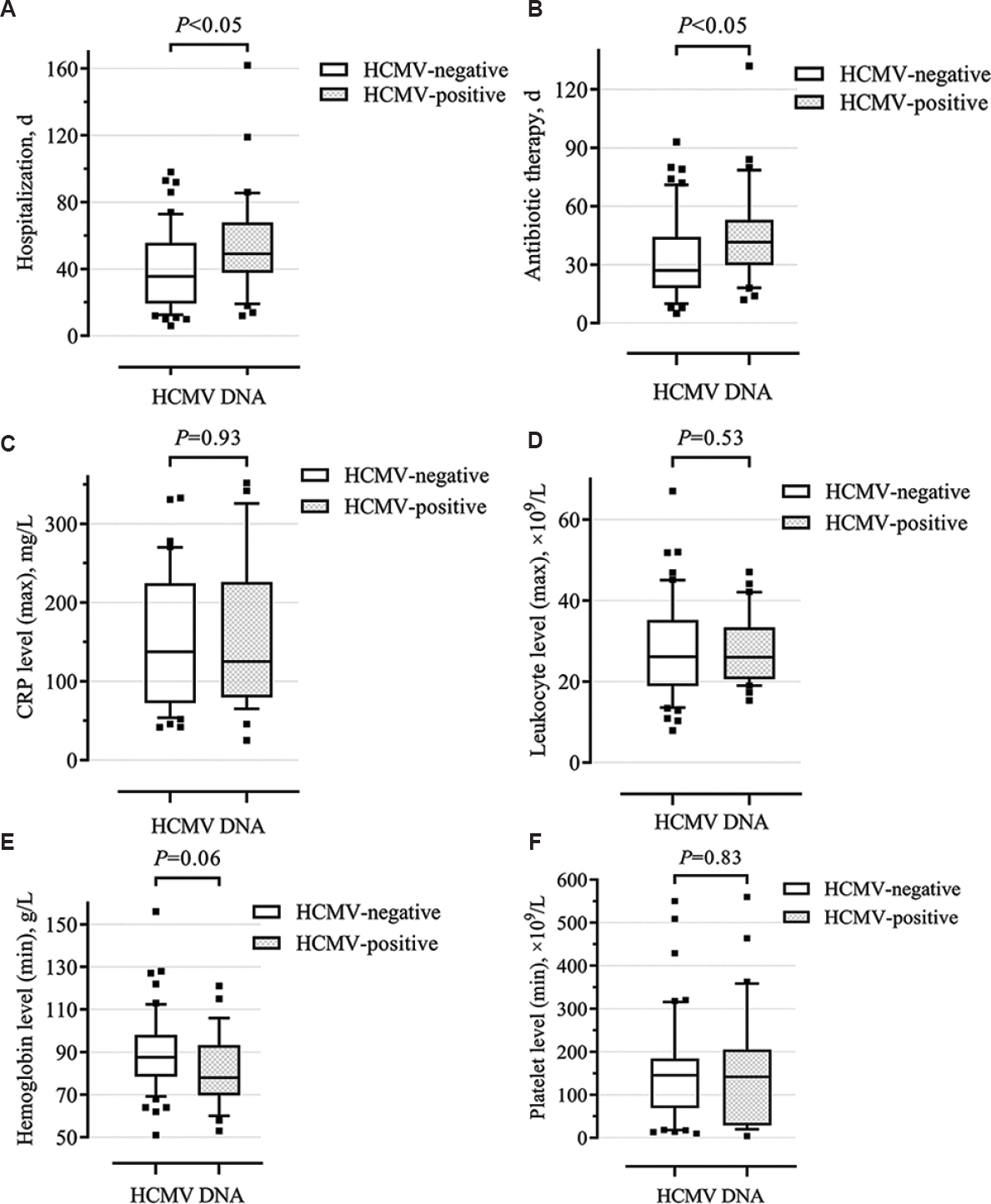
- Box and Whisker plots showing clinical and laboratory shifts in patients with and without HCMV reactivation. (A) Hospitalization (d), (B) antibiotic therapy (d), (C) maximum CRP level (mg/l), (D) maximum leukocyte level (109/l), (E) minimum hemoglobin level (g/l), (F) minimum platelet level (109/l). Line in the middle of the box marks median distribution, box borders are the first and third quartiles. 10-90 per cent values are marked with asterisks.
Immunological shifts were observed in the HCMV-positive group in the form of a decrease in the number of CD-8 T-suppressors (P=0.01, Fig. 5A) and an increase in the immunoregulatory index (P=0.03, Fig. 5B). Besides, these changes were more pronounced when higher a HCMV viral load was detected. Thus, some laboratory and clinical parameters indicate a more severe course of the disease in the HCMV-positive group.
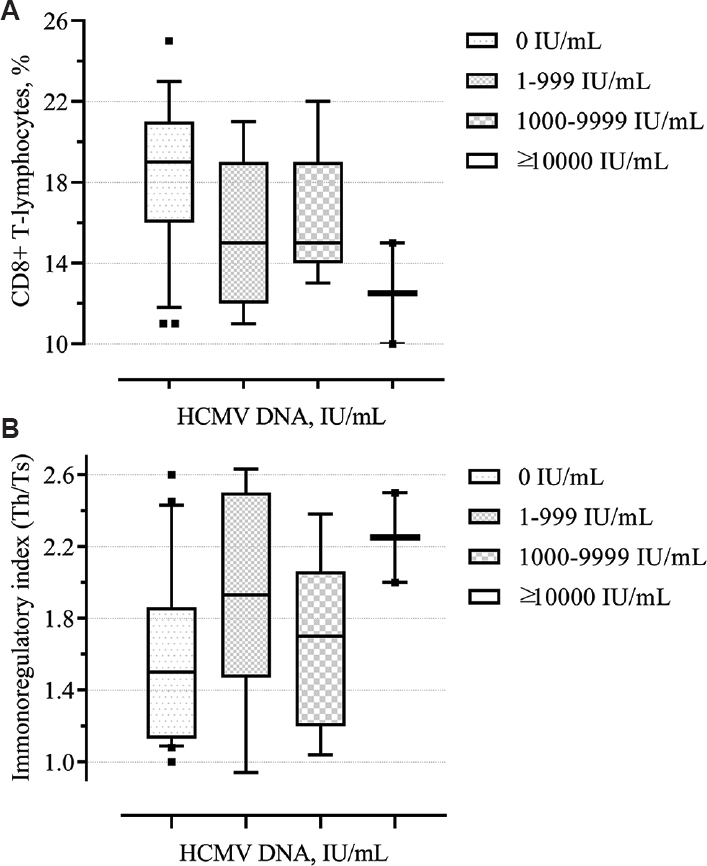
- Box and Whisker plots showing (A) level of CD8+ (%) depending on the HCMV viral load, (B) immunoregulatory index depending on the HCMV viral load.
Consideration of HCMV reactivation in patients with sepsis is of considerable interest12. In the sepsis group, HCMV reactivation was detected in 19 of 45 (42.2%) vs. 11 of 37 (29.7%) patients who did not have sepsis (P<0.001).
HCMV reactivation was subsequently detected in five out of nine patients (55.6%) in whom sepsis occurred at the time of admission. Of the remaining 73 patients, sepsis subsequently developed in 36 cases. Of these 36 patients, preceding HCMV viremia was found in 14 cases (38.9%) compared with 11 of the remaining 37 HCMV-positive patients without sepsis (29.7%) (OR=1.504). HCMV viral load in patients with sepsis was higher both in plasma (mean 3398.5 vs. 369.2 IU/ml; median 998.4 vs. 182.9 IU/ml) and in sputum (mean 39647.4 vs. 7362.6 IU/ml; median 4409 vs. 2990 IU/ml) (Fig. 6).
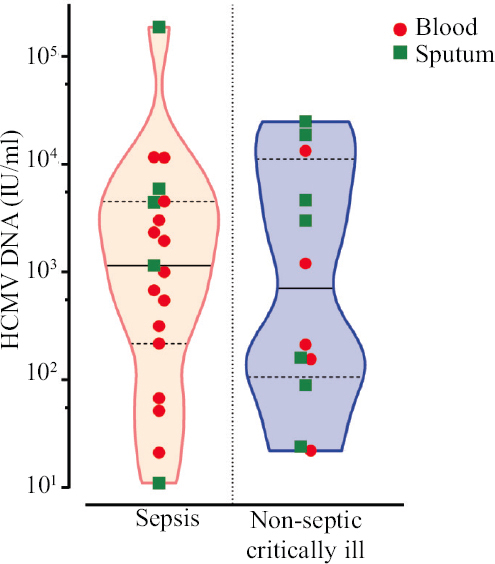
- Level of HCMV DNA (IU/ml) in blood and sputum in patients with and without sepsis. The number of positive samples exceeds the number of HCMV-positive patients because in some patients HCMV DNA could be detected in plasma and sputum.
Discussion
The understanding of the nature of the immune response in septic patients is currently under revision. To date, the study of the cytokine response in systemic inflammatory response syndrome (SIRS) and compensatory anti-inflammatory response syndrome (CARS) has led to the understanding that the mixed antagonistic response syndrome, which is characterized by the simultaneous release of both types of mediators13, is the predominant one. Based on the data obtained in the present study, it seems that HCMV infection can lead to the generation of inflammatory mediators, perpetuating the harmful proinflammatory/anti-inflammatory imbalance seen in septic patients. It was found that some laboratory and clinical parameters indicate a more severe course of the disease in the HCMV-positive group. An association was also found between the presence of HCMV DNA and the subsequent development of sepsis in patients with severe bacterial pathology. It can hence be concluded that sepsis as a state of immunosuppression may be a predisposing factor for the development of HCMV reactivation. In turn, HCMV reactivation itself can trigger a pathological cascade, leading to the development of septic conditions which is proved not only by the more frequent development of sepsis in HCMV-positive patients but also by the higher levels of viral load of HCMV DNA in biological fluids14. At last, the results of the present study indicate an important role of CD8+ T-lymphocytes in the antiviral response to HCMV in patients with sepsis. These results are also confirmed by the results of studies obtained by other authors15-18.
There were several limitations in our study including the relatively small sample size and the different nosologies of the patients included in the study. Difficulties in interpretation were introduced by the complex study design. In addition, retrospective analysis of data did not allow influencing the assignment of some tests of interest to all patients. At last, HCMV reactivation may also be associated with a direct cytopathic effect. Neither immunoassays nor did PCR allow for evaluation of the cytopathic effects of active cytomegalovirus infection. Thus, it should be taken into account in further research.
HCMV is a frequent participant in the infectious process in critically ill patients, contributing to the clinical outcome of the disease. In this study, HCMV was reactivated in 36.6 per cent of the participants. The disease course of HCMV-positive patients was characterized by longer duration of hospitalization, as well as an increased risk of nosocomial infections. No differences in mortality rates were found between patients with and without HCMV reactivation.
An association was found between the presence of sepsis and an HCMV reactivation (P<0.001), as well as higher HCMV viral loads in both blood and sputum of septic patients. There was an association between the presence of HCMV DNA and the subsequent development of sepsis in patients with severe bacterial pathology (OR=1.504).
Overall, the involvement of HCMV in the emerging immunological shifts in critically ill patients with a prevalence of CARS, manifested by a decrease in CD8+ T-lymphocytes (P=0.01) and an increase in the immunoregulatory index (P=0.03). Monitoring the viral load of latent HCMV can be helpful in the assessment of the host immune status, the course of the pathological process, and its clinical prognosis.
Financial support & sponsorship: The study was funded by the Vitebsk State Medical University.
Conflicts of Interest: None.
References
- Cytomegalovirus reactivation and mortality in patients with acute respiratory distress syndrome. Intensive Care Med. 2016;42:333-41.
- [Google Scholar]
- Cytomegalovirus reactivation in the critically ill septic intensive care patient: Pathogen or passenger? Anaesth Intensive Care. 2016;44:535-8.
- [Google Scholar]
- Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis. 2017;64:1204-10.
- [Google Scholar]
- Association between cytomegalovirus reactivation and clinical outcomes in immunocompetent critically ill patients: A systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:ofx029.
- [Google Scholar]
- Active cytomegalovirus infection is common in mechanically ventilated medical Intensive Care Unit patients. Crit Care Med. 2009;37:1850-7.
- [Google Scholar]
- Cytomegalovirus infection in immunocompetent critically ill adults: Literature review. Ann Intensive Care. 2016;6:110.
- [Google Scholar]
- The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801-10.
- [Google Scholar]
- CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-32.
- [Google Scholar]
- Cytomegalovirus infection in critically ill patients: Associated factors and consequences. Chest. 2005;127:233-41.
- [Google Scholar]
- Cytomegalovirus reactivation in a general, nonimmunosuppressed Intensive Care Unit population: Incidence, risk factors, associations with organ dysfunction, and inflammatory biomarkers. J Crit Care. 2015;30:276-81.
- [Google Scholar]
- Cytomegalovirus infection in patients with sepsis due to bloodstream infections: Lower risk and better outcomes in new versus already hospitalised Intensive Care Unit admissions. Anaesth Intensive Care. 2016;44:571-80.
- [Google Scholar]
- Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967-74.
- [Google Scholar]
- Impact of cytomegalovirus load on host response to sepsis. Med Microbiol Immunol. 2019;208:295-303.
- [Google Scholar]
- Role of antibodies in confining cytomegalovirus after reactivation from latency: Three decades'résumé. Med Microbiol Immunol. 2019;208:415-29.
- [Google Scholar]
- Interferon-γproduction by CMV-specific CD8+T lymphocytes provides protection against cytomegalovirus reactivation in critically ill patients. Intensive Care Med. 2016;42:46-53.
- [Google Scholar]
- Impaired polyfunctionality of CD8+ T cells in severe sepsis patients with human cytomegalovirus reactivation. Exp Mol Med. 2017;49:e382.
- [Google Scholar]
- Molecular characterization of HCMV-specific immune responses: Parallels between CD8(+) T cells, CD4(+) T cells, and NK cells. Eur J Immunol. 2015;45:2433-45.
- [Google Scholar]






