Translate this page into:
Rapid detection of drug-resistant Mycobacterium tuberculosis directly from clinical specimens using allele-specific polymerase chain reaction assay
For correspondence: Dr Shampa Anupurba, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221 005, Uttar Pradesh, India e-mail: shampa_anupurba@yahoo.co.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Rapid detection of drug resistance in Mycobacterium tuberculosis (MTB) is essential for the efficient control of tuberculosis. Hence, in this study a nested-allele-specific (NAS) PCR, nested multiple allele-specific PCR (NMAS-PCR) and multiple allele-specific (MAS) PCR assays were evaluated that enabled detection of the most common mutations responsible for isoniazid (INH) and rifampicin (RIF) resistance in MTB isolates directly from clinical specimens.
Methods:
Six pairs of primers, mutated and wild type, were used for the six targets such as codon 516, 526 and 531 of rpoB, codon 315 of katG and C15-T substitution in the promoter region of mabA-inhA using allele-specific (AS) PCR assays (NAS-PCR, NMAS-PCR and MAS-PCR). The performance of AS PCR method was compared with phenotypic drug susceptibility testing (DST).
Results:
The usefulness of AS PCR assays was evaluated with 391 clinical specimens (251 Acid fast bacilli smear positive and MTB culture positive; 93 smear negative and MTB culture positive; 47 smear positive and MTB culture negative) and 344 MTB culture positive isolates. With culture-based phenotypic DST as a reference standard, the sensitivity and specificity of the NAS-PCR, NMAS-PCR and MAS-PCR assay for drug resistance-related genetic mutation detection were 98.6 and 97.8 per cent for INH, 97.5 and 97.9 per cent for RIF and 98.9 and 100 per cent for multidrug resistance (MDR).
Interpretation & conclusions:
The performance of AS PCR assays showed that those could be less expensive and technically executable methods for rapid detection of MDR-TB directly from clinical specimens.
Keywords
Drug susceptibility testing
multiple allele-specific PCR
nested allele-specific PCR
nested multiple allele-specific PCR
rapid diagnosis
The global burden of drug-resistant tuberculosis (TB) and multidrug-resistant TB (MDR-TB), defined as Mycobacterium tuberculosis resistant to rifampicin (RIF) and isoniazid (INH), is increasing and has become a major health problem. Globally, among previously treated TB patients, 18 per cent have MDR-TB and 3.5 per cent of all new TB cases are MDR-TB1. MDR-TB reduces response to anti-tubercular treatment to first-line drugs, leads to higher mortality and treatment failure rate and increases periods of dissemination of MTB complex2. The RIF resistance increases the percentage of MDR-TB because RIF-resistant (RIFr) MTB isolates are more likely to be resistant to several other anti-TB drugs3.
Rapid detection of antimicrobial drug susceptibility pattern in MTB isolates directly from clinical specimens is important for the early administration of proper therapeutic agents to check the development and further dissemination of drug-resistant MTB. In this situation, molecular detection of drug resistance by identifying associated genes that cause mutations will be appropriate for developing a potential rapid molecular drug susceptibility test as an attractive and alternative assay to conventional methods456.
RIF resistance is mostly caused by mutations in rpoB gene. Approximately 98 per cent of RIFr clinical MTB isolates contain point mutations clustered in an 81 bp RIF resistance-determining region (RRDR) between codons 507-533, with the three most common mutations located at codons 516, 526 and 5317. In contrast, INH resistance is caused by mutations in various genes, most commonly in katG gene and in the promoter region of inhA. Different studies have reported that mutations in INH resistant MTB account for 50-95 and 15-34 per cent mutations in katG315 and mabA-inhA promoter region, respectively789. Different molecular methods have been used to detect specific mutations. However, these methods require specialized instrumentation and are not feasible in resource-poor settings. Thus, there is a need for a rapid, reliable and cost-effective technique for detection of drug resistance in M. tuberculosis.
This study was undertaken with the aim to modify the rapid and cost-effective multiple allele specific (MAS) PCR technique to suit the detection of drug-resistant TB directly from clinical specimens, and to evaluate nested MAS (NMAS) PCR assay for direct detection of mutations in katG315 codon, rpoB516, nested allele-specific (NAS) PCR assay for direct detection of mutations in rpoB526 and rpoB531 codons and MAS PCR to detect -15C→T mutation in inhA promoter region of MTB directly from clinical samples.
Material & Methods
This study was conducted in the department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, which is a tertiary care centre of northern region of India. The duration of the study was from May 2013 to March 2015. The study protocol was approved by the institutional ethics committee.
Clinical specimens: A total of 721 clinical specimens were received from clinically suspected pulmonary TB patients that included 656 pulmonary specimens [including 636 sputum speciemns and 20 bronchoalveolar lavage (BAL)] and 65 extra-pulmonary specimens including 20 urine, 15 pus, 15 fine-needle aspirations (FNAs), nine cerebrospinal fluid (CSF), one bone marrow and five pleural fluid. These specimens were obtained from newly diagnosed and previously treated patients. The criteria for patient selection were based on the signs, symptoms, radiological scans, cytology, previous treatment and family history. A total of 721 participants were included, comprising of 429 males and 292 females. Among the 721 participants, 230 were newly diagnosed cases while 302 were previously treated cases and the remaining 189 were unknown. The culture test results were handled in a blinded manner until molecular testing was complete.
The specimens were examined by light microscopy after Ziehl-Neelsen staining. The smear grading was performed using the Revised National Tuberculosis Control Program (RNTCP) recommendations10. The clinical specimens received from non-sterile sites [including sputum, pus and BAL] were processed by the conventional modified Petroff's method1112. The samples received from sterile sites (pleural fluid, blood from bone marrow, CSF) were used directly10. The sediments were inoculated on Lowenstein-Jensen (L-J) medium in duplicate and incubated at 37°C and inspected weekly for bacterial growth up to eight weeks. The blood from bone marrow and FNA samples were collected aseptically and directly inoculated onto a pair of L-J slants.
Conventional drug susceptibility test: Any suspected growth was confirmed by standard biochemical tests such as nitrate reductase, heat stable catalase and sensitivity to p-nitro benzoic acid (PNB)1314. Drug susceptibility of M. tuberculosis isolates to INH (0.2 μg/ml), RIF (40 μg/ml), ethambutol (EMB, 2 μg/ml) and streptomycin (SM, 4 μg/ml) (Sigma-Aldrich, USA) was performed by following standard one per cent proportion method using L-J medium1315.
DNA extraction from clinical specimens and MTB isolates: DNA used for the PCR analysis was extracted from clinical samples and MTB isolates and purified as described by the method of van Embden et al16. The DNA samples were stored at −20°C.
Allele specific PCR assays for drug resistance detection: These allele-specific PCR assays were performed using specific primers to detect the five most common INH and RIF-associated mutations which target codon 315 of katG gene, inhA promoter region and codon 516, 526 and 531 of the rpoB gene171819.
Nested multiple allele-specific (NMAS) PCR for rpoB516 and katG315 codons: NMAS-PCR assay included preliminary amplification of the larger portion of rpoB, katG with outer primers rpoBOF, rpoBOR, katGOF and katGOR (Table I). Each reaction (25 μl) contained 10 pmol (1 μl) of each primers (GeNei, Bengaluru), 0.2 mM (each) dNTPs (2 μl) (GeNei 1U Taq DNA polymerase (1 μl) (GeNei 10× PCR buffer (2.5 μl) (GeNei) and 5 μl purified DNA from clinical samples. The first round was performed under the following reaction conditions: initial denaturation 96°C for three minutes; 30 cycles of 95°C for one minute, 65°C for one minute and 72°C for 40 sec and final elongation at 72°C for five minutes.
| Oligonucleotide primers for rpoB and katG genes mutation detection | |||||
|---|---|---|---|---|---|
| Genes | Primers | Codons H37Rv sequence/amino acid | Sequence (5’- 3’) | Size (bp) | References (primers) |
| rpoB | rpoBOF | GTCGCCGCGATC AAGGA | 249 | Mokrousov et al, 200318 | |
| rpoBOR | TGACCCGCGCGTACAC | ||||
| katG | katGOF | GCAGATGGGGCTGATCTACG | 435 | Mokrousov et al, 200217 | |
| katGOR | AACGGGTCCGGGATGGTG | ||||
| rpoB | Outer R | TGACCC GCG CGT ACA C | Mokrousov et al, 200318 | ||
| Inner F | 516 GAC/Asp | GCT GAG CCA ATT CAT GGA | 214 | ||
| 526 CAC/His | GTC GGG GTT GAC CCA | 181 | |||
| 531 TCG/Ser | ACA AGC GCC GAC TGT C | 167 | |||
| katG | Outer F | 315 AGC/Ser | GCA GAT GGG GCT GAT CTA CG | Mokrousov et al, 200217 | |
| Inner R | 315*ACC/Thr | ATA CGA CCT CGA TGC CGG | 293 | ||
| Inner R | 315*ACA/Thr | ATA CGA CCT CGA TGCCTG | 293 | ||
| Oligonucleotide primers for mutation detection in mabA-inhA promoter region | |||||
| Genes | Primers | Codons H37Rv sequence/amino acid | Sequence (5’- 3’) | Size (bp) | References (primers) |
| mabA-inhA promoter region | Outer F | ACAAACGTCACGAGCGTAACC | 451 | Leung et al, 200619 | |
| Outer R | GTTGGCGTTGATGACCTTCTC | ||||
| Inner R | -15 C-to-T | TCACCCCGAGAACCTATCG | 119 | ||
*For mutant allele ACC/Thr. F, forward; R, reverse
The NMAS-PCR assay for two codons was performed simultaneously at the same cycling conditions. The reaction mixture for NMAS PCR for rpoB516 and katG315 codon contained 10 pmol (1 μl each) of each allele-specific outer forward and inner reverse primers (Table I), 200 μM concentrations of each of dNTPs (2 μl) 1 U Taq DNA polymerase (1 μl), 10× PCR buffer (2.5 μl), 5 μl of purified DNA from clinical samples and final volume was maintained by molecular grade water. The reaction conditions were the same as the first-round PCR. For wild-type strains, two fragments of 249 bp and 214 bp were amplified, while a single 249 bp fragment was amplified for mutants in the case of rpoB 516 and two fragments of 435 bp and 293 bp were amplified for wild type, while a single 435 bp fragment was amplified for mutants in the case of katG 315 codon.
Nested allele-specific PCR (NAS-PCR) assay for rpoB526 and rpoB531 codons: The NMAS-PCR reaction mixture (final volume, 25 μl) for rpoB526 and rpoB531 comprised of 10 pmol of rpoBOR (1 μl), 10 pmol of one AS inner reverse primer for rpoB526 and rpoB531 (1 μl), 200 μM concentrations of each of dNTPs (2 μl), 10× reaction buffer (2.5 μl), 1 U Taq DNA polymerase (1 μl), 5 μl purified DNA from clinical sample, and final volume was maintained by molecular grade water. The reaction was performed in thermocycler (T-100™-Bio-Rad, USA) under the following reaction conditions: initial denaturation at 96°C for three minutes; five cycles of 95°C for 45 sec, 62°C for 50 sec and 72°C for 20 sec; five cycles of 95°C for 40 sec, 60°C for 50 sec and 72°C for 20 sec; 20 cycles of 94°C for 50 sec, 58°C for 40 sec and 70°C for 20 sec and final elongation at 72°C for three minutes. The amplified fragments (5 μl) were electrophoresed in 1.5 per cent agarose gels and visualized under UV light. For wild-type strains, three fragments of 249 bp, 181 bp and 167 bp were amplified, while a single 249 bp fragment was amplified for mutants in the case of rpoB526 and rpoB531.
Multiple allele-specific (MAS) PCR for mabA-inhA promoter region: MAS-PCR was performed in 25 μl reaction mixture containing 2 μl 200 μM concentrations of each of dNTPs, 1 U Taq DNA polymerase and 10 pmol of each primers designed (Table I) by Leung et al19. The amplification was performed using the following cycling conditions: initial denaturation at 96°C for three minutes; five cycles of 95°C for one minute, 68°C for one minute and 72°C for 30 sec; five cycles of 95°C for one minute, 66°C for 40 sec and 72°C for 30 sec; 20 cycles of 94°C for one minute, 64°C for 40 sec and 72°C for 30 sec and final elongation at 72°C for three minutes. For wild-type strains, two fragments of 451 bp and 119 bp were amplified, while a single 451 bp fragment was amplified for mutants.
DNA sequencing and analysis: To validate point mutations detected by AS-PCR assays and to analyze the samples having discrepancy between the results of drug susceptibility testing (DST) and AS-PCR assays, 15 (5 susceptible and 10 resistant) each of 249 bp rpoB and 435 bp katG pre-amplified fragments were randomly selected for DNA sequence analysis. DNA sequencing was performed by Genome Xcelris, Ahmadabad using Sanger sequencing method. The data obtained were compared with sequences from the NCBI database using the alignment tool (Clustal Omega Multiple Sequence Alignment tool, https://www.ebi.ac.uk/Tools/msa/clustalo/).
Details of methods for all specimens were depicted in Fig. 1.
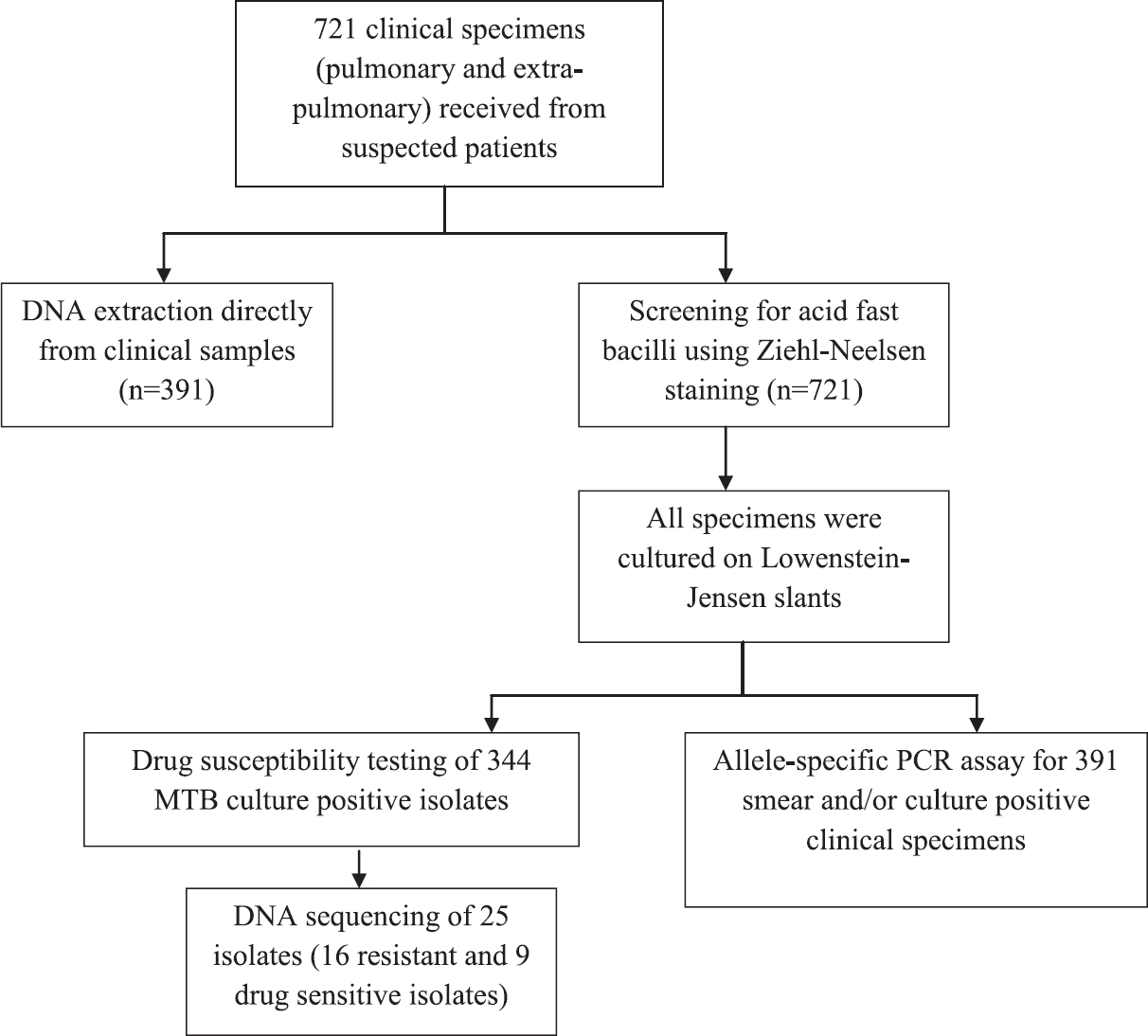
- Flowchart showing available nested multiplex polymerase chain reaction assay, drug susceptibility testing, allele-specific polymerase chain reaction assays and sequencing. MTB, Mycobacterium tuberculosis.
Results
Among the 721 clinical samples, 251 (34.8%) were both smear and culture positive, 93 (12.9%) were smear negative culture positive, 47 (6.5%) were smear positive culture negative and 326 (45.2%) were both smear and culture negative. From 721 specimens, 302 (41.9%) were found to be positive for acid-fast bacilli (AFB) smear, whereas 348 (48.3%) were culture positive for Mycobacteria spp. and 345 (48.7%) were culture negative while 28 (3.9%) were contaminated. Of the 348 culture-positive isolates, 344 (98.8%) were identified as M. tuberculosis while four (1.1%) were identified as non-tubercular mycobacteria.
Drug susceptibility test results by proportion method: Phenotypic DST was performed on 344 M. tuberculosis isolates grown on L-J slants. The results showed that 183 samples contained MDR isolates of M. tuberculosis. One hundred and ninety eight MTB isolates were resistant to RIF (183 MDR and 15 RIF mono-resistant samples), 208 were resistant to INH (183 MDR and 25 INH mono-resistant samples) and 101 were susceptible to both INH and RIF.
Results of AS PCR assays: A total of 391 microbiologically confirmed (251 Acid fast bacilli smear positive and MTB culture positive; 93 smear negative and MTB culture positive; 47 smear positive and MTB culture negative) clinical samples were tested by AS PCR assays. In addition, a total of 344 MTB culture positive isolates were also subjected to AS PCR assays to detect RIF and INH resistance. The three genes, katG, inhA and rpoB, in the 391 microbiologically confirmed clinical specimens and in the 344 MTB isolates were amplified using AS PCR assays (NMAS PCR, MAS PCR and NAS PCR).
Results of AS PCR of microbiologically confirmed clinical specimens (n=391): Of the 391 clinical specimens, 246 [62.9%; 199 MDR, 29 non-MDR INH resistant (INHr) and 18 non-MDR RIFr] harboured INHr and/or RIFr bacilli. The remaining 145 (37.1%) clinical specimens harboured bacilli susceptible to INH and RIF drugs. Distinct-banding patterns were obtained for different mutation profiles at the five targeted loci (Figs 2 and 3).
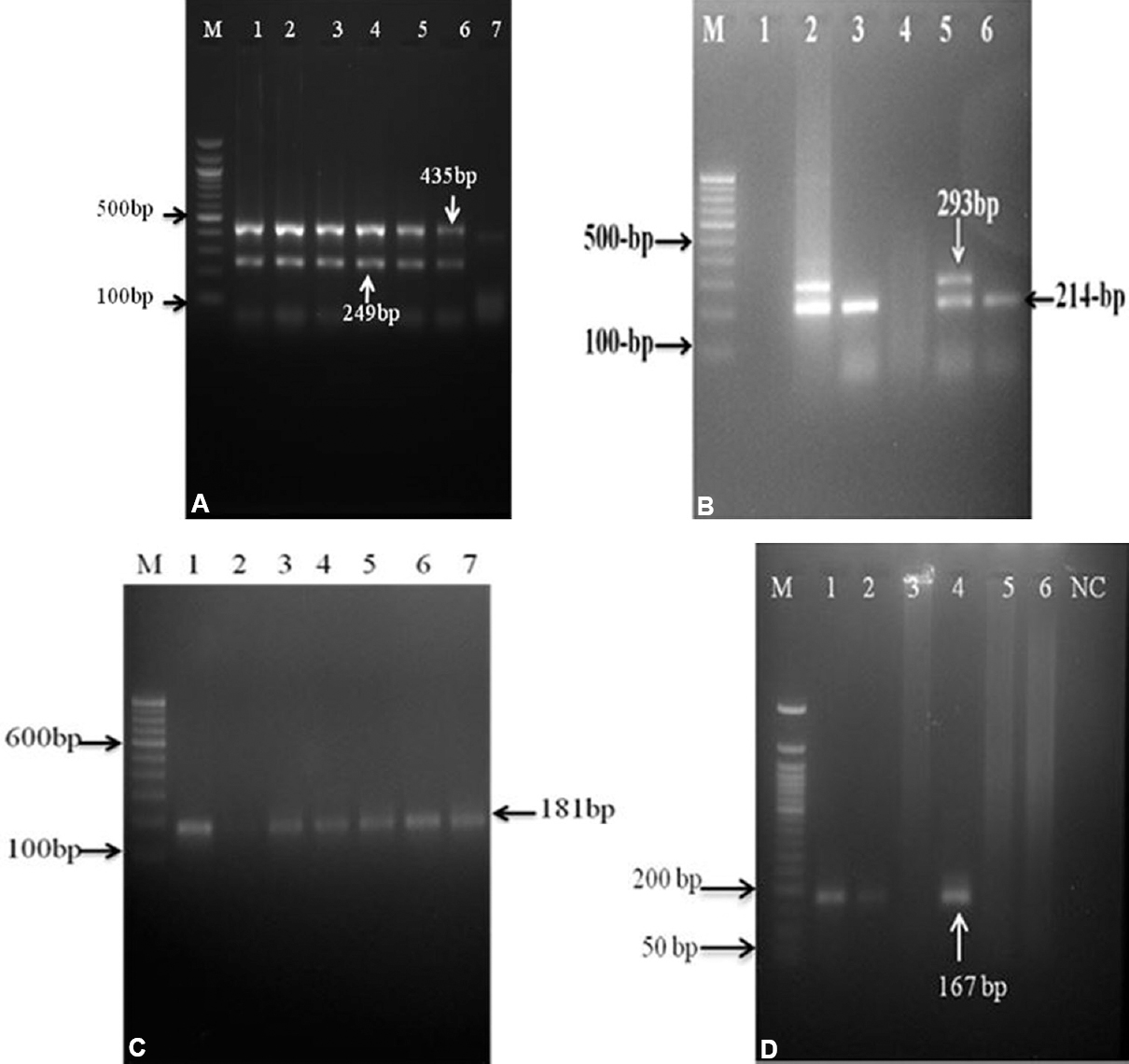
- Gel images of allele-specific PCR assays with clinical specimens DNA preparation: (A) First step nested multiple allele-specific PCR with outer rpoB and katG derived primers. M- 100 bp ladder; lane 1- H37Rv strain; lanes 2 to 6- strains with outer region of codon rpoB and katG genes; lane 7- negative control. (B) Analysis of rpoB516 codon and katG315 codon by nested multiple allele-specific PCR assay. Lane 1- negative control ; lane 2- H37Rv strain; lane 3- isolate with katG315 mutant allele (AGC-ACC); lane 4 - isolate with rpoB516 mutant allele (GAC-GTC, TAC and GGC) and katG315 mutant allele (AGC-ACC); lane 5- isolate with rpoB516 wild-type allele and katG315 wild-type allele; lane 6- isolate with rpoB516 wild-type allele and katG315 mutant allele (AGC-ACA); M- 100 bp DNA ladder; (C) Analysis of rpoB526 codon by nested allele-specific-PCR assay. Lane 1- H37Rv strain; lanes 2 to 7- isolates with rpoB526 wild-type allele; lane 3- isolate with rpoB526 mutant allele (CAC-GAC, TAC and CTC); (D) Analysis of rpoB531 codon by nested allele-specific PCR assay. M- 50 bp DNA ladder; lane1- H37Rv strain; lanes 2 and 4- isolates with rpoB531 wild-type allele; lanes 3, 5 and 6- isolates with rpoB531 mutant allele (TAC-TCG and); NC, negative control.
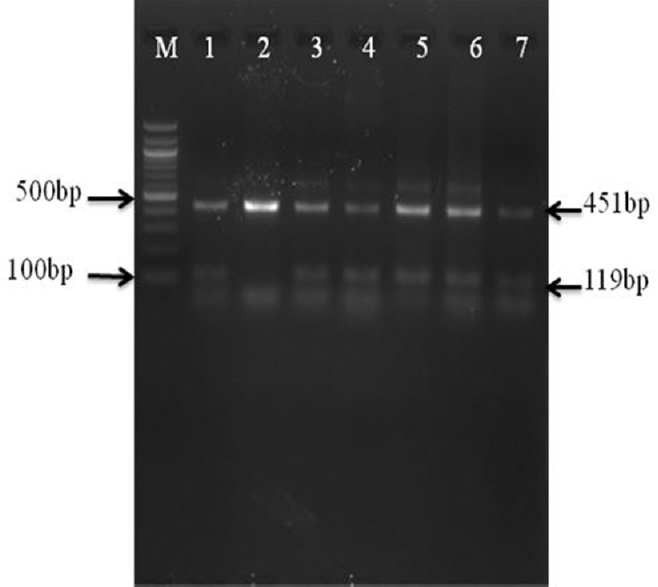
- Gel image of mabA single step multiple allele-specific PCR assay with clinical specimens DNA preparation: Analysis of mabA-inhA promoter region by multiple allele-specific PCR assay M-100 bp DNA ladder; lane 1- H37Rv strain; lane 2- isolate with a-15 C-T substitution in the promoter region of the inhA gene; lanes 3-7- isolates with the mabA-15 wild-type allele.
Mutations in rpoB gene: Of the 391 microbiologically confirmed samples, 154 (39.4%) harboured RIF-resistant bacilli had a single nucleotide alteration in codon 531, resulting in the amino acid substitution of Ser to Leu (S531L). The second most affected codons were 516, resulting in the amino acid substitution of Asp to Val (D516V) and His to Asp (H526D) at 526 codon, which were found in 16 (4.1%) and 47 (12.0%) samples, respectively (Table II and Fig. 2). It included nine (2.3%) and 16 (4.1%) isolates harboured double mutations in two separate codons, i.e. codons 516 and 526 and codons 526 and 531, respectively. One hundred and seventy four (44.5%) specimens containing bacilli had no mutations in the target codons of rpoB gene.
| Drugs | DNA target | Nucleotide change | Amino acid change | Number of isolates with mutation (n=391) (%) |
|---|---|---|---|---|
| RIF (n=217) | rpoB516* | GAC→GTC | Asp→Val | 16 (4.1) |
| rpoB526* | CAC→GAC | His→Asp | 47 (12.0) | |
| rpoB531* | TCG→TTG | Ser→Leu | 154 (39.4) | |
| rpoB516 and rpoB526 | GAC→GTC and CAC→GAC | Asp→Val and His→Asp | 9 (2.3) | |
| rpoB526 and rpoB531 | CAC→GAC and TCG→TTG | His→Asp and Ser→Leu | 16 (4.1) | |
| $Wild type | 174 (44.5) | |||
| Isoniazid (n=228) | katG 315* | AGC→ACC | Ser→Thr | 180 (46.0) |
| katG 315* | AGC→AAC | Ser→Asn | 18 (4.6) | |
| inhA-15* | C→T | NA | 30 (7.7) | |
| katG315 and inhA-15 | AGC→ACC and C→T | Ser→Thr NA | 5 (1.3) | |
| Wild type$ | 163 (41.7) |
*Including double and triple mutations; $There were no mutations in katG 315 codon (Ser315Thr and Ser315Leu) and inhA promoter. NA, not applicable; RIF, rifampicin
Mutations in katG encoding region and inhA promoter region: According to the AS PCR assays results, 180 (46.0%) of the 391 samples harboured INHr bacilli, had mutations in katG gene at codon 315 (Ser315Thr) and 18 (4.6%) had Ser-Asn (Ser315Asn) (Table II and Fig. 3). Mutations in the inhA promoter region were observed in 30 (7.7%) MTB isolates, which had a mutation at position -15 in the inhA promoter region (Table II and Fig. 2). Among those with mutation in inhA promoter, five had additional mutation in katG315 (Ser315Thr). One hundred and sixty three (41.7%) specimens had no mutations in the target codons studied.
Results of AS PCR of culture isolates (n=344)
Analysis of rpoB gene for mutations in rifampicin (RIF)-resistant MTB isolates: Mutations in the RRDR of the rpoB gene were identified using NAS PCR assay and NMAS PCR assay in 193 (97.5%) of the 198 RIFr isolates. A single nucleotide alteration in codon 531, resulting in the amino acid substitution of amino acid substitution of Ser to Leu (Ser531Leu), was most prevalent and observed in 142 isolates (71.7%). The second most affected codons were 516 (Asp516Val) and 526 (His526Asp), which were found in 15 (7.6%) and 36 (18.2%) isolates of M. tuberculosis, respectively (Table III). Four (2.0%) and six (3.0%) isolates carried double mutations in two separate codons, i.e. codons 516 and 526 and codons 526 and 531, respectively. No mutations were detected in the remaining five (2.5%) RIFr isolates.
| Drugs | DNA target | Nucleotide change | Amino acid change | RIFr/INHr (%) | RIFs/INHs (%) |
|---|---|---|---|---|---|
| RIF (n=198) | rpoB516* | GAC→GTC | Asp→Val | 15 (5.5) | 0 |
| rpoB526* | CAC→GAC | His→Asp | 36 (15.1) | 1 | |
| rpoB531* | TCG→TTG | Ser→Leu | 142 (71.7) | 2 | |
| rpoB516 and rpoB-526 | GAC→GTC and CAC→GAC | Asp→Val and His→Asp | 4 (2.0) | 0 | |
| rpoB526 and rpoB531 | CAC→GAC and TCG→TTG | His→Asp and Ser→Leu | 6 (3.0) | 0 | |
| Wild type$ | 5 (2.5) | 143 | |||
| Isoniazid (n=208) | katG 315* | AGC→ACC | Ser→Thr | 169 (81.2) | 2 |
| katG 315* | AGC→ACG | Ser→Asn | 8 (3.8) | 0 | |
| inhA-15* | C→T | NA | 28 (13.5) | 1 | |
| katG315 and inhA-15 | AGC→ACC and C→T | Ser→Thr and NA | 5 (2.4) | 0 | |
| Wild type$ | 3 (1.4) | 133 |
*Including double and triple mutations, $There were no mutations in katG 315 codon (Ser315Thr and Ser315Leu) and inhA promoter. NA, not applicable; RIF, rifampicin; INH, isoniazid
One hundred and twenty three of the 146 phenotypically RIFs isolates showed the expected wild-type AS patterns while three of the 146 non- RIFr isolates showed a mutant pattern for the Ser531Leu (n=2) and His526Asp (n=1).
Analysis of mutations in katG encoding region and inhA promoter region: Among 208 phenotypically INHr isolates, 177 (85.1%) had katG mutations, the vast majority (169; 81.2%) of which was the commonly described substitution katG (Ser315Thr) (Table III). Eight isolates had Ser to Asn (Ser315Asn) substitution at katG position 315. Mutations in the inhA promoter region were observed in 28 (13.5%) INHr isolates, which had a mutation at position -15 in the inhA promoter region. Among those with mutation in inhA promoter, five had additional mutation in katG315. Three INHr (1.4%) isolates had no mutation at katG codon 315 and mabA-inhA C-15 position.
One hundred and thirteen of the 136 phenotypically INHs isolates showed the expected wild-type AS patterns while three of the 136 non-INH showed a mutant pattern for the katGSer315Asn and -15 position of the mabA-inhA promoter region.
DNA sequence analysis: Results of DNA sequencing showed that all nine INH, RIF-susceptible and eight of 16 INH, RIFr isolates produced same results as compared to phenotypic method and AS-PCR assays. Another three isolates that were detected INHr by phenotypic method and susceptible by AS-PCR showed no mutation in 435 bp katG region targeting codon 315. These INHr isolates had mutations at katG Gly299Ser and katG Gln295Ser codons. Another five RIFr isolates (susceptible by AS PCR) had mutation in rpoB 522 (TCG →TGG, Ser→Trp), 511 (TCG→TGG, Ser→Trp) and 533 codon (CTG→CCG, Leu→Pro).
Sensitivity and specificity: The sensitivity and specificity of the NAS-PCR, NMAS-PCR and MAS-PCR for detecting RIF and INH resistance were assessed using conventional DST results as a reference. When compared to DST, the sensitivity and specificity of AS PCR assay for INH resistance were determined to be 98.6 and 97.8 per cent, for RIF resistance were determined to be 97.5 and 97.9 per cent and for MDR were determined to be 98.9 and 100 per cent, respectively (Table IV).
| NAS PCR | DST results | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Number of resistant | Number of susceptible | |||||
| INH resistance | ||||||
| Detected | 205 | 3 | 98.6 | 97.8 | 98.6 | 97.8 |
| Not detected | 3 | 133 | ||||
| RIF resistance | ||||||
| Detected | 193 | 3 | 97.5 | 97.9 | 98.5 | 96.6 |
| Not detected | 5 | 143 | ||||
| MDR mutations | ||||||
| Detected | 181 | 0 | 98.9 | 100 | 100 | 98.7 |
| Not detected | 2 | 161 | ||||
DST, drug susceptibility testing; NAS, nested allele-specific; PPV, positive predictive value; NPV, negative predictive value
Discussion
Anti-TB drug resistance causes a significant risk to human health, which usually develops due to chromosomal mutations in drug targets in M. tuberculosis genes7. The AS PCR assays offer a rapid, sensitive and cost-effective molecular techniques to accurately screen the most common mutations associated with resistance to RIF and INH. These provide the multiple quality assurance to control for false-negative results due to lack of amplification and especially useful for direct analysis of human samples. Due to PCR inhibitors, the quality and quantity of DNA from clinical specimens may be poor, and the first step PCR is necessary to generate a sufficient template for subsequent AS PCR assay20.
Resistance mutations observed in a majority of RIFr isolates (>95%) were due to small deletions or insertions within an 81-base pair RRDR of the rpoB gene, between codons 507 and 5339 and that most of these mutations occurred exclusively at codons 516, 526 and 531. In this study, mutations in rpoB region were found in 97.5 per cent of RIFr isolates using AS PCR assays. The most frequently mutated codon in our study was codon 531 followed by mutations at codons 526 and 516, which was similar to those reported in clinical isolates from India212223. Panama24, China25, Pakistan26, Nepal4 and other geographical regions171819. Café Oliveira et al5 from Brazil showed 62.8 and, 7 per cent mutations at codons 531 and 526, respectively. Five phenotypically RIFr isolates have no mutations in studied rpoB codons using AS PCR assays, similar to those reported previously45. This indicated that most of the RIFr isolates could be rapidly found by screening of the most common genetic mutations in RRDR region of rpoB gene, even though it was necessary to detect the presence of isolates lacking mutations at studied codons.
Earlier studies designated that INH resistance was mediated by mutations in several genes, most commonly katG, particularly in codon 315, and the promoter region of inhA4672930. Accordingly, we found that 85.1 per cent of phenotypically INHr clinical isolates had point mutations in katG gene, and the frequencies were similar to those reported by other researchers4. Similarly, 77.6, 71.4, 79, 55.8 and 41.9 per cent of INHr isolates in India6, South Africa31, Pakistan22, China32 and Brazil5, respectively, have been reported for harbouring mutation at the same genetic location. In the present study, 28 isolates had C to T transition at point -15 for inhA promoter region reported to be associated with INH resistance as described by Poudel et al4, and mutation frequency in inhA was also comparable with other studies showing frequencies varying from 10 to 34 per cent59262933. The three phenotypically INHr isolates had no mutations in katG 315 codon and inhA promoter region and confirmed that resistance in these isolates could be due to mutations present in other codons of katG gene430.
Three phenotypically RIF susceptible isolates have mutations at rpoB526 (n=1) and rpoB531 (n=2) codons. Similarly, three phenotypically INH susceptible isolates have mutations at katG315 codon (n=2) and at point -15 inhA promoter region. It could be possible that these isolates comprised a heterogeneous population of organisms with both wild-type and mutated alleles in the rpoB, katG and inhA promoter region encoding gene, leading to amplification of the corresponding mutated alleles PCR product.
Thirumurugan et al34 have detected the RIF resistance in 127 MTB clinical isolates in Puducherry, South India. In this study, distinct PCR banding patterns were observed for different mutation profiles, and the correlation between MAS-PCR results and phenotypic drug susceptibility test was 96.7 per cent34. The major obstructions for utilization of MAS-PCR method in case of clinical samples is the presence of lower amount of mycobacterial DNA in specimens. According to Chia et al24, MAS-PCR method required as high as 20 ng of template DNA per 25 μl of reaction mixture for successful amplification, whereas Vadwai et al35 could not utilize single-tube MAS-PCR assay when amount of DNA was low resulting in amplification failure due to increased competition among primers. To solve this problem, in this study, nested AS-PCR assay was used in which the rationale of first-round PCR was to increase the amount of template DNA for second-round PCR from clinical specimens20.
The NAS-PCR assay demonstrates multiple quality assurance to check false-negative results due to lack of amplification. This assay is predominantly helpful for direct analysis of human samples, and a wild-type strain (H37Rv) included in each run as a positive control for amplification of the allele-specific fragment. Thus, the absence of a wild-type AS fragment in the tested strain is considered to indicate the presence of mutation and hence a drug-resistant phenotype.
A major limitation to molecular genetic detection of drug resistance by any technique is that such tests generally only detect known mutations. The sensitivity and specificity of a molecular detection assay may vary when being used in different geographic regions, depending on the presence of genetic mutations targeted by a molecular detection assay in the drug-resistant M. tuberculosis isolates. Though the molecular methods cannot completely replace culture-based method, but will allow more rapid and decentralized detection of drug resistance and may successfully complement conventional methods.
AS-PCR assay might be a practical and relatively cost-effective molecular method for rapid detection of considerable proportion of RIFr, INHr and MDR-TB directly from clinically confirmed samples in India and other developing countries with resource-poor settings.
Financial support & sponsorship: This study was financially supported by Department of Science and Technology, New Delhi (Grant no. DST/INSPIRE Fellowship/2011). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of Interest: None.
References
- Global Tuberculosis Report 2018. Geneva: WHO; 2018.
- WHO group 5 drugs and difficult multidrug-resistant tuberculosis: A systematic review with cohort analysis and meta-analysis. Antimicrob Agents Chemother. 2013;57:4097-104.
- [Google Scholar]
- WHO treatment guidelines for drug-resistant tuberculosis: 2016 update. Available from: https://apps.who.int/iris/bitstream/handle/10665/250125/97 89241549639-webannexes-eng.pdf
- Molecular character ization o f multidrug-resistant Mycobacterium tuberculosis isolated in Nepal. Antimicrob Agents Chemother. 2012;56:2831-6.
- [Google Scholar]
- Detection of multidrug-resistant Mycobacterium tuberculosis strains isolated in Brazil using a multimarker genetic assay for katG and rpoB genes. Braz J Infect Dis. 2016;20:166-72.
- [Google Scholar]
- Early detection of multidrug resistant (MDR) Mycobacterium tuberculosis in a single tube with in-house designed fluorescence resonance energy transfer (FRET) probes using real-time PCR. Indian J Exp Biol. 2016;54:229-36.
- [Google Scholar]
- Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis. 1998;79:3-29.
- [Google Scholar]
- Mechanisms of action and resistance of antimycobacterial agents. In: Mayers DL, ed. Antimicrobial drug resistance, infectious disease. New York: Humana Press; 2009. p. :271-91.
- [Google Scholar]
- Mutation profiling for detection of isoniazid resistance in Mycobacterium tuberculosis clinical isolates. J Antimicrob Chemother. 2015;70:3214-21.
- [Google Scholar]
- Manual for laboratory technician. New Delhi: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare; Available from: https://ntiindia.kar.nic.in/cddistrictlevel/Ielearn/CATEGORY/RNTCP%20Modules/LABMANUAL.pdf
- A new and rapid method for the isolation and cultivation of tubercle bacilli directly from the sputum and feces. J Exp Med. 1915;21:38-42.
- [Google Scholar]
- Public health mycobacteriology. In: A guide for the level III laboratory. Atlanta: Centers for Disease Control; 1985. p. :21-44.
- [Google Scholar]
- Mycobacteria: Laboratory methods for testing drug sensitivity and resistance. Bull World Health Organ. 1963;29:565-78.
- [Google Scholar]
- Differential identification of mycobacteria. VII. Key features for identification of clinically significant mycobacteria. Am Rev Respir Dis. 1973;107:9-21.
- [Google Scholar]
- Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41:21-43.
- [Google Scholar]
- Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: Recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406-9.
- [Google Scholar]
- Detection of isoniazid-resistant Mycobacterium tuberculosis strains by a multiplex allele-specific PCR assay targeting katG codon 315 variation. J Clin Microbiol. 2002;40:2509-12.
- [Google Scholar]
- Allele-specific rpoB PCR assays for detection of rifampin-resistant Mycobacterium tuberculosis in sputum smears. Antimicrob Agents Chemother. 2003;47:2231-5.
- [Google Scholar]
- Molecular characterization of isoniazid resistance in Mycobacterium tuberculosis: Identification of a novel mutation in inhA. Antimicrob Agents Chemother. 2006;50:1075-8.
- [Google Scholar]
- Evaluation of efficiency of nested multiplex allele-specific PCR assay for detection of multidrug resistant tuberculosis directly from sputum samples. Lett Appl Microbiol. 2016;62:411-8.
- [Google Scholar]
- Molecular characterisation of drug resistance in Mycobacterium tuberculosis isolates from North India. Int J Tuberc Lung Dis. 2013;17:251-7.
- [Google Scholar]
- Characterization of katG and rpoB gene mutations in multi drug resistant Mycobacterium tuberculosis clinical isolates. Int J Curr Microbiol Appl Sci. 2014;3:1072-80.
- [Google Scholar]
- Detection of multi-drug resistance & characterization of mutations in Mycobacterium tuberculosis isolates from North- Eastern states of India using GenoType MTBDRplus assay. Indian J Med Res. 2014;140:501-6.
- [Google Scholar]
- Use of multiplex allele-specific polymerase chain reaction (MAS-PCR) to detect multidrug-resistant tuberculosis in Panama. PLoS One. 2012;7:e40456.
- [Google Scholar]
- Molecular characterization of multidrug-resistant Mycobacterium tuberculosis isolates from China. Antimicrob Agents Chemother. 2014;58:1997-2005.
- [Google Scholar]
- Molecular characterization of multidrug-resistant isolates of Mycobacterium tuberculosis from patients in Punjab, Pakistan. Pak J Zool. 2013;45:93-100.
- [Google Scholar]
- Detection of isoniazid and rifampin resistance of Mycobacterium tuberculosis by a multiplex allele-specific polymerase chain reaction (PCR) assay. Int J Mycobacteriol. 2012;1:34-9.
- [Google Scholar]
- Extensive transmission of isoniazid resistant M. tuberculosis and its association with increased multidrug-resistant TB in two rural counties of Eastern China: A molecular epidemiological study. BMC Infect Dis. 2010;10:43.
- [Google Scholar]
- Molecular characterization of INH-resistant Mycobacterium tuberculosis isolates by PCR-RFLP and multiplex-PCR in North India. Infect Genet Evol. 2009;9:1352-5.
- [Google Scholar]
- Detection of mutations associated with isoniazid resistance in multidrug-resistant Mycobacterium tuberculosis clinical isolates. J Antimicrob Chemother. 2014;69:2369-75.
- [Google Scholar]
- Molecular detection and characterization of resistant genes in Mycobacterium tuberculosis complex from DNA isolated from tuberculosis patients in the Eastern Cape province South Africa. BMC Infect Dis. 2014;14:479.
- [Google Scholar]
- Molecular characterization of multidrug- and extensively drug-resistant Mycobacterium tuberculosis strains in Jiangxi, China. J Clin Microbiol. 2012;50:2404-13.
- [Google Scholar]
- Minor contribution of inhA-15 mutations to the rapid detection of isoniazid resistance in Mycobacterium tuberculosis isolates. Iran J Med Sci. 2016;41:161-3.
- [Google Scholar]
- Molecular analysis of rpoB gene mutations in rifampicin resistant Mycobacterium tuberculosis isolates by multiple allele specific polymerase chain reaction in Puducherry, South India. J Infect Public Health. 2015;8:619-25.
- [Google Scholar]
- Multiplex allele specific PCR for rapid detection of extensively drug resistant tuberculosis. Tuberculosis (Edinb). 2012;92:236-42.
- [Google Scholar]






