Translate this page into:
Quantitative & qualitative analysis of endothelial cells of donor cornea before & after penetrating keratoplasty in different pathological conditions
Reprint requests: Dr Aruna Kumari R. Gupta, Department of Ophthalmology, C.U. Shah Medical College & Hospital, Dhudrej Road, Surendranagar 363 001, Gujarat, India e-mail: arunagupta.eye@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Endothelial cells of the donor cornea are known to be affected quantitatively and qualitatively in different pathological conditions after penetrating keratoplasty (PK) and this has direct effect on the clarity of vision obtained after PK. This study was undertaken to analyze the qualitative and quantitative changes in donor endothelial cells before and after PK in different pathological conditions.
Methods:
A prospective investigational analysis of 100 consecutive donor corneas used for penetrating keratoplasty between June 2006 and June 2008, was conducted. The patients were evaluated on the first day, at the end of first week, first month, third and six months and one year.
Results:
A decrease was observed in endothelial cell count in all pathological conditions. After one year of follow up the loss was 33.1 per cent in corneal opacity, 45.9 per cent in acute infective keratitis (AIK), 58.5 per cent in regrafts, 28.5 per cent in pseudophakic bullous keratopathy (PBK), 37 per cent in descemetocele, 27 per cent in keratoconus and 35.5 per cent in aphakic bullous keratopathy (ABK) cases.
Interpretation & conclusions:
The endothelial cell loss was highest in regraft cases which was significant (P<0.05), while the least endothelial cell loss was seen in keratoconus cases. The cell loss was associated with increase in coefficient of variation (CV), i.e. polymegathism and pleomorphism. Inspite of this polymegathism and pleomorphism, the clarity of the graft was maintained.
Keywords
Coefficient of variation
endothelial cell loss
eye bank
keratoanalyser
pathological conditions
penetrating keratoplasty
pleomorphism
polymegathism
specular microscope
Penetrating keratoplasty (PK) is a full-thickness corneal transplantation. The primary goal after corneal transplantation is preservation of a clear graft which is maintained with the help of corneal endothelium1. PK can visually rehabilitate many of those who suffer from visual impairment due to corneal diseases. After successful PK, the transplanted corneal endothelial cells remain viable as a true chimera for years. Corneal endothelial cell density is a commonly reported indicator of the outcome for corneal grafts2. Endothelial structure in corneal transplants cannot be examined by the usual histological methods but clinical specular microscope can examine, photograph and quantitatively evaluate the corneal endothelium in vivo without disturbing the cornea. Qualitative morphometric analysis of specular images provides a rapid clinical evaluation of the endothelium. Qualitative cellular analysis identifies abnormal endothelial structures and grades the endothelium either according to the number or size of the abnormal structures present or on the basis of an overall visual assessment of endothelial appearance. Quantitative morphological parameters are cell size (cell area or cell density), pleomorphism per cent of hexagonal cells and polymegathism (coefficient of variation- CV)3. Studies have shown that the prognosis of PK is dependent on the pathology responsible for causing corneal blindness4567.
In our study, the preoperative morphometric analysis of endothelial cell of donor cornea was done by an eyebank keratoanalyser before PK and subsequently followed up by specular microscope in recipients for various distinct pathological conditions. As similar studies are not well documented using this methodology, the purpose of this study was to report the qualitative and quantitative changes in donor endothelial cells before and after PK in different pathological conditions.
Material & Methods
In this prospective study 100 consecutive donor corneas procured by Sant Punit Chakshu Bank, Navsari, Gujarat, and used for penetrating keratoplasty in Rotary Eye Institute, Navsari, Gujarat, between June 2006 and June 2008, were included to analyze the endothelial cell density of the donor cornea before and after penetrating keratoplasty. Enucleation of the eye was done after noting the details such as age, gender, cause of death, history of surgery done on the eye and past history of any ocular or systemic disease. The whole globe was subjected to gross examination and slit lamp biomicroscopy for grading as per established guideline8910. The tissue blood samples were screened for human immunodeficiency virus, hepatitis B, hepatitis C and syphilis. When found suitable for keratoplasty, the sclero-corneal rim was preserved under strict aseptic condition, appropriately labelled and stored in Mc Carey-Kaufman (M-K) medium at 4°C (Ramayamma International Eye Bank, Hyderabad, India). Endothelial cell count and morphological analysis of donor cornea were done using non-contact eye bank specular microscope (Konan Keratoanalyser EKA-98 Konan, Japan)1112. The morphology of endothelial cells was observed and presence of any pathology such as guttate, folds, snail tracks, etc. were looked for at the same time. One hundred cells were selected and marked.
Inclusion criteria for donor cornea were grade ‘excellent’, ‘very good’, and ‘good’ by slit lamp examination and those with endothelial cells >2000 cells/mm2 on eye bank keratoanalyser. Exclusion criteria included donor cornea of grade ‘fair’ and ‘poor’ on slit lamp examination, cornea with endothelial cells <2000 cells/mm2 on eye bank keratoanalyser, donor tissue removed more than six hours after death and viable storage period of corneo-scleral button more than three days.
Pre-operative assessment of recipients included details of patient, chief complaints, presence of any predisposing factors such as ocular surface disorders, trauma, contact lens use, systemic history, past history of ocular surgery and graft infection. Clinical examination included uncorrected visual acuity, best corrected visual acuity (International Statistical Classification of Diseases and Related Health Problems, WHO 1992)13 cycloplegic refraction with cyclopentolate 1 per cent or tropicamide 0.8 per cent and phenylephrine 5.0 per cent (not done in infective keratitis cases), slit lamp biomicroscopy to determine any ocular pathology, applanation tonometry (not done in infective keratitis cases), dilated fundus examination to rule out posterior segment pathology and Sac syringing. Investigations included tear film status and gonioscopy. Ultrasonography of the posterior segment was performed to rule out vitreous exudation suggestive of endophthalmitis. Specular microscopy if possible was done in cases of PBK and ABK (pseudophakic and aphakic bullous keratopathy) preoperatively and was used to study the postoperative endothelial cell count in all cases using non-contact specular microscope (Topcon SP-2000P, Topcon, Japan)111214.
The preoperative counselling of 100 patients (97 adults, 3 children) was done and informed written consent was obtained from all patients. The study protocol was approved by the institutional ethics committee. All PKs were performed under peribulbar anaesthesia (xylocaine 2.0% and bupivacaine 0.5%) except in children where general anaesthesia was used. An anterior vitrectomy was performed in a few cases when required. All cases received amikacin (25 mg), cefazolin (100 mg) and dexamethasone (4 mg) subconjunctivally at the end of the operation. In cases of infective keratitis dexamethasone was not given.
Postoperatively, the eyes were patched and corticosteroids were not administered topically until the epithelium was intact over the transplant. Topical antibiotic and steroid combination drops were given as required. The patients who completed one year of follow up and the graft remained clear were included in the study. The patients were evaluated on the first day, the first week and then on the first, third and sixth month and one year postoperatively in the same manner as in the preoperative assessment. The results were statistically analyzed using paired and unpaired t tests.
Results
The mean age of the donors (n=100) was 46.55± 13.85 (range 10 to 83 yr). Male donors were 62 per cent (n=62) and female donors 38 per cent (n=38). The mean age of recipients was 43.04 ± 17.67 (range 6 to 78 yr). The male recipients were 60 per cent (n=60) and female recipients were 40 per cent (n=40).
The medical records were reviewed for indications for keratoplasty. The indications were corneal opacity (43%), acute infective keratitis (AIK) (25%), PBK (14%), regrafts (7%), descemetocele (5%), ABK (3%) and keratoconus (3%). A decrease in endothelial cell count was observed in all pathological conditions. After one year of follow up the loss was 33.1 per cent in corneal opacity, 45.9 per cent in AIK, 58.5 per cent in regrafts, 28.5 per cent in PBK, 35.5 per cent in ABK cases, 37 per cent in descemetocele and 27 per cent in keratoconus. Count showed maximum decrease in regraft cases from 2762.2 to 1120.5 cells/mm2; and the minimum decrease in keratoconus cases from 2942.7 to 2218 cells/mm2 (Table I).
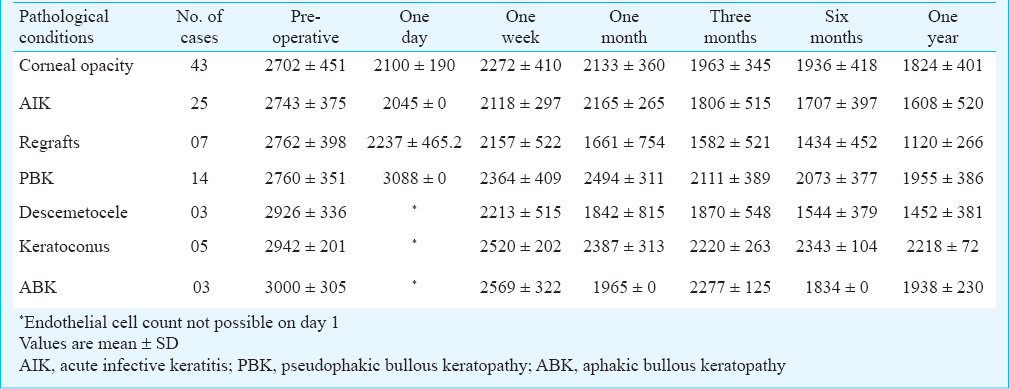
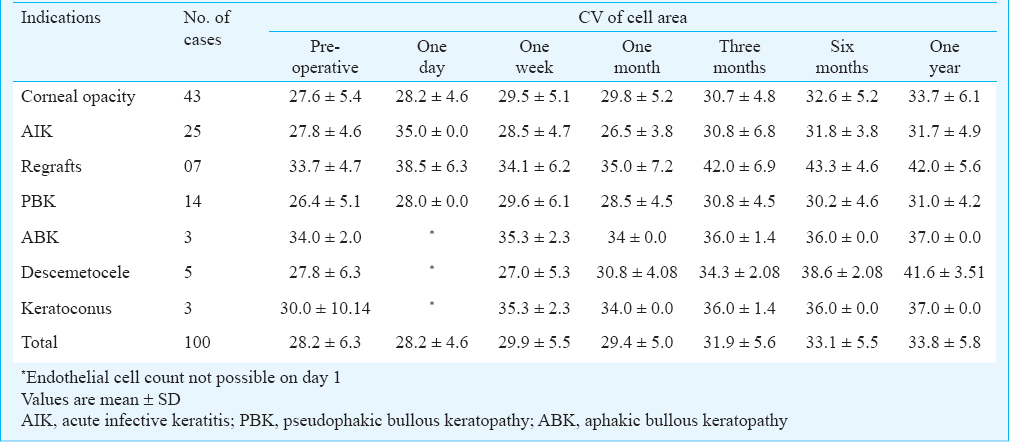
At one year the coefficient of variation (CV) was found to increase in all the cases from average preoperative CV of 28.2±6.3 to 33.8±5.8. The maximum increase in CV after one year was recorded in descemetocele cases, followed by regraft cases and keratoconus while the least increase in CV was recorded in ABK and AIK cases. There was an increase of CV in corneal opacity cases and PBK patients (Table II).
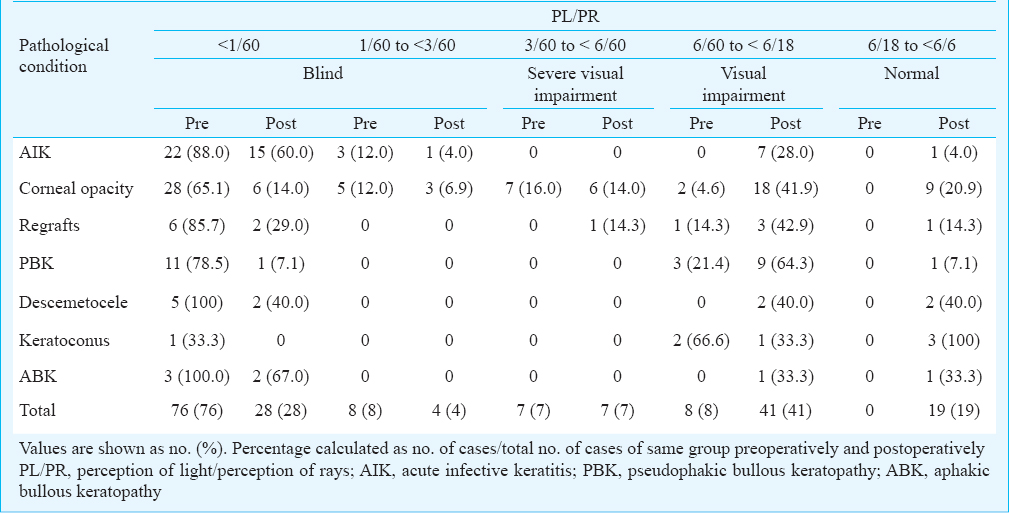
Improvement in visual acuity was observed to “Normal” from 0 per cent cases preoperatively to 19 per cent postoperatively. The “Blind” category patients decreased significantly from 84 per cent preoperatively to 32 per cent postoperatively, from 8 per cent cases in visually impaired group preoperatively to 41 per cent after PK. The cases of worse categories preoperatively improved significantly to better categories postoperatively. As shown in Table III patients having normal VA increased from 0 per cent cases preoperatively, in all categories to 20.9 per cent in corneal opacity, 14.3 per cent in regrafts, 7.1 per cent in PBK, 40 per cent in descemetocele, 100 per cent in keratoconus, 33.3 per cent in ABK, and 4 per cent in AIK after one year. The gain in VA was more in cases of optical (keratoconus, PBK, corneal opacity and regrafts, ABK) PK as compared to therapeutic (AIK) PK. The beneficial effect of PK on VA was observed by the percentage of cases who improved from the blind category to other better categories in each pathological condition. The AIK patients improved 36 per cent, corneal opacity cases improved 56.2 per cent, regraft patients 56.7 per cent, in descemetocele cases 60 per cent, in keratoconus 33.3 per cent improved, in PBK there were 78.5 per cent cases who improved and in ABK the improvement was 33 per cent. The improvement in VA was seen more in optical PK, as compared to therapeutic PK patients indicating that visual gain was less if PK was performed in inflamed eyes.
Discussion
Endothelial cell loss after PK is an ongoing process. The mean annual rate of endothelial cell loss during the first three to five years after PK (7.8%/year) is higher than the physiological endothelial cell loss (0.52%/year)1516. Despite a small degree of continuous cell loss, corneal grafts have a favourable prognosis for long-term clinical stability. This “idiopathic” cell loss after PK is postulated to result from a subclinical immunological graft reaction17. Recent advances in corneal graft technology, including donor tissue retrieval, storage and surgical techniques, have greatly improved the clinical outcome of corneal grafts. Despite these advances, immune mediated corneal graft rejection remains the single most important cause of corneal graft failure17. Several factors like more than two quadrant vascularisation, herpes simplex keratitis; uveitis; silicone oil keratopathy; failed grafts; “hot eyes”; young recipient age; large grafts and multiple surgical procedures at the time of grafting have been identified as “high risk” cases18.
Various mechanisms have been discussed as possible explanations for the chronic endothelial cell loss after PK. Contributing factors may include discrete inflammation of the anterior segment of the eye, surgical trauma, cell exchange between donor and recipient19 cell ageing, acute immune reactions20 and chronic subclinical immune reaction212223. Studies have shown that the prognosis of PK is dependent on the pathology responsible for causing corneal blindness or visual impairment4567.
In our study, the loss of endothelial cell density was analyzed in different pathological conditions of corneal opacity, active infectious keratitis, regraft, pseudophakic bullous keratopathy, aphakic bullous keratopathy, descemetocele and keratoconus. After one year the endothelial cell loss was highest in regraft cases. It was found to be significantly more (P<0.05) than in cases of corneal opacity, PBK, ABK, and keratoconus. Bertelmann et al24 found that endothelial cell loss was lower in keratoconus patients than in patients with bullous keratopathy, but these differences were not significant. This was similar to our finding. Kim et al25 reported the lowest rate of endothelial cell loss in keratoconus similar to our study. The reason for low cell loss may be due to lesser cell migration from donor grafted cornea to the peripheral host cornea which has healthy endothelium25. Similar to our study another study revealed cumulative endothelial cell density (ECD) loss rates of 14.90 per cent at the first year26.
Price et al27 evaluated the relationship between preoperative diagnosis and postoperative endothelial cell morphology and concluded that the preoperative diagnosis seemed to be one of the major determinants of the endothelial cell loss in penetrating keratoplasty. Obata et al28 reported the rate of cell loss in patients with keratoconus as 1.9 per cent at two weeks, 1.2 per cent at one month, 9.9 per cent at three months, 30.6 per cent at six months and 33.4 per cent at 12 months, whereas in the bullous keratopathy group, these values were 13.8, 25.9, 52.6, 47.2, and 66.9 per cent, respectively. The same group also reported that cell loss in the postoperative first year was due to primary disease and that the rate of cell loss in patients in the bullous keratopathy eyes showed higher values compared to those in the keratoconus and the corneal opacity eyes, and have concluded that cell loss in penetrating keratoplasty during the first postoperative year depends on the host diseases.
In our study the endothelial cell loss was highest in regraft cases. This may be attributed to allogenic graft rejection in regraft cases. Large grafts in cases of AIK and descemetocele by virtue of being closer to the host limbus, with its complement of vessels and antigen-presenting Langerhans cells, are more susceptible to rejection. The migration of corneal endothelium from the graft to the host as a repair mechanism is thought to be significantly more pronounced in bullous keratopathy than in other conditions29. In our study the least endothelial cell loss was seen in keratoconus cases. The reason for low cell loss may be due to lesser cell migration from donor grafted cornea to the peripheral host cornea which has healthy endothelium.
The coefficient of variation (CV) of cell area (standard deviation of cell area divided by the mean cell area) provides a quantitative index of the variability of cell area or polymegathism. In the present study, the CV was found to increase in all the cases on an average of 5.6 at the end of one year (from 28.2 preoperatively to 33.8 at the end of one year). The maximum increase in CV after one year was recorded in descemetocele cases, while the least increase in CV was recorded in ABK and AIK cases. Patel et al30 recorded the CV as 26 ± 6 preoperatively, 25 ± 6 at end of three months and 26 ± 6 at end of one year. Similar findings were reported in another study31 where the coefficient of variation in cell area stayed relatively constant after five years. Harper et al3 documented no change in CV after one year in their study.
To conclude, the ultimate goal of PK is the maintenance of clear graft which is a marker for final visual acuity. The improvement in VA was seen more in optical PK, as compared to therapeutic PK patients indicating that visual gain was less if PK was performed in inflamed eyes. The endothelial cell loss was significantly more in the first quarter after PK. Incipient “hot eye”, increased surgical time, complicated surgery cause increased inflammation which may be the cause of more cell loss in the first quarter in regrafts, descemetocele, AIK and corneal opacity. Once the inflammation decreases, the cell loss stabilizes but the idiopathic cell loss from a subclinical immunological graft reaction, immune mediated corneal graft rejection remains the single most important cause of chronic cell loss in all pathological conditions. The cell loss was associated with increase in CV in all cases. In spite of polymegathism and pleomorphism, the clarity of the graft was maintained.
Acknowledgment
Authors thank Drs Falguni Mehta and O.P. Billore, Rotary Eye Institute, Navsari, for their guidance and support during the study.
Conflicts of Interest: None.
References
- Corneal physiology. In: Stephen Foster C, Dimitri T. Azar, Claes H. Dohlman, eds. Text book of Smolin and Thoft's the cornea: Scientific foundations and clinical practice (4th ed). Philadelphia, USA: Lippincott Williams & Wilkins; 2005. p. :46-52.
- [Google Scholar]
- The corneal endothelium after keratoplasty for keratoconus. Clin Exp Optom. 2013;96:201-7.
- [Google Scholar]
- Endothelial viability of organ-cultured corneas following penetrating keratoplasty. Eye. 1998;12:834-8.
- [Google Scholar]
- Five-year corneal graft survival: large, single-center patient cohort. Arch Ophthalmol. 1993;111:799-805.
- [Google Scholar]
- Graft survival in four common groups of patients undergoing penetrating keratoplasty. Ophthalmology. 1991;98:322-8.
- [Google Scholar]
- An analysis of corneal endothelium and graft survival in pseudophakic bullous keratopathy. Trans Am Ophthalmol Soc. 1989;87:762-801.
- [Google Scholar]
- Tissue Banks International (TBI) In: Eye banking manual of technical policies and procedures. Baltimore, USA: TBI; 1994.
- [Google Scholar]
- Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices and new intraocular drugs and solutions. Cornea. 2008;27:1-16.
- [Google Scholar]
- Donor age and endothelial cell loss after corneal transplantation. Ophthalmology. 2008;115:627-32.
- [Google Scholar]
- World Health Organization (WHO). International statistical classification of diseases and related helath problems, 10th version. Geneva: WHO; 1992. p. :456-7.
- [Google Scholar]
- Further analysis of assessments of the coefficient of variation of corneal endothelial cell areas from specular microscopic images. Clin Exp Optom. 2008;91:438-46.
- [Google Scholar]
- Corneal endothelium five years after transplantation. Am J Ophthalmol. 1994;118:185-96.
- [Google Scholar]
- Regression analysis of corneal endothelium after nonmechanical penetrating keratoplasty. Klin Monbl Augenheilkd. 2000;216:393-9.
- [Google Scholar]
- Corneal allograft rejection: risk factors, diagnosis, prevention, and treatment. Indian J Ophthalmol. 1999;47:3-9.
- [Google Scholar]
- Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex chromosomes. Exp Eye Res. 1999;68:341-6.
- [Google Scholar]
- The effect of allograft rejection after penetrating keratoplasty on central endothelial cell density. Am J Ophthalmol. 1991;111:739-42.
- [Google Scholar]
- Pathology of late endothelial failure: late endothelial failure of penetrating keratoplasty. Study with light and electron microscopy. Cornea. 2000;19:40-6.
- [Google Scholar]
- Outcome of rotational keratoplasty: comparison of endothelial cell loss in autografts versus allografts. Arch Ophthalmol. 2004;122:1437-40.
- [Google Scholar]
- Endothelial cell loss after autologous rotational keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2005;243:57-9.
- [Google Scholar]
- Risk factors for endothelial cell loss post-keratoplasty. Acta Ophthalmol Scand. 2006;84:766-70.
- [Google Scholar]
- Endothelial cell changes after penetrating keratoplasty. J Korean Ophthalmol Soc. 2000;41:1124-31.
- [Google Scholar]
- Corneal endothelium after deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus: A four-year comparative study. Indian J Ophthalmol. 2012;60:35-40.
- [Google Scholar]
- Descemet's stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the cornea donor study. Ophthalmology. 2010;117:438-44.
- [Google Scholar]
- Corneal endothelial cell damage in penetrating keratoplasty. Jpn J Ophthalmol. 1991;35:411-6.
- [Google Scholar]
- Chronic endothelial cell loss of the graft after penetrating keratoplasty: influence of endothelial cell migration from graft to host. Klin Monatsbl Augenheilkd. 2002;219:410-6.
- [Google Scholar]
- Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Trans Am Ophthalmol Soc. 2004;102:57-66.
- [Google Scholar]
- Long-term morphologic changes in the endothelium of transplanted corneas. Arch Ophthalmol. 1985;103:1343-6.
- [Google Scholar]






