Translate this page into:
Protein quality & amino acid requirements in relation to needs in India
For correspondence: Dr Anura V. Kurpad, Department of Physiology, St. John's Medical College, Sarjapur Road, Bengaluru 560 034, Karnataka, India e-mail: a.kurpad@sjri.res.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The relevance of protein and its constituent amino acids (AAs) in the structure and function of the human body is well known. Accumulating evidence has conferred specific functional and regulatory roles for individual AAs, adding relevance to their requirements across different age groups. The methods for measuring AA requirements have progressed from the classical nitrogen balance to the current stable isotope-based AA balance methods. Requirements of most of the indispensable AA (IAA) have been estimated in healthy Indian population by the best available balance method and has shown to be higher than earlier 1985 WHO/FAO/UNU (World Health Organization/Food and Agriculture Organization/United Nations University) recommendations. In addition, potential changes in the requirement, through adaptation to chronic undernutrition or to infection, have also been evaluated. In 2007, the WHO/FAO/UNU released a recommendation that increased the daily IAA requirement, based on primary evidence from Indian balance studies. This meant that to ensure that the new IAA requirements were met, individual foods or mixed diets needed to be assessed for their protein quality, or their ability to deliver the required amount of IAA. The recent FAO report on protein quality evaluation recommends the use of a new chemical AA score, the digestible IAA score (DIAAS), to replace the earlier protein digestibility corrected AA score. The DIAAS requires the determination of individual AA digestibility at the ileal level. A minimally invasive dual stable isotope tracer-based approach has been developed in India and has been used to determine digestibility of various foods in Indian adults and children. The increase in IAA requirements and subsequent protein quality requirements have implications for national regulatory frameworks, growth and development, and in turn, for economic and agricultural policy.
Keywords
Amino acid requirement
digestible indispensable amino acid score
dual stable isotope tracer approach
ileal digestibility
protein quality
Introduction
Protein is structurally and functionally the most important proximate nutrient required by the body. The functions of protein are myriad; acting as enzymes, hormones, blood transport molecules and forming essential component of cell membranes and intracellular matrices. The amino acids (AAs) are precursors of different coenzymes, hormones and biologically active molecules1. Moreover, with emerging evidence on regulatory roles of individual AAs and their implications on various physiological functions, the traditional classification of indispensable AA (IAA) and dispensable AA has led to new concepts of conditionally dispensable and functional AA. For example, among dispensable AAs, glutamine, glutamate and arginine are important for intestinal health and overall foetal and neonatal growth1. For the IAA, emphasis is placed on branched-chain AA, particularly leucine, in regulation of key functions linked to glucose metabolism, intestinal development and mucin production as well as enterocyte AA transport, mammary health, embryo growth and immune responses2.
An imbalance in AA could be detrimental to the body. For example, excessive leucine intake has been implicated in the pathogenesis of pellagra3 and high branched-chain AA intake has been associated with obesity and insulin resistance in humans4. A study in mice observed reversal of obesity and metabolic dysfunction on reduced intake of branched-chain AA5. Further, the quality of protein intake is important for early child growth and may be essential in the prevention of linear growth faltering678. Collectively, this underscores the importance of individual AA in health and disease, and the relevance of the composition (quantity and quality) of foods in meeting the body's metabolic demand for AAs. In view of the significance attached to individual AA and treating them as separate nutrients, it is important to understand the individual IAA requirements and the type and quantity of foods that should be eaten to meet these requirements.
Indispensable amino acid (IAA) requirements
The older and classical method of measuring the IAA requirement from estimations of nitrogen (N) balance9 was flawed in terms of the excessive energy intake that the experimental subjects received, since energy spares the protein requirement. Another problem was that the miscellaneous nitrogen losses (that is, other than urine and faecal, from desquamated skin, for example) were accounted for in the N balance measurements. Since the slope of the N balance-IAA intake curve is shallow near the zero N balance point (which defines the value for requirement of IAA), small differences in the values assumed for miscellaneous loss would have a large impact on the zero N balance point. When re-evaluations of the classic N balance experiments for the lysine intake were made using either 5 or 8 mg N/kg/day as the assumed value for miscellaneous loss, these suggested an approximate 50 per cent increase in lysine requirement101112. Therefore, the estimates of IAA requirement from the classic N balance studies remained uncertain.
Obligatory loss model
A theoretical model derived by Young et al13, was called the predicted obligatory AA losses model. In this, the basal rate of N excretion in an individual receiving a zero protein diet was used to estimate the requirement for specific AA by relating it to the composition of AA in body proteins. Since there is always a loss of N from the body when zero protein is administered, as IAA is indispensable, it is called the obligatory loss of N. An individual receiving zero protein intake would therefore, breakdown his/her own body protein, to meet the daily requirement for IAA, resulting in a negative N balance and a subsequent loss of body tissue and weight. While some of the released IAA would be recycled back into protein synthesis, some would be irreversibly oxidized and be excreted as N (it is impossible to bring the irreversible oxidation rate down to zero, due to inefficiencies in metabolic cycling) or the obligatory loss. In theory, the breakdown rate of protein would be determined by that IAA which had the highest rate of obligatory loss and its concentration in the body protein pool13. The other IAAs would be released based on their concentration in the protein that is broken down, and the excess over their daily requirement would be oxidized. In effect, the amount of obligatory loss of N is an indication of how much body protein was broken down, and the pattern of IAA loss is then assumed to be reflected by the IAA composition of body protein. The calculation of the amount of IAA needed to be fed to balance the daily obligatory loss required an assumption of the efficiency of utilization of dietary IAA. If dietary IAAs were used completely to balance the loss in the synthesis of body protein, this would result in an efficiency of 100 per cent; whereas in reality, the efficiency is much lower. This theoretical model pointed to a possible error in the empirically determined N balance estimates of the IAA requirement. It could not be considered to provide primary data needed to make recommendations; however, it led to a sustained effort to find more accurate ways that used isotopic methods, to measure the IAA requirement.
Direct amino acid (AA) balance
A better method of estimating the IAA requirement is by the measurement of AA balance (direct AA balance-DAAB) rather than the N balance14. If IAA oxidation could be accurately measured, then a balance could be constructed as the difference between the 24 h IAA intake and the 24 h irreversible oxidation. This can be done by the use of stable isotope tracers, where a stable isotope of carbon [13C] is used to label the studied (test) IAA at the position where it is released on oxidization, such that the tracer would appear in the breath and quantified. Since the labelled CO2 is mainly released through the breath, and if the quantitation of breath loss was accurate, it would provide a more accurate measurement of IAA oxidation compared to the N loss method, where N could be lost through routes that were difficult to quantify. When different levels of the test IAA are fed to adult human volunteers, the minimum level of intake of the test IAA that result in a zero IAA balance is considered to be the daily requirement of that particular AA (Fig. 1A). An important requirement of this tracer-based technique is the precise measurement of the isotopic enrichment of the IAA pool being subject to oxidation, and this is difficult to determine for most amino acids except leucine15 and probably methionine16.
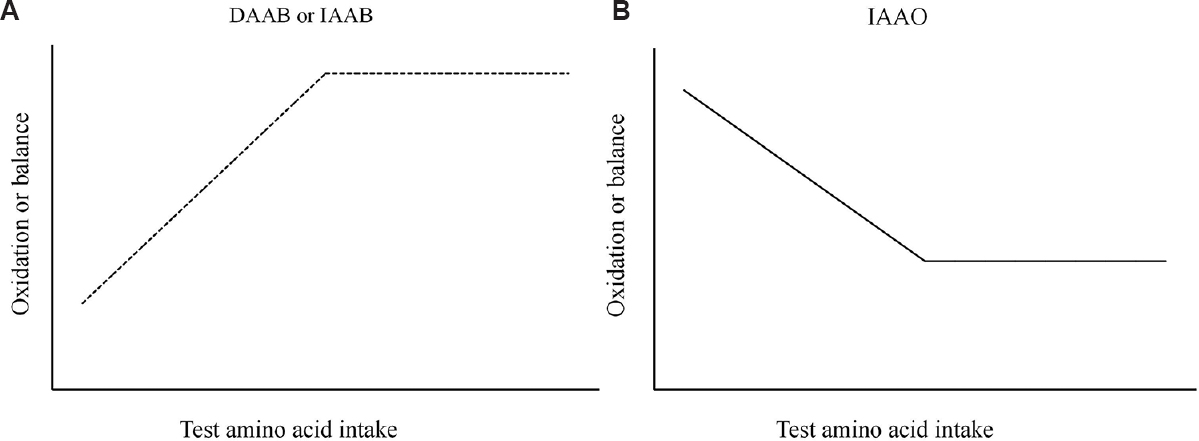
- Different tracer methods used in measuring indispensable amino acid requirements. (A) direct amino acid balance (DAAB) or indicator amino acid balance (IAAB) - increasing balance towards zero balance of either ‘test’ or ‘indicator’ amino acid with increasing test amino acid intake levels. Note that at supramaintenance intake levels, balance stays at zero. (B) indicator amino acid oxidation (IAAO)-decreasing oxidation of ‘indicator’ amino acid at supramaintenance test amino acid intake levels. Inflection or breakpoint on the lines indicates the measured requirement level.
Because the kinetics of leucine are well established17 and can be measured for direct determination of leucine balance over 24 h, this became the first IAA whose requirement was determined accurately over a day. A significant development was the demanding 24 h tracer balance protocol after adapting individuals to levels of test AA intake for one week1819. The value determined for the requirement of leucine (40 mg/kg/day) was similar to that predicted by the theoretical obligatory N loss model13. Other validations included short term leucine balance studies, which showed that the extrapolated daily requirement for leucine was greater than the 1985 FAO/WHO/UNU (World Health Organization/Food and Agriculture Organization/United Nations University) value of 14 mg/kg/day and in the range of about 40 mg/kg/day920. The leucine requirements of healthy, well-nourished Indians were measured in this way, by the 24 h technique using L-AA mixtures supplying graded intake levels of leucine, and where the individuals were adapted to these intakes for one week21, and found a similar value of 40 mg/kg/day.
Indicator amino acid (AA) oxidation and balance
The developments in tracer methods ascertained a level of confidence in leucine measurements. However, the requirement of the other IAA was more difficult, as the measurement of the intracellular precursor pool of the labelled test AA was not easy to measure. A technique which had been applied earlier in animals that advanced the measurement of the IAA requirement was the indicator AA oxidation technique (IAAO). In this method, two IAA are considered; the test IAA whose requirement is to be determined, and a tracer labelled ‘indicator’ IAA whose oxidation and balance could be accurately measured when graded test IAAs were fed. This would result in a curve of oxidation or balance, whose inflection indicates the test IAA requirement (Fig. 1A and B). At suboptimal (limiting) intakes of the test IAA, protein synthesis is inefficient, resulting in increased oxidation of the other IAA which is consumed at its normal requirement level. In this approach, the inflection point of the oxidation of the indicator IAA (whose intake is constant) at different test IAA intakes is a nadir, since the indicator IAA oxidation rate would reduce as the intake of the test IAA increased until it reached its minimum; it would stay at that minimum even as the intake of the test IAA increased further. The indicator IAA balance can also be evaluated in a similar way [called the indicator amino acid balance (IAAB) method], where the balance would improve from a negative value towards zero, as the intake of the test IAA increased, and remained at zero for test IAA intakes above the requirement. This would also yield an inflection point at zero balance that would indicate the requirement of the test IAA. It is important to note that oxidation and balance are measured over a full 24 h cycle, such that this method is more correctly called the 24 h IAAO or IAAB method, and is demanding since individuals are adapted to their test IAA intakes for at least a week before IAAO and IAAB are measured22. However, the technical problems of precursor pool identification that are related to the DAAB method are not present, since the indicator IAA chosen is one (leucine) in which the precursor pool enrichment is measurable.
This indicator method has effectively been simplified to a breath test, when it is used as a short-term IAAO method23, in contrast to the longer-term 24 h IAAO and IAAB method described above22. The short-term IAAO method has been used with lysine or phenylalanine as the indicator IAA2425, where the recovery of the oxidized [13C] label in the breath, that appears after the oxidation of the 1-13C-labelled IAA, is measured postprandially for a few hours. The adaptation period to the test IAA is much shorter, usually over two days. However, the non-invasive breath test with a short adaptation period allows for repeated measurements of IAA oxidation at graded levels of test IAA intake in the same individual, and yields a variance term of the requirement. The possible problems related to the short dietary adaptation period, and the short-term nature of the postprandial evaluation may also influence the requirement estimate.
These studies using the 24 h IAAO and IAAB measurements with an adequate dietary adaptation period have been considered as the best available measurement for the determination of IAA requirements, and based on these measurements as the primary source of data222627282930313233, the 2007 WHO/FAO/UNU expert committee on protein and AA requirements34 recommended a generally increased pattern (2 to 3 times) of IAA requirements for adults (Fig. 2), but in turn, showed that many foods (particularly cereals) that were hitherto thought to be adequate in their IAA content, were limited in lysine. These IAA requirements were determined in healthy, well-nourished Indians222627282930313233, considered representative of well-nourished and healthy populations globally. Similar studies using the short-term IAAO method were also carried out in children to validate the factorial method that was used to determine their IAA requirement36. Since a significant proportion of the population in India is undernourished, these measurements, particularly for lysine, methionine and leucine were performed in chronically undernourished individuals with a low but stable BMI and were found to be reasonably similar to well-nourished individuals373839. In addition, the effect of infections and intestinal function and parasites on the IAA requirement was also studied40, where it was found that the presence of mixed parasites increased the lysine requirement by almost 50 per cent in children and adults4142.
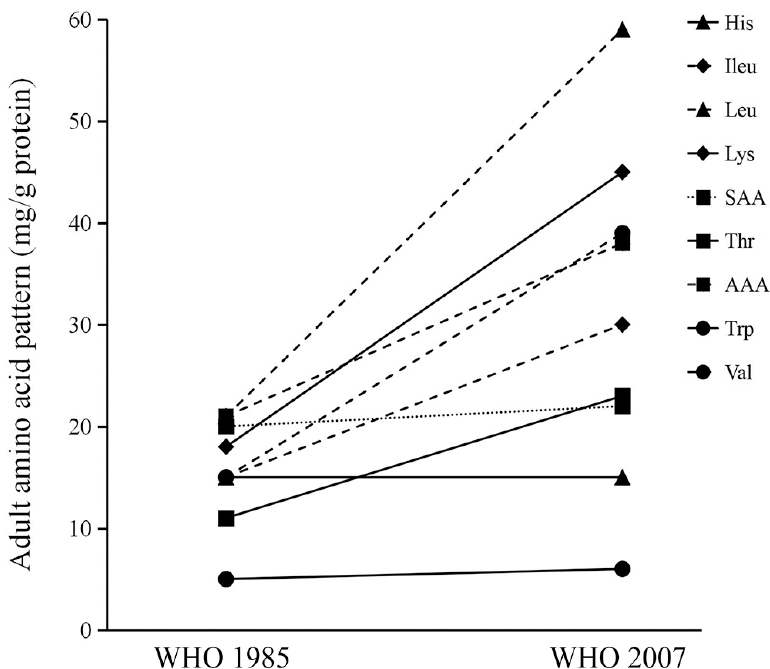
- Comparison of adult amino acid pattern (mg/g protein) between 2007 and 1985 FAO/WHO/UNU recommendation. Amino acids include His, Histidine; Ileu, Isoleucine; Leu, Leucine; Lys, Lysine; SAA, Sulphur Amino Acids (Methionine and Cysteine); Thr, Threonine; AAA, Aromatic Amino Acid (Phenylalanine and Tyrosine); Trp, Tryptophan; Val, Valine.
-
Source: Refs 34, 35.
Protein quality
The protein quality of a food reflects its capability to meet the daily AA requirements, and is determined in turn, by the content, composition and the metabolic availability of its constituent AAs. Several methods have been employed over the years to assess protein quality of foods or diets, such as protein efficiency ratio, net protein utilization, net protein retention, biological value, nitrogen balance, in vitro or in vivo protein digestibility; with the most accepted method being the chemical AA score (AAS) corrected for digestibility43.
Protein digestibility corrected amino acid score
The AAS is the ratio between the quantity of each IAA in the food or mixed diet protein (mg/g protein) and a reference requirement pattern of IAA (mg/g protein) for each age group. The lowest (limiting) AAS of the food or mixed diet, when corrected for crude protein faecal N digestibility, is called the protein digestibility-corrected AAS (PDCAAS). This index was introduced by the 1989 Joint FAO/WHO Expert Consultation on Protein Quality Evaluation and has been in use for 20 years now35. The PDCAAS has been subject to constant criticism, of using a single crude protein or N digestibility value instead of a specific digestibility value of each IAA, using faecal instead of true ileal digestibility, the truncation of score and others4344.
Digestible indispensable amino acid score
To overcome the concerns related to PDCAAS, a FAO report recommended replacing the PDCAAS with the new scoring system termed the digestible IAA score (DIAAS)45. In the DIAAS, the limiting AAS is corrected for the specific true ileal digestibility of each IAA. A detailed account of the DIAAS calculation with an evaluation of aspects influencing the calculation is available46. For the computation of DIAAS, there is a need to determine true ileal IAA digestibility of habitually consumed foods and mixed diets, preferably in humans, although a porcine model has been suggested as its digestive physiology is very similar to humans4748.
True ileal digestibility in humans
The direct determination of true ileal protein (nitrogen) or AA digestibility entails the collection of digesta at the level of the ileum, in the animal models or humans49. In the animals, either growing rats or pigs, the method used is slaughter or ileal cannulation technique, with different types of cannulations being used for the latter. These methods have advantages and disadvantages, with key issues related to inaccuracy in quantitative digesta collection and the disruption of normal gut physiology5051.
In humans, mainly two models were adopted; naso-ileal intubation and the ileostomy model (in ileostomates)5253. These methods have made use of intrinsically and uniformly 15N or 13C-labelled (stable isotopes) test foods that allow for true ileal digestibility calculations, which adjust for endogenous protein contribution from the intestine545556. True N and AA digestibility is calculated from the cumulated amounts recovered at the ileal level and thus not absorbed in the small intestine. However, there are considerable methodological limitations concerning both the techniques. The naso-ileal intubation is fraught with technical challenges; such as guided intubation to ensure correct placement of the double-lumen catheters, and the use of only homogenized foods requiring markers to quantify ileal flow of effluents; it is thus time-consuming and invasive. The presence of a tube in situ may disrupt the normal physiological functioning of the gastrointestinal system by increasing small intestinal motility, which could further compromise nutrient absorption57. The ileostomy model involves individuals with surgically exteriorized terminal ileum (stoma), indicated for various pathological conditions of the colon, such as ulcerative colitis, familial polyposis or colonic cancer, and follows the classical oro-ileal balance method58. This method shares the same concerns as the porcine cannulation models.
In order to address these issues non-invasive or minimally invasive methods are recommended, using stable isotopes49. These methods include the non-invasive IAAO technique and the minimally invasive dual stable isotope tracer approach. The IAAO follows the same principle used for IAA requirements but measures the metabolic availability of the limiting AA in the test protein5960. Although non-invasive in nature, IAAO requires repeated experiments in a single individual, and a strict adherence to an adaptation diet that could affect compliance, which limits its use in vulnerable population.
The dual isotope tracer method is based on a similar principle of other dual-tracer approaches such as those used for starch digestion61. This tracer technique was used in protein digestion, but for a single AA, where the phenylalanine digestibility was measured in humans with cystic fibrosis, using uniformly labelled 15N-spirulina62. To determine the true ileal IAA digestibility of protein source foods, they first have to be intrinsically labelled with a stable isotope. There are multiple ways in which a plant or animal protein can be selectively labelled555663646566 depending on the label of interest (2H, 13C or 15N). The animals are either enterally fed or intravenously infused with labelled isotopes (single or multiple) that in turn intrinsically labels the protein source (eggs, milk and meat)56646566. Deuterium (2H2O) watering or atmospheric labelling using 13CO2 gas mixtures are used for plants67. A pulsed-dose protocol with 2H2O for plants, has been standardized to obtain sufficient AA enrichments for use in human digestibility studies63. These intrinsically stable isotope-labelled test proteins are simultaneously fed with a differentially labelled ‘standard’ protein of a pre-determined digestibility, or with crystalline AAs that do not require digestion and are therefore 100 per cent digestible. As standard and test proteins are delivered simultaneously, it is assumed that their splanchnic extraction will be the same. Then, the postprandial ratio of the appearance of differently labelled AAs in the blood in relation to their ratio in the food ingested will allow for the evaluation of digestibility of the test protein. The measurement of IAA enrichment in the blood reflects absorption only from the small intestinal epithelium since colonic AA absorption is controversial. In addition, as the method only measures the appearance of labelled AAs from the intrinsically labelled test and standard protein, it is not affected by the contribution from endogenous protein sources and is therefore, a measure of true ileal digestibility. The technique is promising for determination of true ileal IAA digestibility, as it is minimally invasive, and can be applied across age groups, in pregnancy and in pathological conditions. Studies have been conducted using this approach in south Indian adults where the true ileal IAA digestibility of spirulina, hen egg and meat protein has been determined (Table)6364. The same technique was applied in children (1.5-2 yr) to obtain mean true ileal IAA digestibility of rice (79%), finger millet (68%), mung bean (65%) and whole boiled hen egg (87%). Of particular importance was the digestibility of the two limiting AAs, methionine and lysine. The mean digestibility was, for methionine 80, 60, 54, 86 per cent and 78, 75, 65, 94 per cent for lysine in rice, finger millet, mung bean and whole boiled hen egg, respectively(unpublished data).
| IAA | Spirulina (%)63 | Egg white (%)64 | Whole boiled egg (%)64 | Chicken meat (%)64 |
|---|---|---|---|---|
| Methionine | 84 | 80 | 86 | 93 |
| Phenylalanine | 95 | 93 | 96 | 94 |
| Threonine | 82 | 89 | 96 | 94 |
| Lysine | 78 | 89 | 89 | 96 |
| Leucine | 86 | 88 | 88 | 89 |
| I-Leucine | 84 | 84 | 85 | 89 |
| Valine | 87 | 82 | 87 | 90 |
| Mean IAA | 85 | 86 | 89 | 92 |
Superscript numerals indicate reference numbers
Use of protein quality
Protein quality indexes are used in regulatory frameworks for countries and industries, as criteria to identify and endorse foods or formulations to be of a certain quality68. Expert consultations by the WHO/FAO on protein quality considerations have advocated the use of DIAAS where data are available, if not PDCAAS, to member nations3445. These promotions on the use of protein quality assessments are particularly relevant in feeding vulnerable populations such as children, either for optimal growth or when undernourished. For example, ensuring good protein quality of ready-to-use therapeutic food or similar formulations is critical to meet the metabolic needs of the severe acute malnutrition children.
Implications
The requirement value for the limiting IAA in cereal-based diets (lysine) that is, now established is substantially higher and has important implications with respect to the protein quality assessment of diets, particularly in developing regions, where such diets supply the major proportion of the IAA intake6970. Cereals offer low-quality protein and are limiting in lysine. Thus, the populations expected to be at a higher risk of a lysine inadequacy from the diet are those in the low- and middle-income countries70. There is evidence that the quality of protein influences linear growth in children717273. On the other hand, it is also evident that populations consuming diets containing evidently poor-quality cereal protein have survived quite effectively. Assuming that the new IAA requirements are correct, the question that remains is, are these populations physically and functionally healthy? Or, was it possible that populations adapted to low-quality protein intakes without any consequences, or after bearing some cost in terms of body composition? Did they have adaptations through contributions of nutritionally significant amounts of IAA from gut microbes to supplement the IAA from the diet?
The first two questions could be answered by correlating with criterion of health with IAA intake. For instance, the physical (anthropometric) characteristics and activity (functional) patterns of an individual could be used to diagnose a state of chronic energy deficiency. However, there is no specific anthropometric or functional criterion that can be used to define a minimum but safe level of intake of IAA. Populations eating low lysine diets have been shown to have a lower level of selected immune markers as well as responses to stress7475, and in some cases, a relatively higher lysine intake over three weeks resulted in a slightly improved muscle function32. Clearly, more functional studies are required. In addition, there are concerns related to the possibility that the environmental and parasitic burden imposed on poorer, undernourished communities, may actually result in a higher lysine requirement3741. The splanchnic uptake of leucine is better in those with a higher body mass index, indicating that gut function may well be an area of research into the adaptive price paid by undernourished humans76.
It is also possible that the gut microbes contribute significantly to intestinal lysine (and other IAA) intake. For example, there is evidence of significant absorption of IAA synthesized by the gut microbiome in single stomach animals and humans7778. Experiments using15NH4Cl incorporation into microbial lysine in normal humans estimated that lysine absorption from synthesis in the microbiome was 29-68 mg/kg/day, which was similar to the adult daily lysine requirement79. This suggests that nutritionally significant amounts of microbially derived lysine are available for absorption although quantifying this is difficult, related to the difficulties of measuring the enrichment of the label in the lumen of the small intestine7980. Using the IAAO technique, the potential leucine (and by extension, other IAA) input from the gut microbiota was of the order of about 20 per cent in adult men, and in the potential range of nutritional relevance81.
The protein quantity and quality of complementary foods become crucial during the period of rapid growth and development. Consumption of high-quality protein through animal source foods (ASFs) has shown beneficial effects on linear growth and development8283848586. This underscores the importance of providing quality foods, especially in poor conditions with ongoing environmental insults (high pathogen burden, either bacterial or parasitic infestations) compounded by societal and family issues. The environmental enteric dysfunction resulting from unsanitary environments is one such condition, with the potential to impair absorption of nutrients probably due to exaggerated intestinal immune activation and permeability87. Ecological and biochemical analyses suggest that poor protein quality may be associated with childhood stunting67. However, it is an overreach to attribute this association to protein quality alone, as suboptimal intakes are often accompanied by other micronutrient inadequacies and covaries with determinants and consequences of poverty8.
Economic and policy implications for India
An analysis using lysine as a measure for quality of protein found that the overall proportion of the population at risk of quality protein inadequacy ranged from 4-26 per cent for different age groups belonging to rural and urban sectors of India88. While these rates are determined in relation to protein requirements with good health and clean environment, the estimates are likely to be higher for developing countries with majority of population living in suboptimal sanitary and hygiene conditions. To account for this, by imposing an additional protein requirement of 20 per cent42, the risk of inadequacy increased and varied between 6 and 42 per cent for different age groups and sectors in India. These relatively high risks of inadequacy are not surprising, since majority of protein in the Indian diet is derived from cereals, which offer relatively low-quality protein and are limiting in lysine. On the other hand, foods such as pulses, milk, egg, fish and meat are consumed in limited quantity despite their high protein content. Together, these quality foods contribute <36 per cent of the total protein intake in India88.
These patterns in consumption are expected given the heavy focus of food subsidy programmes on cereals; the National Food Security Act 2013, which seeks to cover up to 75 per cent (rural) and 50 per cent (urban) of the Indian population, provides an entitlement of 5 kg per person per month of cereals at subsidized rate for the economically poor sections88. On the other hand, the volatility in prices of pulses continues to pose a challenge. And even though the demand for ASF is expected to increase, these are not affordable by all.
The consumption pattern is also commensurate to the trends in production. Fig. 3A presents the trends in food grain production in India from 1950-1951 to 2014-2015. The annual production for rice has steadily increased from 20.6 million tonnes in 1950-1951 and reached 105.5 million tonnes in 2014-2015, with the growth rate averaging at 3.2 per cent over this period89. Wheat production registered a higher average growth rate of 4.6 per cent during the same period. The period of green revolution saw high input usage, provision of high yielding varieties of seeds and extension services for these crops; as a result of this, the yield of these crops have more than tripled over these years. In comparison, the yield for pulses increased marginally from 0.4 tonnes/hectare in 1950-1951 to 0.8 tonnes/hectare in 2014-2015 with the annual production touching 17.1 million tonnes89. There is clearly a need to shift the policy focus from cereals to pulses. Having a minimum support price (MSP) for pulses is not enough; this price is hardly applicable if it is not accompanied by assured procurement. Equally, investment in technology as well as research and development are important for improving the yield of pulses. Fig. 3B presents the production of major ASF in India over the last 25 years90. Although the production of all these foods has steadily increased, the gains in milk production have been most dramatic. This can be attributed to ‘Operation Flood’ which bought the milk producers together to form cooperatives. Further, an analysis of supply projections for major protein sources in India found that despite the expected increase, the futuristic supply of pulses, milk, egg, fish and meat (EFM) may not be sufficient to reduce the risk of deficiency described above to <5 per cent till 2026 (unpublished data). Alternative measures such as fortification of cereals with AA may be considered, but with a high-cost factor, to bring down the risk of inadequacy.
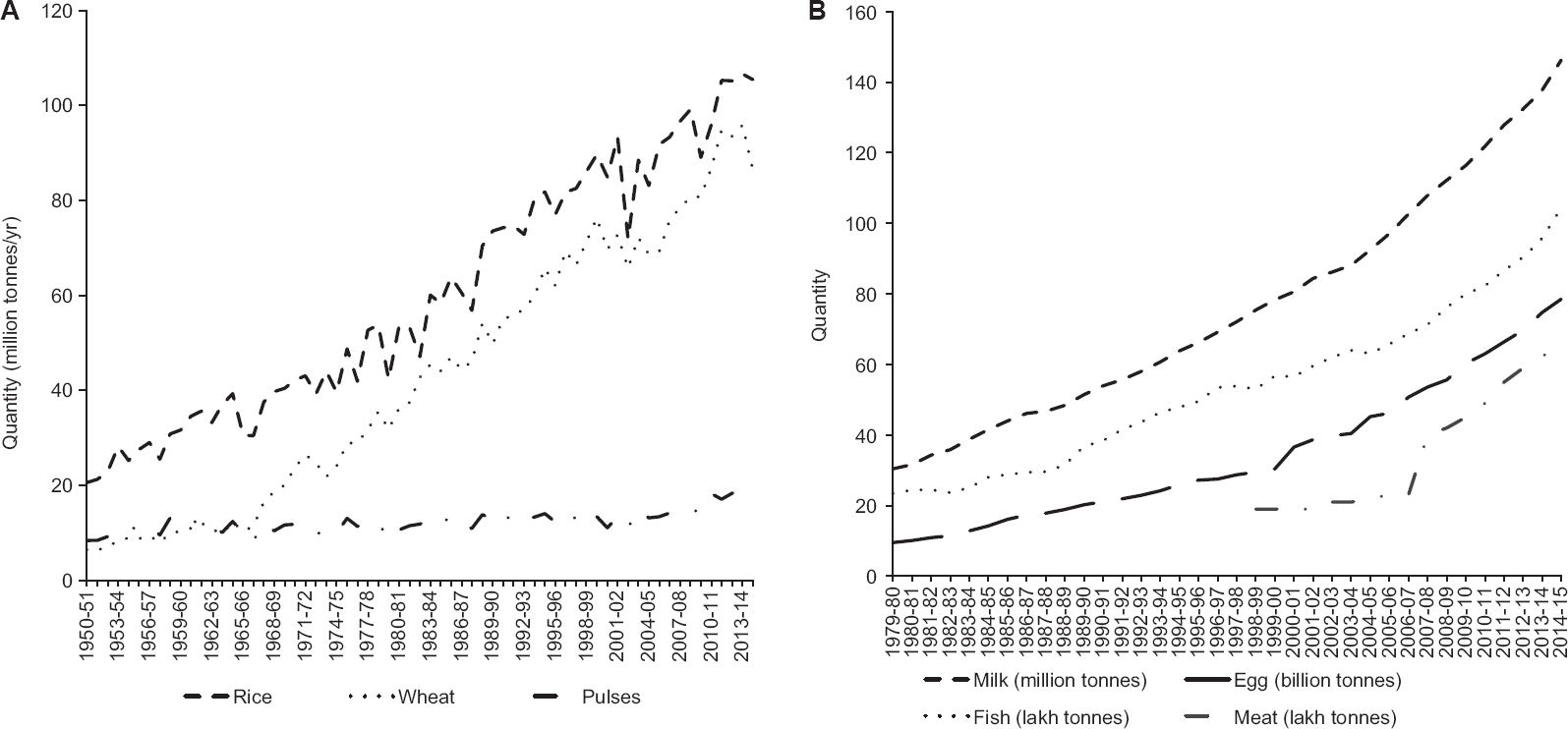
- (A) Trends in food grain production in India from 1950-1951 to 2014-2015. Source: Ref. 89. (B) Trends in production of animal sources foods in India from 1979-1980 to 2014-2015. Source: Ref. 90.
Conclusion
The aim of this review was to provide an overview on the methods used to estimate the IAA requirements, protein quality and ileal digestibility, with focus on an Indian setting. It is now clear that the IAA requirement is higher than previously thought, which has emphasized the supply of quality protein in the diet. Measuring protein quality is not easy, as it has to be measured in the small intestine alone. With the introduction of the new index for protein quality, the DIAAS, considerable work is still needed for the operationalization of the metric. The dual isotope tracer approach, with properties of being minimally invasive and applicability across populations, may be promising in this context to generate more data to inform DIAAS. Efforts to determine ileal digestibility of foods need to be continued and should inform agricultural and feeding policies, as there are cultural differences in food consumption, and protein quality of the diets is important for growth, development and functionality and in mitigating risks associated with metabolic dysregulation. Feeding trials are required based on optimization of foods using protein quality information. If the proportion of population at risk of quality protein inadequacy in India has to be reduced, the agricultural and animal husbandry policies need to focus on increasing production of pulses and ASF, supported by reduced prices by food subsidy programmes.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Novel metabolic and physiological functions of branched chain amino acids: A review. J Anim Sci Biotechnol. 2017;8:10.
- [Google Scholar]
- Possible role for dietary leucine in the pathogenesis of pellagra. Lancet. 1969;1:197-9.
- [Google Scholar]
- Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723-36.
- [Google Scholar]
- Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596:623-45.
- [Google Scholar]
- Child stunting is associated with low circulating essential amino acids. EBioMedicine. 2016;6:246-52.
- [Google Scholar]
- Protein quality in the first thousand days of life. Food Nutr Bull. 2016;37((Suppl 1)):S14-21.
- [Google Scholar]
- Nutrition, infection and stunting: The roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr Res Rev. 2017;30:50-72.
- [Google Scholar]
- Metabolic demands for amino acids and the human dietary requirement: Millward and Rrvers (1988) revisited. J Nutr. 1998;128:2563S-76S.
- [Google Scholar]
- Statistical analysis of nitrogen balance data with reference to the lysine requirement in adults. J Nutr. 1999;129:1920-6.
- [Google Scholar]
- A theoretical basis for increasing current estimates of the amino acid requirements in adult man, with experimental support. Am J Clin Nutr. 1989;50:80-92.
- [Google Scholar]
- Human indispensable amino acid requirements: New paradigms of measurement and the implication for protein quality. Indian J Physiol Pharmacol. 1999;43:5-24.
- [Google Scholar]
- Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: A method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982;31:1105-12.
- [Google Scholar]
- Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 2001;280:E947-55.
- [Google Scholar]
- Leucine kinetics at graded intakes in young men: Quantitative fate of dietary leucine. Am J Clin Nutr. 1988;48:998-1009.
- [Google Scholar]
- Validation of the tracer-balance concept with reference to leucine: 24-h intravenous tracer studies with L-[1-13C]leucine and [15N-15N]urea. Am J Clin Nutr. 1994;59:1000-11.
- [Google Scholar]
- The 24-h pattern and rate of leucine oxidation, with particular reference to tracer estimates of leucine requirements in healthy adults. Am J Clin Nutr. 1994;59:1012-20.
- [Google Scholar]
- Leucine kinetics at graded leucine intakes in young men. Am J Clin Nutr. 1986;43:770-80.
- [Google Scholar]
- Daily requirement for and splanchnic uptake of leucine in healthy adult Indians. Am J Clin Nutr. 2001;74:747-55.
- [Google Scholar]
- An initial assessment, using 24-h [13C]leucine kinetics, of the lysine requirement of healthy adult Indian subjects. Am J Clin Nutr. 1998;67:58-66.
- [Google Scholar]
- Recent advances in methods of assessing dietary amino acid requirements for adult humans. J Nutr. 1995;125:2907-15.
- [Google Scholar]
- Dietary lysine requirement of young adult males determined by oxidation of L-[1-13C]phenylalanine. Am J Physiol. 1993;264:E677-85.
- [Google Scholar]
- Tyrosine requirement of healthy men receiving a fixed phenylalanine intake determined by using indicator amino acid oxidation. Am J Clin Nutr. 2001;73:276-82.
- [Google Scholar]
- The daily phenylalanine requirement of healthy Indian adults. Am J Clin Nutr. 2006;83:1331-6.
- [Google Scholar]
- Branched-chain amino acid requirements in healthy adult human subjects. J Nutr. 2006;136:256S-63S.
- [Google Scholar]
- The daily valine requirement of healthy adult Indians determined by the 24-h indicator amino acid balance approach. Am J Clin Nutr. 2005;82:373-9.
- [Google Scholar]
- Threonine requirements of healthy Indian men, measured by a 24-h indicator amino acid oxidation and balance technique. Am J Clin Nutr. 2002;76:789-97.
- [Google Scholar]
- Effect of cystine on the methionine requirement of healthy Indian men determined by using the 24-h indicator amino acid balance approach. Am J Clin Nutr. 2004;80:1526-35.
- [Google Scholar]
- Daily methionine requirements of healthy Indian men, measured by a 24-h indicator amino acid oxidation and balance technique. Am J Clin Nutr. 2003;77:1198-205.
- [Google Scholar]
- Lysine requirements of healthy adult indian subjects receiving long-term feeding, measured with a 24-h indicator amino acid oxidation and balance technique. Am J Clin Nutr. 2002;76:404-12.
- [Google Scholar]
- Protein and amino acid requirements in the elderly. Eur J Clin Nutr. 2000;54((Suppl 3)):S131-42.
- [Google Scholar]
- World Health Organization. Technical series No- 935. In: Joint WHO/FAO/UNU Expert Consultation on protein and amino acid requirements in human nutrition, 2002. Geneva: WHO; 2007.
- [Google Scholar]
- Food and Agriculture Organization/World Health Organization. In: Protein quality evaluation: Report of the joint FAO/WHO expert consultation; 1989 December 4-8; Bethesda, Maryland, USA. Rome: Food and Agriculture Organization; 1991.
- [Google Scholar]
- Lysine requirement of healthy, school-aged Indian children determined by the indicator amino acid oxidation technique. J Nutr. 2010;140:54-9.
- [Google Scholar]
- Lysine requirements of chronically undernourished adult Indian men, measured by a 24-h indicator amino acid oxidation and balance technique. Am J Clin Nutr. 2003;77:101-8.
- [Google Scholar]
- Leucine requirement and splanchnic uptake of leucine in chronically undernourished adult Indian subjects. Am J Clin Nutr. 2003;77:861-7.
- [Google Scholar]
- Daily requirement for total sulfur amino acids of chronically undernourished Indian men. Am J Clin Nutr. 2004;80:95-100.
- [Google Scholar]
- The requirements of protein & amino acid during acute & chronic infections. Indian J Med Res. 2006;124:129-48.
- [Google Scholar]
- Intestinal parasites increase the dietary lysine requirement in chronically undernourished Indian men. Am J Clin Nutr. 2003;78:1145-51.
- [Google Scholar]
- Lysine requirements of moderately undernourished school-aged Indian children are reduced by treatment for intestinal parasites as measured by the indicator amino acid oxidation technique. J Nutr. 2015;145:954-9.
- [Google Scholar]
- Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br J Nutr. 2012;108((Suppl 2)):S183-211.
- [Google Scholar]
- Advantages and limitations of the protein digestibility-corrected amino acid score (PDCAAS) as a method for evaluating protein quality in human diets. Br J Nutr. 2012;108((Suppl 2)):S333-6.
- [Google Scholar]
- Food and Agricultural Organization. Dietary protein quality evaluation in human nutrition. In: Report of an FAO Expert Consultation. Food and Nutrition Paper No. 92. Rome: FAO of the United Nations; 2013.
- [Google Scholar]
- Protein quality as determined by the digestible indispensable amino acid score: Evaluation of factors underlying the calculation. Nutr Rev. 2016;74:584-99.
- [Google Scholar]
- Animal models for determining amino acid digestibility in human - A review. Br J Nutr. 2012;108((Suppl 2)):S273-81.
- [Google Scholar]
- Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr Res Rev. 2010;23:4-22.
- [Google Scholar]
- Food and Agricultural Organization. Research approaches and methods for evaluating the protein quality of human foods. In: Report of a FAO Expert Working Group. Rome: FAO; 2014.
- [Google Scholar]
- Amino acids - The collection of ileal digesta and characterization of the endogenous component. In: Feed evaluation: Principles and practice. Wageningen, The Netherlands: Wageningen Academic Publishers; 2000. p. :105-24.
- [Google Scholar]
- Methodological aspects of the in vivo measurement of ileal amino acid digestibility in pigs - A review. Asian Aust J Anim Sci. 1996;9:495-502.
- [Google Scholar]
- High true ileal digestibility but not postprandial utilization of nitrogen from bovine meat protein in humans is moderately decreased by high-temperature, long-duration cooking. J Nutr. 2015;145:2221-8.
- [Google Scholar]
- Digestibility of cooked and raw egg protein in humans as assessed by stable isotope techniques. J Nutr. 1998;128:1716-22.
- [Google Scholar]
- In vivo determination of amino acid bioavailability in humans and model animals. J AOAC Int. 2005;88:923-34.
- [Google Scholar]
- Postprandial metabolic utilization of wheat protein in humans. Am J Clin Nutr. 2005;81:87-94.
- [Google Scholar]
- A pilot study for the intrinsic labeling of egg proteins with 15N and 13C. Rapid Commun Mass Spectrom. 2012;26:43-8.
- [Google Scholar]
- Effect of gastrointestinal intubation on the passage of a solid meal through the stomach and small intestine in humans. Gastroenterology. 1983;84:1568-72.
- [Google Scholar]
- An acute ileal amino acid digestibility assay is a valid procedure for use in human ileostomates. J Nutr. 2005;135:404-9.
- [Google Scholar]
- Metabolic availability of the limiting amino acids lysine and tryptophan in cooked white African cornmeal assessed in healthy young men using the indicator amino acid oxidation technique. J Nutr. 2018;148:917-24.
- [Google Scholar]
- Lysine from cooked white rice consumed by healthy young men is highly metabolically available when assessed using the indicator amino acid oxidation technique. J Nutr. 2013;143:302-6.
- [Google Scholar]
- An explorative study of in vivo digestive starch characteristics and postprandial glucose kinetics of wholemeal wheat bread. Eur J Nutr. 2008;47:417-23.
- [Google Scholar]
- New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr. 2014;33:1024-32.
- [Google Scholar]
- Measurement of protein digestibility in humans by a dual-tracer method. Am J Clin Nutr. 2018;107:984-91.
- [Google Scholar]
- Ileal digestibility of intrinsically labeled hen's egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am J Clin Nutr. 2018;108:980-7.
- [Google Scholar]
- Hens produce artificially enriched 13C egg proteins for metabolic tracer studies. Int J Biol. 2013;5:69-74.
- [Google Scholar]
- Development of intrinsically labeled eggs and poultry meat for use in human metabolic research. J Nutr. 2016;146:1428-33.
- [Google Scholar]
- Intrinsic stable isotope labeling of plants for nutritional investigations in humans. J Nutr Biochem. 1997;8:164-71.
- [Google Scholar]
- Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr Rev. 2017;75:658-67.
- [Google Scholar]
- World balance of dietary essential amino acids related to the 1989 FAO/WHO protein scoring pattern. Food Nutr Bull. 1995;16:166-77.
- [Google Scholar]
- Wheat proteins in relation to protein requirements and availability of amino acids. Am J Clin Nutr. 1985;41:1077-90.
- [Google Scholar]
- Nutritional influences on linear growth: A general review. Eur J Clin Nutr. 1994;48((Suppl 1)):S75-89.
- [Google Scholar]
- Is complete catch-up possible for stunted malnourished children? Eur J Clin Nutr. 1994;48((Suppl 1)):S58-70.
- [Google Scholar]
- Malnutrition and dietary protein: Evidence from China and from international comparisons. Food Nutr Bull. 2003;24:145-54.
- [Google Scholar]
- Lysine-fortified wheat flour improves the nutritional and immunological status of wheat-eating families in Northern China. Food Nutr Bull. 2004;25:123-9.
- [Google Scholar]
- Lysine fortification reduces anxiety and lessens stress in family members in economically weak communities in Northwest Syria. Proc Natl Acad Sci U S A. 2004;101:8285-8.
- [Google Scholar]
- The effect of nutritional status on the leucine requirement and splanchnic uptake of leucine in adult Indian men. [SIIC En Internet Section Expertos Invitados Expertos del Mundo] 2003. Available from: https://www.siicsalud.com/dato/experto.php/20045
- [Google Scholar]
- Contribution of microbial amino acids to amino acid homeostasis of the host. J Nutr. 2000;130:1857S-64S.
- [Google Scholar]
- Availability of intestinal microbial lysine for whole body lysine homeostasis in human subjects. Am J Physiol. 1999;277:E597-607.
- [Google Scholar]
- Pigs’ gastrointestinal microflora provide them with essential amino acids. J Nutr. 2003;133:1127-31.
- [Google Scholar]
- Lysine synthesized by the gastrointestinal microflora of pigs is absorbed, mostly in the small intestine. Am J Physiol Endocrinol Metab. 2003;284:E1177-80.
- [Google Scholar]
- Intestinal microbial contribution to metabolic leucine input in adult men. J Nutr. 2008;138:2217-21.
- [Google Scholar]
- Diet quality and risk of stunting among infants and young children in low- and middle-income countries. Matern Child Nutr. 2017;13((Suppl 2)):e12430.
- [Google Scholar]
- Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. Int Arch Med. 2013;6:29.
- [Google Scholar]
- Eggs in early complementary feeding and child growth: A randomized controlled trial. Pediatrics. 2017;140 pii: e20163459
- [Google Scholar]
- Eggs early in complementary feeding increase choline pathway biomarkers and DHA: A randomized controlled trial in Ecuador. Am J Clin Nutr. 2017;106:1482-9.
- [Google Scholar]
- High protein intake from meat as complementary food increases growth but not adiposity in breastfed infants: A randomized trial. Am J Clin Nutr. 2014;100:1322-8.
- [Google Scholar]
- Dietary protein and the health-nutrition-agriculture connection in India. J Nutr. 2017;147:1243-50.
- [Google Scholar]
- Government of India. In: Agricultural statistics at a glance 2016. New Delhi: Government of India; 2016.
- [Google Scholar]
- Government of India. In: Basic animal husbandry & fisheries statistics 2017. New Delhi: Government of India; 2017.
- [Google Scholar]






