Translate this page into:
Protective effect of pioglitazone on cardiomyocyte apoptosis in low-dose streptozotocin & high-fat diet-induced type-2 diabetes in rats
Reprint requests: Dr Uma Bhandari, Department of Pharmacology, Faculty of Pharmacy, Hamdard University, New Delhi 110 062, India e-mail: uma_bora@hotmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Cardiomyocyte apoptosis is one of the pathologic phenomena associated with diabetes and related conditions including obesity, insulin resistance and hyperlipidaemia. In the present study, the protective effects of pioglitazone on cardiomyocyte apoptosis was evaluated in experimental diabetes induced by low dose of streptozoticin (STZ) combined with high fat diet (HFD) in rats.
Methods:
Male Wistar rats (150-200 g) were injected with low-dose STZ (45 mg/kg, i.v., single dose) and orally fed with a HFD (20 g/day/rat) for a period of 28 days and simultaneously treated with pioglitazone (20 mg/kg/p.o.) for a period of 21 days (from 8th day to 28th day). On 29th day blood was collected, serum separated and used for biochemical parameters. Heart tissue was used for cardiomyocyte apoptosis measurement and also for histopathological examination.
Results:
Pioglitazone treatment resulted in a decrease in cardiomyocyte apoptosis as revealed by a decrease in cardiac caspase-3, lactate dehydrogenase (LDH) levels and DNA fragmentation, and an increase in Na+K+ATPase levels in diabetic rats. Cardiac histology of diabetic control rats showed dense focal fatty infiltration in the myocardial cells whereas normal architecture with regular morphology and well preserved cytoplasm was observed with pioglitazone treatment. Pioglitazone treatment significantly reduced the heart rate, mean arterial blood pressure, body mass index (BMI) and levels of serum glucose, leptin, insulin, HOMA-IR, total cholesterol (TC) and triglycerides (TGs), apoliproprotein-B glycosylated haemoglobin (HbA1c) levels and atherogenic index, and increased the levels of serum high density lipoprotein cholesterol (HDL-C) and cardiac antioxidant enzymes.
Interpretation & conclusions:
The present study results suggest that pioglitazone possesses cardiac anti-apoptotic potential in diabetic rat model and can be further explored for its use for treatment of diabetic cardiomyopathy.
Keywords
Cardiomyocyte apoptosis
caspase-3
diabetes
DNA laddering
pioglitazone
Diabetic cardiomyopathy is one of the leading causes of death in patients with diabetes1. Cardiomyocyte apoptosis plays a critical role in the pathogenesis of diabetic cardiomyopathy2. Hyperglycaemia, dyslipidaemia, excessive generation of reactive oxygen species (ROS), inflammation and accumulation of cardiac fibrosis, and apoptosis are probably involved in the pathophysiology of diabetic cardiomyopathy34.
Thiazolidinediones (TZDs) are insulin-sensitizing agents, widely used for the treatment of patients with type-2 diabetes (T2D). TZDs were introduced in the late 1990s as an adjunctive therapy for T2D5. TZDs reduce myocardial infarct size and recover the contractile function in intact rat hearts after ischaemia and reperfusion in both normal and insulin resistant models6789.
Pioglitazone, a derivative of TZDs, is a peroxisome proliferator-activated receptor-γ (PPARγ) agonist that is used to treat T2D by increasing insulin sensitivity10. Pioglitazone has been shown to reduce the myocardial infarct size via activation of various pathways viz. PPAR-γ, PI3-kinase, Akt, and eNOS11. Pioglitazone has earlier been shown to restore the endothelial function in aorta of streptozotocin (STZ)- induced diabetic rats12.
Cardiovascular safety of these drugs has been a cause of concern. Recently, rosiglitazone was withdrawn due to increased liability to cardiotoxicity13. Despite numerous studies showing the cardioprotective effect of pioglitazone14, the concern about its safety on heart is still not clear. Therefore, this study was undertaken to evaluate the effect of pioglitazone on cardiomyocyte apoptosis in diabetic rats wherein experimental diabetes was induced by low dose of STZ combined with high fat diet.
Material & Methods
Drugs and chemicals: The designed and standardized HFD containing (g/kg) (casein-342, L-cystine-3, starch-172, sucrose-172, cellulose-50, ground nut oil-25, tallow-190, AIN salt mix-35 and AIN vitamin mix-10) was procured from National Institute of Nutrition, Hyderabad, India. Pioglitazone (Sun Pharma, Mumbai, India); glucose, total cholesterol (TC) and triglycerides (TGs) kits (Span diagnostic Ltd., Udhna, Surat); high density lipoprotein-cholesterol (HDL-C) and lactate dehydrogenase (LDH) kit (Reckon Diagnostics Pvt. Ltd., Vadodara, Gujrat, India); insulin ELISA kit (Alpco Diagnostics, Salem, USA); leptin ELISA kit (Bio Vendor, Germany); apolipoprotein-B kit (apo-B) (Immunoturbidimetric method; Randox Laboratories Ltd., Antrim, UK); glycosylated haemoglobin (HbA1c) kit (Asritha Invitro Diagnostic Reagents, Hyderabad, India); Caspase-3/CPP32 colorimetric assay kit (BioVision, USA) were used for the study. All chemicals were of analytical grade and chemicals required for sensitive biochemical assay were purchased from Sigma Chemical Co., USA, Hi Media, and SD Fine Chemicals, Mumbai, India.
Experimental design: This study was performed at the department of Pharmacology, Jamia Hamdard University, and department of Pathology, Vardhman Mahavir Medical College and Safdarganj Hospital, New Delhi, India. Male adult Wistar albino rats weighing 150-200 g, procured from the Central Animal House Facility were acclimatized under standard laboratory conditions at 25 ± 2°C, 50 ± 15 per cent relative humidity (RH) and normal photoperiod (12 h light: dark cycle) for seven days. The animals were fed with commercial normal pellet diet (NPD) and water ad libitum. After acclimatization, except normal control rats (group 1), all rats were injected with STZ (45 mg/kg, iv, single dose, given in 0.1 M citrate buffer, pH 4.5), those rats having fasting blood glucose (FBG) level more than 200 mg/dl were selected for further pharmacological studies. The animals were randomized and divided into the following six groups of eight animals each. Group 1: normal control [rats treated with 1% carboxy methyl cellulose solution in 0.9% w/v normal saline (2 ml/kg, po) daily + normal diet for 28 days]; Group 2: HFD control (rats fed HFD 20 g/day/rat for a period of 28 days]; Group 3: STZ control [rats treated with STZ (45 mg/kg, iv, single dose)]; Group 4: STZ+HFD control [rats treated with STZ (45 mg/kg, iv, single dose) + HFD (20 g/day/rat) orally in pellet form for a period of 28 days]; Group 5: STZ+pioglitazone treated [rats treated with STZ (45 mg/kg, iv, single dose) + from 8th day pioglitazone (20 mg/kg/po) treatment to 28th days]; Group 6: STZ+HFD+pioglitazone treated [rats treated with STZ (45 mg/kg, iv single dose) + HFD 20 g/day/rat for a period of 28 days + from 8th day pioglitazone (20 mg/kg/po) treatment to 28th days]. Animals were subjected to biochemical estimations on 29th day.
Estimation of anthropometric and haemodynamic parameters: Body weight gain, body mass index (BMI), food and water consumption were recorded in each group. Body weight gain was recorded weekly while food and water consumption was recorded each day. Haemodynamic parameters (systolic, diastolic, mean arterial blood pressure and heart rate) were recorded by non-invasive blood pressure recorder using rat's tail-cuff method (Kent Scientific Corporation, USA) on 29th day.
Estimation of biochemical parameters: On 29th day, blood was collected from the retro-orbital plexus of overnight fasted rats using microcapillary tube and serum was separated. Serum TC, HDL-C, TGs, glucose, insulin, leptin, LDH, apo-B and HbA1c levels were estimated by enzymatic kits. Homeostasis Model Assessment (HOMA-IR), an index of insulin resistance, i.e. fasting glucose (mg/dl) x fasting insulin (mU/ml)/2430 was calculated15. Low density lipoprotein (LDL) cholesterol and very low density lipoprotein (VLDL) cholesterol levels were calculated from the formula of Friedewald et al16, and atherogenic index was calculated by formula TC/HDL-C and LDL-C/HDL-C. The rats were sacrificed by cervical dislocation and the heart were rinsed, and weighed. The isolated heart was used for biochemical estimations of malondialdehyde (MDA)17, glutathione (GSH)18, glutathione peroxidase (GPx)19, glutathione reductase (GR)20, glutathione-S-transferase (GST)21, superoxide dismutase (SOD)22, catalase (CAT)23 and Na+ K+ ATPase24.
Cardiomyocyte apoptosis measurement: Caspase-3 (CPP-32) activity in heart tissue supernatant was measured spectrophotometrically and caspase-3 activity was calculated as nmole/h/mg protein25. Apoptosis was evaluated by examining the characteristic pattern of DNA laddering generated in the apoptotic myocardium using gel electrophoresis. Myocardial samples were homogenized in solution containing 50 mmol/l Tris-HCl (pH 8.0), 100 mmol/l EDTA, 100 mmol/l NaCl, and 1 per cent sodium dodecyl sulphate. The tissue homogenate was digested with 5 µl of proteinase K (stock solution 20 mg/ml) at 56 °C for two h and incubated with RNase A (1µl/ml) at 37°C for one h. After that phenol/chloroform (1:1) extraction was performed twice. The tubes were shaken and kept at room temperature (5-10 min). The tissues were precipitated and centrifuged in cold centrifuge at 4 °C; 7000 g for 10 min. Supernatant containing DNA was precipitated with 600 µl isopropanol and the resulting DNA pellets after centrifugation were washed with 75 per cent chilled ethanol and dissolved in 100 µl of TE buffer solution [10 mmol/l Tris HCl (pH 8.0), 1 mmol/l EDTA]. DNA samples (5 µl DNA+1 µl gel loading dye) were subjected to electrophoresis on 2 per cent agarose gel, stained with ethidium bromide. DNA laddering, an indicator of tissue apoptotic nucleosomal DNA fragmentation was visualized and photographed under ultraviolet transilluminator26.
Histopathological examination: The animals were killed and heart tissue samples were collected, fixed in 10 per cent formalin buffered solution, cut into 5 µm sections and stained with haematoxylin/eosin.
Statistical analysis: Comparisons between the treatment groups and control group were performed by one-way ANOVA followed by Dunnett's test.
Results
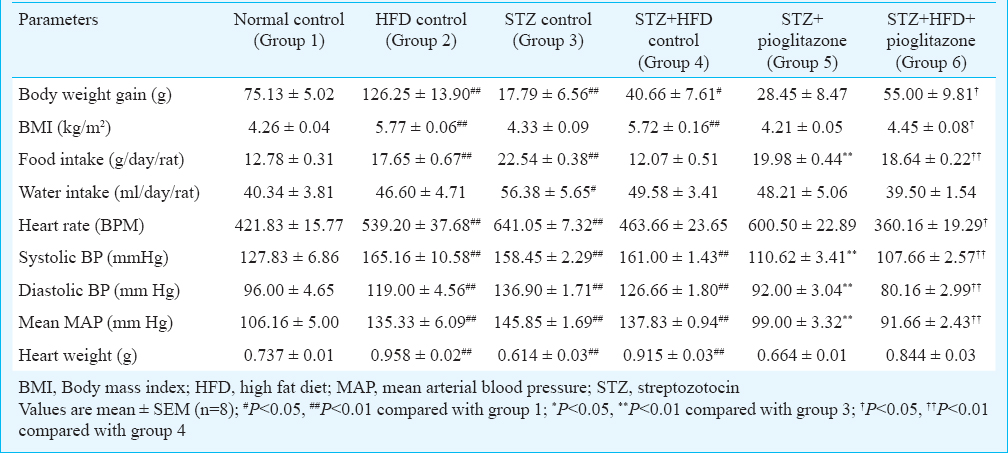
Effect of pioglitazone on anthropometric parameters: Table I Iillustrates the effects of pioglitazone on anthropometric parameters. A significant (P<0.01) increase in the body weight gain, BMI, food intake, blood pressure and heart weight was observed in the HFD control group; a significant (P<0.05 or P<0.01) increase in food intake, water intake and blood pressure while a significant (P<0.01) decrease in the body weight gain and heart weight were observed in STZ control; and a significant (P<0.01) increase in BMI, blood pressure and heart weight while a significant decrease (P<0.05) in body weight gain were observed in STZ+HFD control as compared to the normal control group. Treatment with pioglitazone (20 mg/kg, bw, orally) for 21 days significantly (P<0.01) decreased food intake and blood pressure in STZ treated group (group 5) when compared with STZ control rats (group 3). Pioglitazone (20 mg/kg, bw, orally) treatment significantly increased food intake and body weight gain (P<0.05 or P<0.01) in STZ+HFD treated (group 6) rats and significantly decreased BMI, blood pressure, and heart rate when compared with STZ+HFD treated rats (group 4).
Effect of pioglitazone on metabolic parameters: As shown in Table II, the levels of TC, TG, LDL-C, VLDL-C, atherogenic index, glucose, HOMA-IR, LDH, Apo-B and HbA1c in the HFD control or STZ control or STZ+HFD control rats were significantly (P<0.01) higher; the level of HDL-C in HFD control or STZ control were significantly (P<0.05 or P<0.01) decreased; and the levels of insulin were significantly (P<0.01) increased in HFD control group than those of the normal control group. The enhanced levels of TC, TG, LDL-C, VLDL-C, atherogenic index, glucose, HOMA-IR, LDH, Apo-B and HbA1c were brought down significantly (P<0.01) after 21 days of pioglitazone treatment in STZ-treated (group 5) and STZ+HFD-treated group (group 6) with a significant increase in HDL-C in the treated rats.

Effect of pioglitazone on cardiac myocyte apoptosis, Na+ K+ ATPase and oxidative stress parameters: As shown in Table III, the mean cardiac caspase-3 levels were significantly increased (P<0.01) in the HFD control or STZ control (groups 2, 3, 4) as compared to the normal control rats (group 1), while these levels were significantly reduced in STZ+ treated rats (group 5) compared with STZ control rats (group 3) or STZ+HFD pioglitazone treated rats (group 6) when compared with STZ+HFD control (group 4). Electrophoresis of DNA extracted from the left ventricle region of the heart of the HFD control or STZ control or STZ+HFD control rats (groups 2, 3, 4) showed DNA laddering indicating apoptotic inter-nucleosomal DNA fragmentation. Ladders were not detected in normal control rats, and STZ+pioglitazone or STZ+HFD+pioglitazone treated rats, where genomic DNA band was preserved. The mean Na+ K+ ATPase levels were significantly decreased (P<0.01) in the HFD control or STZ control or STZ+HFD control rats (groups 2, 3, 4) compared to the normal control rats (group 1). Treatment with pioglitazone significantly increased (P<0.01) the Na+ K+ ATPase levels in the cardiac tissue in STZ + pioglitazone treated rats (group 5) compared with STZ control rats (group 3) or STZ+HFD + pioglitazone treated rats (group 6) when compared with STZ+HFD control (group 4).
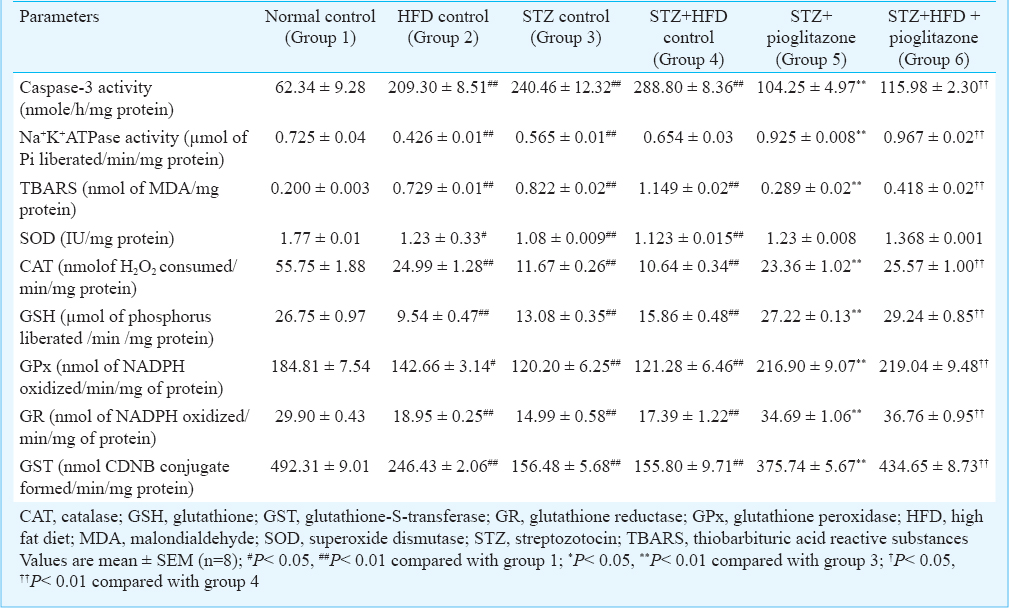
The mean TBARS levels were significantly increased (P < 0.01) in HFD control or STZ control or STZ+HFD control rats (groups 2, 3, 4) compared with the normal control rats (group 1), while treatment with pioglitazone significantly decreased (P<0.01) the TBARS levels in the cardiac tissue in STZ + pioglitazone treated rats (group 5) compared with STZ control rats (group 3) or STZ+HFD pioglitazone treated rats (group 6) compared with STZ+HFD control (group 4). The mean SOD, catalase, glutathione, glutathione peroxidase, glutathione reductase and glutathione-S-transferase levels were significantly (P<0.01) decreased in HFD control or STZ control or STZ+HFD control rats (groups 2, 3, 4) compared with the normal control rats (group 1), while these levels were significantly increased (P<0.01) in the STZ + pioglitazone compared with STZ control rats or STZ+HFD+ pioglitazone treated rats compared with STZ control rats.
Effect of pioglitazone on histopathological studies: The histopathology analysis of the heart tissue of HFD control or STZ control or STZ+HFD control rats (groups 2, 3, 4) showed dense focal fatty infiltration in the myocardial cells compared with normal control rat's heart (group 1) which showed normal architecture with regular morphology of myocardial cell membrane and well preserved cytoplasm (Figure). STZ+pioglitazone or STZ+HFD+pioglitazone treatment (groups 5, 6) showed normal morphology and no other pathological changes within the myocardium.

- Effect of pioglitazone on hematoxylin and eosin stained heart tissues sections of diabetic rats (x 100). (A) Normal control group showed no pathological changes with normal architecture (B) HFD control group showed deposition of fat globules in myocardial cells (arrow). (C) STZ control group showed a few calcified lesions with fatty particles in myocardial cell (arrow). (D) STZ+HFD control group showed dense focal fatty infiltration in myocardial cells (arrow). (E) STZ+pioglitazone showed no pathological changes in myocardial cells. (F) STZ+HFD+pioglitazone treated group showed no pathological changes with regular architecture of myocardium.
Discussion
In the present study, the oral administration of pioglitazone for a period of 21 days resulted in significantly lowering of the serum TC, TG, LDL-C, and apo-B, LDH, HbA1c, glucose, leptin levels in rats with STZ or STZ+HFD-induced diabetes. The reduction of TC or LDL-C in serum has been reported to lower the risk of coronary heart disease27 because high TC or LDL-C concentrations are a risk factor for coronary heart disease28. The clinical complications of atherosclerosis could be diminished when serum lipid concentration was lowered by hypocholesterolaemic agents29.
Increased blood pressure causes diastolic pressure overload, heart failure and instigates cardiac remodelling3031. The treatment with pioglitazone ameliorated both the increase in blood pressure and cardiac remodelling. Pioglitazone decreased the oxidative stress and increased the levels of antioxidant enzymes as reported earlier31. Moraes et al32 showed that neuronal apoptosis was induced by the fat-rich diet. Caspase-3 is a key player involved in the caspase-dependant apoptotic pathway33. In the present study, pioglitazone treatment was found to improve the cardiomyocyte apoptosis, induced by HFD in diabetic rats by lowering the cardiac caspase-3 levels and DNA laddering and increasing the cardiac Na+ K+ ATPase activity.
Due to safety concerns regarding the cardiovascular effect of rosiglitazone, the effect of pioglitazone on cardiovascular outcomes has been closely evaluated in recent years. A meta-analysis of 19 trials with pioglitazone in 16,390 patients with diabetes showed that pioglitazone was associated with a significantly lower risk of death, myocardial infarction, or stroke34. PPAR-γ activators have been shown to prevent cardiac hypertrophy, improve diastolic function, decrease collagen accumulation (fibrosis) and prevent myocardial ischaemic injury35. Our study findings indicate the cardiovascular safety profile of pioglitazone by showing decreased focal fatty infiltrations in diabetic rats.
In conclusion, our study showed a marked state of insulin resistance and obesity in STZ+HFD treated rats associated with various defects in glucose and lipid metabolism. Despite the persistence of obesity in these animals, pioglitazone treatment led to an apparent improvement in overall insulin sensitivity by ameliorating hyperleptinemia, dyslipidaemia, hyperinsulinaemia, hyperglycaemia, HOMA-IR index and oxidative stress, as well as protecting cardiomyocyte apoptosis and histopathological changes. The present results demonstrated that the pioglitazone exhibited a protective effect on cardiomyocyte apoptosis in diabetic rats.
Acknowledgment
Authors acknowledge the University Grants Commission, New Delhi, India for financial support.
Conflicts of Interest: None.
References
- Management of cardiac fibrosis in diabetic rats; the role of peroxisome proliferator activated receptor gamma (PPAR-gamma) and calcium channel blockers (CCBs) Diabetol Metab Syndr. 2011;3:4.
- [Google Scholar]
- Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptoticthioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2009;296:E1133-9.
- [Google Scholar]
- Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. 2009;58:1373-81.
- [Google Scholar]
- Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17:325-44.
- [Google Scholar]
- Rosiglitazone, a peroxisome proliferator-activated receptor-ã, inhibits the Jun (NH)2-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 2002;51:1507-14.
- [Google Scholar]
- Thiazolidinedione treatment normalizes insulin resistance and ischemic injury in the Zucker fatty rat heart. Diabetes. 2002;51:1110-7.
- [Google Scholar]
- Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002;16:1027-40.
- [Google Scholar]
- In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2001;104:2588-94.
- [Google Scholar]
- Pioglitazone retrieves hepatic antioxidant DNA repair in a mice model of high fat diet. BMC Mol Biol. 2008;9:82-91.
- [Google Scholar]
- Antidiabetic drug pioglitazone protects the heart via activation of PPAR-ã receptors, PI3-kinase, Akt, and eNOS pathway in a rabbit model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1558-65.
- [Google Scholar]
- Pioglitazone, a PPARgamma agonist, restores endothelial function in aorta of streptozotocin-induced diabetic rats. Cardiovasc Res. 2005;66:150-61.
- [Google Scholar]
- Evaluation of drugs with specific organ toxicities in organ-specific cell lines. Toxicol Sci. 2012;126:114-27.
- [Google Scholar]
- Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269-76.
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-8.
- [Google Scholar]
- Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984;44:5086-90.
- [Google Scholar]
- Glutthione-S-transferase. The first enzymatic step in mercaptouric acid formation. J Biol Chem. 1974;249:7130-9.
- [Google Scholar]
- Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469-74.
- [Google Scholar]
- Catalase activity. In: Greenwald RA, ed. Handbook of methods for oxygen radical research. Boca Raton: CRC Press; 2003. p. :283-4.
- [Google Scholar]
- Sodium potassium activated adenosine triphosphatase and cation transport. In: Bitler EE, ed. Membrane and ion transport. Vol 1. London: Interscience Wiley; 1970. p. :257-63.
- [Google Scholar]
- Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem. 1997;251:98-102.
- [Google Scholar]
- Total flavones from Elsholtzia blanda reduce infarct size during acute myocardial ischemia by inhibiting myocardial apoptosis in rats. J Ethnopharmacol. 2005;101:169-75.
- [Google Scholar]
- Anti-obesity and hypolipidemic effects of a proprietary herb and fiber combination (S&S PWH) in rats fed high-fat diets. J Med Food. 2008;11:169-78.
- [Google Scholar]
- Effect of genistein with carnitine administration on lipid parameters and obesity in C57Bl/6J mice fed a high-fat diet. J Med Food. 2006;9:459-67.
- [Google Scholar]
- Pioglitazone prevents cardiac remodeling in high-fat, high-calorie-induced Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2006;291:H81-7.
- [Google Scholar]
- Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension. 2004;43:48-56.
- [Google Scholar]
- Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;8:2784-91.
- [Google Scholar]
- Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta- analysis of randomized trials. JAMA. 2007;298:1180-8.
- [Google Scholar]






