Translate this page into:
Protective effect of antigen excess immune complex in guinea pigs infected with Mycobacterium tuberculosis
Reprint requests: Dr Harshavardhan Shakila, Department of Molecular Microbiology, School of Biotechnology, Madurai Kamaraj University, Madurai 625 021, Tamil Nadu, India e-mail: mohanshakila@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Immune complexes (ICs) play a crucial role which can either be beneficial or pathological to the host. Involvement of circulating immune complexes (CICs) has been shown in tuberculosis (TB) cases (adults and neonates form), but its immunomodulatory effect has not been studied in vivo. Hence, this study was carried out to understand and explore the prognostic therapeutic potential of CICs on the host immune system in guinea pigs animal TB model.
Methods:
In this study, the guinea pigs (group I) were immunized with in vitro synthesized antigen excess IC (AgX-IC), group II with antibody excess IC (AbX-IC) and group III with phosphate-buffered saline. All these animals were sensitized with Mycobacterium tuberculosis H37Rv before immunization and subsequently infected with M. tuberculosis H37Rv strain post-immunization with IC.
Results:
Mortality was observed in animals belonging of groups II and III, while all animals in group I survived. A steady increase in the body weight of animals immunized with AgX-IC was observed when compared to the other groups. The infection load in the spleen and lungs was less in animals from group I when compared to the other groups. The CICs were found to be in higher concentration in serum of IC-immunized guinea pigs when compared to ICs non-immunized animals.
Interpretation & conclusions:
Based on our findings, it can be speculated that the ICs may have a protective immunomodulatory role pertaining to disease progression and development of pathology. As a new perspective, with further insight into the underlying mechanism of action and correlation with clinical data, ICs may also be used as a potential tool for assessing the immune status of the infected individuals, especially the close contacts of TB patients.
Keywords
Guinea pigs
immune complex
immunomodulation
infection load
Mycobacterium tuberculosis
Immune complexes (ICs) are commonly known for their role in the pathophysiology of diseases such as systemic lupus erythematosus, rheumatoid arthritis, various infectious diseases and malignancies1. In a study, Baldwin et al2 blocked lymphocyte-mediated cytotoxicity using specific ICs in rat hepatoma cells. Similar findings were also reported by Sjögren et al3 in tumour-affected individuals. In Leishmania infections, IgG ICs have been reported to boost the immune system by inducing interleukin-10 (IL-10) production from macrophages4. Such beneficial role of ICs pertaining to tuberculosis (TB) has not been much studied.
Two to three billion people are infected with TB as per the WHO Global Tuberculosis Report for the year 20175. Among the infected, 5-15 per cent develop TB disease annually, which accounted for 1.5 million deaths in 20175. Development of drug resistance makes the treatment further difficult. Hence, the development of new vaccine strategies, treatment methods and a better understanding of host immune response against TB are important. The presence of ICs has been demonstrated in TB patients at various stages of the disease. Simonney et al6 demonstrated circulating ICs in paediatric TB cases. These ICs have implication in the evaluation of treatment, drug efficacy and progression of disease1. ICs were observed to activate certain effector mechanisms of granulocytes to induce chemotaxis, phagocytosis and to have a profound effect on its function7. This study was aimed at exploring the prognostic therapeutic potential of circulating immune complexes (CICs) in guinea pigs which is a competent animal model for mimicking human TB infection.
Material & Methods
Mycobacterium tuberculosis strain H37Rv was used for infection and sensitization experiments. The culture was maintained in Middlebrook 7H9 medium (Sigma-Aldrich, USA) supplemented with glycerol, OADC (Oleic Albumin Dextrose Catalase) and antibiotics. The following reagents were obtained through Biodefense and Emerging Infections Research Resources Repository (BEI Resources), National Institute of Allergy and Infectious Diseases (NIAID), NIH, USA: M. tuberculosis strain H37RV-culture filtrate proteins (CFP) - NR-14825 and polyclonal anti-M. tuberculosis CFP (antiserum rabbit) - NR-13808. The study was carried out from September to December 2014.
Sensitization of guinea pigs with M. tuberculosis strain H37RV: Outbred Dunkin Hartley strain Cavia porcellus (guinea pigs) were used. Animal maintenance and experiments were carried out in a BSL3 animal house facility at the National JALMA Institute of Leprosy and other Mycobacterial Diseases, Agra, India. A total of 15 male guinea pigs divided into three groups were used. All animals were acclimatized for the laboratory environment. Proper feed, temperature, humidity and light cycle were ensured and housed in an air-filtered environment. All the animals of each group were sensitized with 103 colony-forming units (cfu) of M. tuberculosis H37Rv strain. Sensitization was carried out in an inhalation exposure system (Allen Bradley, USA; Model no: A4224) which carries out the process in four steps, namely pre-heating of the chamber to 37°C (15 min), nebulization (45 min), cloud decay (30 min) and decontamination by ultraviolet (15 min). The body weight of each animal was monitored during the entire period of the study. All procedures were reviewed and approved by the Institutional Animal Ethical Committee of National JALMA Institute of Leprosy and other Mycobacterial Diseases, Agra, India.
Immune complex (IC) preparation: In vitro preparation of CFP and the anti-CFP IC was carried out at the department of Molecular Microbiology, Madurai Kamaraj University, Madurai, India, using protocol by Geffner et al8. Antigen and antibody mixture was incubated at 37°C for one hour, followed by overnight incubation at 4°C. The mixture was centrifuged and the supernatant was used as soluble ICs. ICs were prepared at two proportion, one with antigen excess (AgX) and another with antibody excess (AbX) at 1:2 ratio. After the preparation of ICs, estimation of total protein concentration was done9.
Immunization of guinea pigs with immune complexes (ICs): Sensitized guinea pigs were divided into three groups with each having five animals. After 30 days of post-sensitization with M. tuberculosis H37Rv, the first group was immunized with AgX-IC (n=5), the second group with AbX-IC (n=5) and the third group (n=5) was kept as control which was given mock immunization with phosphate-buffered saline. The total protein concentration of ICs given to the first and second group was kept same as 375 μg per animal. One guinea pig was kept as a naïve animal without subjecting it to neither immunization nor infection.
Infection of guinea pigs with M. tuberculosis strain H37RV: Seven day post-immunization, all the three groups of animals except the naïve animal were subjected to infection with 106 bacilli of M. tuberculosis strain H37Rv using the inhalation exposure system as mentioned previously.
Euthanization and harvesting the organs: On 60th day post-immunization, all the animals were euthanized as per recommendations of the Committee for the Purpose of Control and Supervision of Experiments on Animals (http://cpcsea.nic.in/Auth/index.aspx) and vital organs such as the spleen and lungs were collected for further analysis. Euthanization and collection of organ materials were carried out in BSL3 workstation to ensure safe handling and to prevent contamination of the environment with the TB pathogen.
Enumeration of colony-forming units (cfu): Harvested tissues were homogenized using tissue homogenizer (Polytron Corporation, Canada). One millilitre of the homogenate was further diluted and plated onto Middlebrook 7H11 medium supplemented with glycerol, OADC and antibiotics and incubated at 37°C. Total colony-forming unit (cfu) per gram of tissue was calculated as a log10 value using the following formula and graphs were plotted.
Total cfu/ml=cfu×Volume factor×Dilution factor×Volume of the homogenate
Total cfu/g=Total cfu/weight of tissue (g)
Isolation and quantification of circulating immune complex from serum: Blood was collected from guinea pigs on the 7th and 60th day post-infection by the retro orbital sinus bleeding method. Serum was collected and stored at −80°C till further use. ICs were isolated using Przybylski and Gołda10 method using 7 per cent polyethylene glycol. The absorbance was read at 280 nm and 260 nm using a spectrophotometer and total protein concentration was estimated using 280/260 nm ratio9.
Sample size calculation and power of the study: The sample size was calculated using online software G*Power version 3.1.9.2 (Franz, Universitat Kiel, Germany). With the power of 95 per cent, the significance of 0.05 level and the effect size of 0.70, sample size was calculated to be 16. These 16 guinea pigs were randomly assigned into three experimental groups as mentioned earlier.
Statistical analysis: Data were expressed as mean±standard deviation from individual guinea pigs within each group (n=5). All statistical analysis was performed using GraphPad Prism software version for Windows (GraphPad Software Inc., La Jolla, CA, USA). As indicated by Shapiro–Wilk test, the samples were normally distributed within each group. The difference in body weight, cfu count and CIC concentration was determined by Student's t test between the experimental groups and control group.
Results
Effect of immune complex on mortality: Post-sensitization, guinea pigs from the three groups were monitored for any symptoms of TB and fatality. Post-infection, animals from the third group which was given mock immunization succumbed to death first and later death was observed in the second group which was immunized with AbX-IC. At the end of 60 day post-infection among the three groups, the first group (AgX-IC) did not show any mortality, whereas mortality was 20 and 40 per cent with the groups II and III, respectively.
Effect of immune complex on body weight: The body weight of each animal was recorded between initiation and end of the study before euthanization. Post-infection, there was a significant difference in the pattern of change in body weight. The second and third group showed a decline in average body weight, whereas the animals from the first group showed a steady increase (Fig. 1).
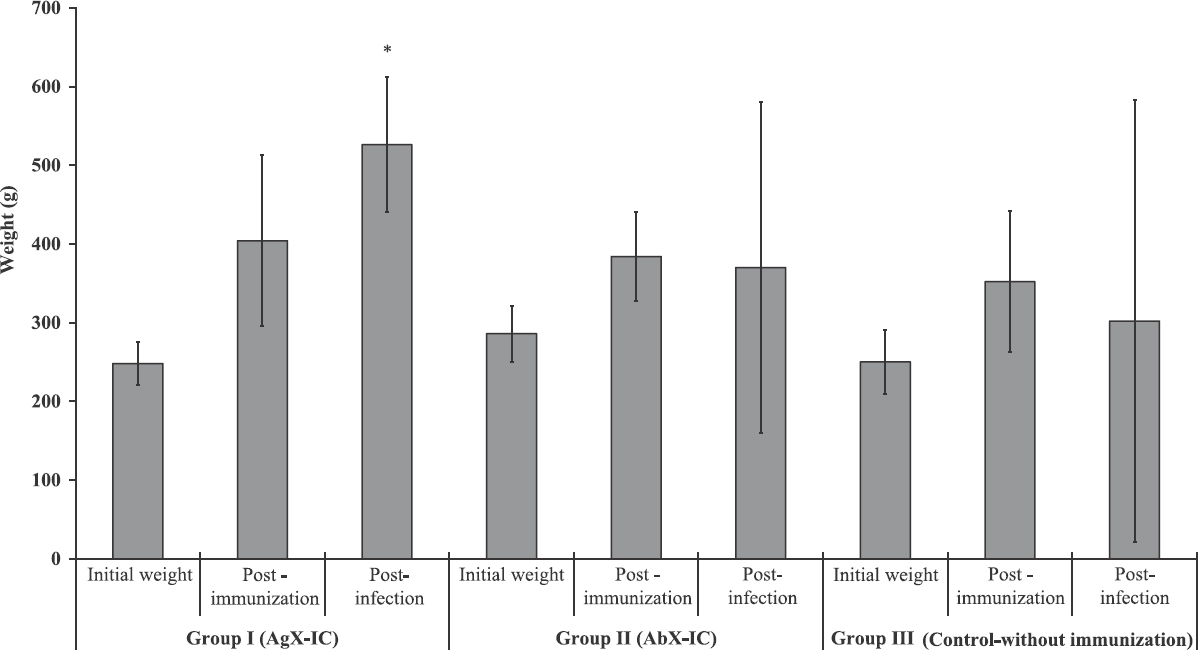
- Mean±standard deviation (n=5) of body weight of guinea pigs. The group I antigen excess IC-immunized guinea pigs showed a sustained increase in body weight whereas group II immunized with antibody excess ICs stopped progressing. The control group showed a decline in body weight. Group I (antigen excess IC), guinea pigs immunized with antigen excess immune complex; Group II (antibody excess IC), guinea pigs immunized with antibody excess immune complex; Group III (control-without immunization), non-immunized control guinea pigs.*P<0.05 compared to Group III (Control without immunization).
Effect of immune complexes (ICs) on bacterial load: Number of M. tuberculosis cfu was enumerated in the lungs and spleen. The cfu was recorded as log10 value. Naïve animal showed no detectable level of cfu showing that the aerosol contamination in the laboratory environment was considerably negligible. In all the three groups of animals, spleen showed an increased cfu count when compared with the lungs. In both the lungs and spleen, animals immunized with AgX-IC showed lesser infection load, followed by animals immunized with AbX-IC and animals without immunization. In the lungs (Fig. 2), the average log10 value of cfu was 4.59, 4.77 and 5.02 for groups I, II and III, respectively, whereas, in spleen the values were 5.22, 5.39 and 5.53 (Fig. 2). The values of AgX-IC group were found to be significant (P<0.05) when compared with the control group which was not immunized with ICs but infected. This evidently accentuates that the ICs do have an effect on the establishment of infection load in the organs. In our study, antigen excess IC found to have a better control over the infection load.
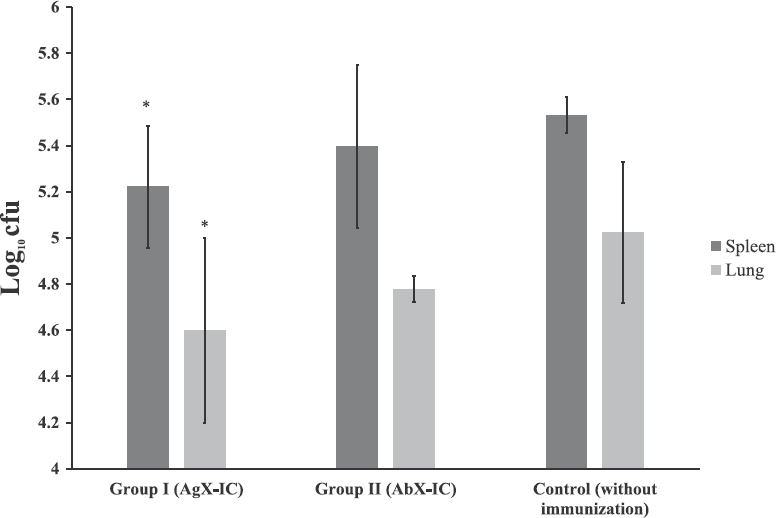
- Mean±standard deviation (n=5) of Mycobacterium tuberculosis infection load in guinea pigs infected post-immunization. The infection load is expressed as log10 cfu. Group I (antigen excess IC), animals immunized with antigen excess IC; Group II (antibody excess IC), animals immunized with antibody excess immune complexes; control (without immunization), non-immunized animals.*P<0.05 compared with control group.
Pathophysiology of tissues from guinea pigs infected with M. tuberculosis: External physiological appearances of the internal organs of guinea pigs were analyzed based on the visual appearance (Fig. 3). Pathological conditions such as lung enlargement, pale lung, splenomegaly, hepatomegaly, swollen lymph nodes, necrotic spots, surface tubercles and caseous spots on various organs were observed. Conditions such as lung enlargement with fluid accumulation and splenomegaly were commonly found in all animals of group I immunized with AgX-ICs apart from necrotic spots and surface tubercles. Group II animals displayed splenomegaly prominently rather than other conditions. Necrotic spots were also observed diffusely. As expected, IC non-immunized guinea pigs from group III displayed almost all prominent symptoms of TB showing the severity of infection and poor host response. The lungs became pale with a few necrotic spots and enlarged with full infection. The animals not immunized with the ICs showed severe pathology when compared to the animals which were immunized with the ICs. Group I animals showed lesser pathology when compared to group II animals.
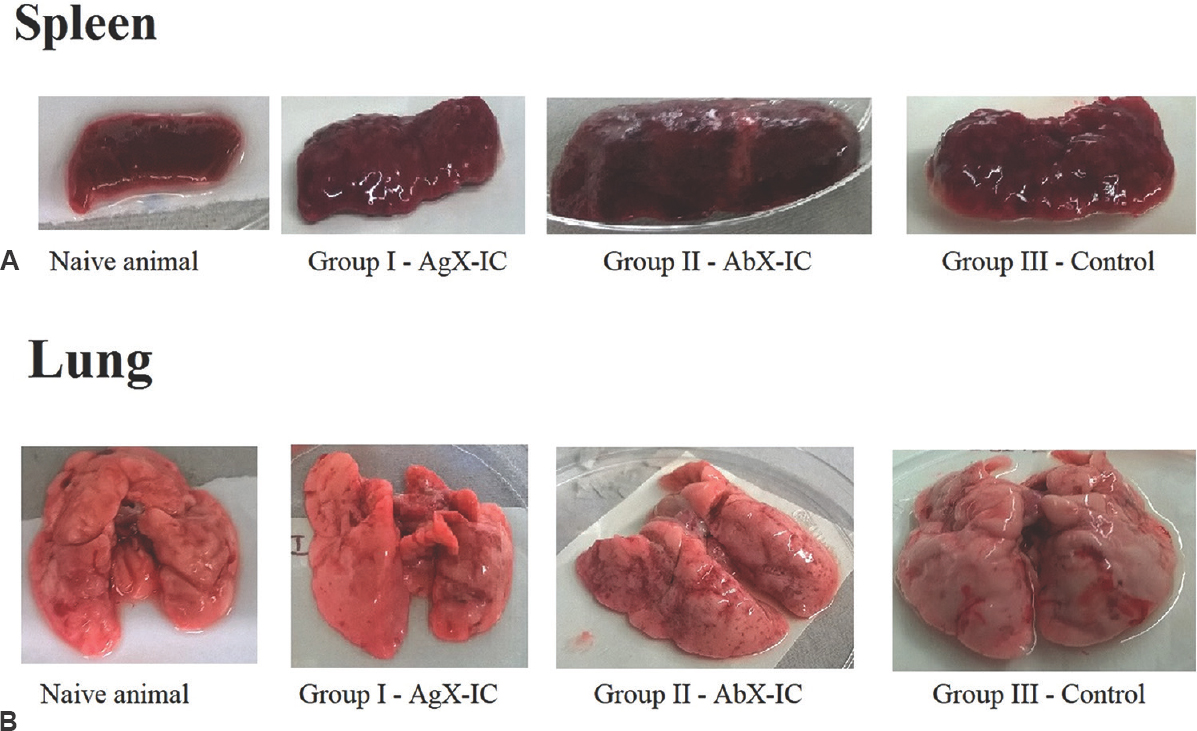
- Pathophysiology of guinea pig spleen (A) and lung (B) infected with Mycobacterium tuberculosis. Typical tuberculosis signs such as tubercles, necrosis and inflammation were observed in the spleen whereas caseation, haemorrhage, necrosis and inflammation were observed in the lungs of all guinea pigs subjected to the study. Guinea pigs immunized with antigen excess ICs showed lesser degrees of pathogenicity. Group I - guinea pigs immunized with antigen excess immune complex; Group II - guinea pigs immunized with antibody excess immune complex; Group III - non-immunized control guinea pigs.
Immune complexes (ICs) immunization increases circulating immune complexes (CICs) concentration: IC-immunized groups showed a remarkable increase in the concentration of CIC isolated from serum. There was an increase in the CIC level between initial days of post-infection and during the time of euthanization in groups I and II. Such increase in CIC level was not observed in the control group (Fig. 4). The level of CICs in the non-IC-immunized control group was also lower than that of immunized groups. There was no significant difference in ICs concentration between groups I and II.
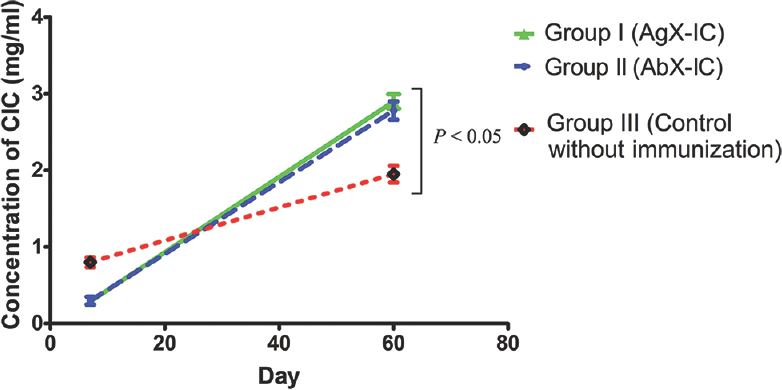
- Mean±standard deviation (n=5) of the level of circulating immune complexes (CICs) from guinea pigs serum. Group I (antigen excess IC), guinea pigs immunized with antigen excess immune complex; Group II (antibody excess IC), guinea pigs immunized with antibody excess immune complex; Group III (Control without immunization), non-immunized control guinea pigs. The difference between immunized groups and non-immunized control group was significant (P<0.05).
Discussion
CICs and complement system are the mediators of host humoral defense11. The ICs are eliminated by phagocytes from circulation by binding to the complement receptor CR1, 3, 4 localized on erythrocyte, granulocyte, monocyte and macrophage surface12. Formation of ICs is immunologically a positive effect13. However, in some cases, CICs are not eliminated and stored in tissues and may be the cause of various complications14.
The presence of CICs has been demonstrated in a variety of diseases and disorders, especially in the case of childhood TB6. CICs level has been correlated with the severity of the disease, for instance, in dermal onchocercal lesions, CICs level is higher in infected individuals compared to the uninfected15. Patients with chronic lymphatic obstruction or uninfected controls showed lower levels of CICs compared to asymptomatic infected patients16. It has been shown that the CICs levels and occurrence are high in most of the patients with active TB117. The presence of CICs might be connected with persisting, not fully recovered TB. Johnson et al18 reported that 1-2 months of therapy did not have any influence on the level of CICs, whereas Raja et al19 reported a decrease in the level of ICs after six months of treatment.
In our study, it was found that the ICs with excess antigen protected the animals from developing symptoms of the disease with subsequent infection, whereas the animals immunized with antibody excess ICs were relatively more susceptible. All the animals of AgX-IC-immunized group survived and had lesser infection load when compared to control group. The pathophysiological condition of the AgX-ICs immunized group was better when compared with the other groups. Body weight is an important factor to be considered in TB. The AgX-IC-immunized animals showed an increase in body weight, whereas that of control group declined. The ICs non-immunized control group showed a significantly lower level of CICs compared to IC-immunized animals. This indicated modulatory effects of ICs on the immune system pertaining to TB infection. This protective effect may be the result of the involvement of cell-mediated immunity instigated by tissue antigen presenting cells (APCs), which subsequently leads to a cascade of immune reaction20. Further molecular studies are required to understand this modulatory effect and the kind of protection established by immunization with ICs.
The variation in ICs level between the IC-immunized and non-immunized groups can be extrapolated to be the reason behind the asymptomatic condition of infected close contacts of TB infection. As it is known that not all infected individuals do not develop the disease, many remain asymptomatic throughout the life and some may become latent TB cases21. ICs level may be an important factor for the host to progress and develop symptoms of the disease. Currently, there are no gold standard methods for discriminating individuals who are infected carriers and diseased and also to predict the fate of disease progression in close contacts of TB patients. Hence, the immunomodulatory potential of ICs can be used as a tool for assessing the immune status of the close contacts. Gonçalves et al22 suggested that ICs screening could be used as an alternative for early diagnosis of strongyloidiasis in immunocompromised individuals. Since the ICs have an immunomodulatory effect on the host immune system and act as a molecular platform for adjuvant-free vaccine delivery23, these may be considered as one of the vaccine candidates or carrier of vaccine for TB. Using guinea pig as an animal model had limitations, especially the availability of immunological reagents and animal maintenance. Hence as an alternate, studies using Zebrafish as a model organism for TB is currently being attempted in our laboratory.
In conclusion, immunization of guinea pigs with AgX-ICs conferred a protective effect by decreasing the mortality, increasing the body weight and lowering the level of infection load. Our study indicates that ICs may have some modulatory roles in the development of disease conditions, and especially in the case of TB, it is towards a protective direction; however, further deep insights at the molecular level with clinical correlation are required to substantiate this indication.
Acknowledgment
The authors thank Council of Industrial and Scientific Research (CSIR), New Delhi, India, for the financial support. The complimentary supply of CFP antigen and anti-CFP antibody by BEI resources, NIAID, NIH is acknowledged. The authors acknowledge the support of technical staffs from National JALMA Institute of Leprosy, Agra, India.
Conflicts of Interest: None.
References
- Significance of circulating immune complexes in pulmonary tuberculosis. Clin Exp Immunol. 1984;58:317-24.
- [Google Scholar]
- Blocking of lymphocyte mediated cytotoxicity for rat hepatoma cells by tumor-specific antigen-antibody complexes. Nat New Biol. 1972;238:185-7.
- [Google Scholar]
- Suggestive evidence that the “blocking antibodies” of tumor-bearing individuals may be antigen-antibody complexes. Proc Natl Acad Sci U S A. 1971;68:1372-5.
- [Google Scholar]
- A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med. 2005;201:747-54.
- [Google Scholar]
- Global Tuberculosis Report - 2017. Geneva: WHO; 2017.
- Analysis of circulating immune complexes (CICs) in childhood tuberculosis: Levels of specific antibodies to glycolipid antigens and relationship with serum antibodies. Int J Tuberc Lung Dis. 2000;4:152-60.
- [Google Scholar]
- Immune complexes isolated from patients with pulmonary tuberculosis modulate the activation and function of normal granulocytes. Clin Vaccine Immunol. 2012;19:1965-71.
- [Google Scholar]
- Neutrophil cytotoxicity induced by immune complexes prepared with cationized antibodies. Scand J Immunol. 1993;37:187-93.
- [Google Scholar]
- Spectrophotometric determination of protein concentration. In: Wrolstad RE, ed. Current Protocols in Food Analytical Chemistry. New York: John Wiley & Sons, Inc; 2002. p. :B131-B137.
- [Google Scholar]
- Research on the occurrence of Mycobacterium tuberculosis antigens in the circulating immune complexes, isolated from serum of patients with tuberculosis. Med Sci Monit. 2014;20:6-10.
- [Google Scholar]
- The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679-91.
- [Google Scholar]
- Microbial immunology: A new mechanism for immune subversion. Curr Biol. 1998;8:R99-101.
- [Google Scholar]
- Immune complex nephritis complicating miliary tuberculosis. Br Med J (Clin Res Ed). 1983;287:1593-4.
- [Google Scholar]
- Associations between clinical disease, circulating antibodies and C1q-binding immune complexes in human onchocerciasis. Parasite Immunol. 1987;9:447-63.
- [Google Scholar]
- Heightened measures of immune complex and complement function and immune complex-mediated granulocyte activation in human lymphatic filariasis. Am J Trop Med Hyg. 2011;85:89-96.
- [Google Scholar]
- Presence of circulating immune complexes in serum of tuberculosis patients. Med Sci Monit. 1999;5:1123-8.
- [Google Scholar]
- Characterization of mycobacterial antigens and antibodies in circulating immune complexes from pulmonary tuberculosis. J Lab Clin Med. 1995;125:581-7.
- [Google Scholar]
- Immunobiology: The Immune System in Health and Disease. (5th ed). New York: Garland Science; 2001. p. :3-38.
- [Google Scholar]
- Antigen, antibody and immune complex detection in serum samples from rats experimentally infected with Strongyloides venezuelensis. Exp Parasitol. 2012;130:205-8.
- [Google Scholar]
- Immune-complex mimics as a molecular platform for adjuvant-free vaccine delivery. PLoS One. 2013;8:e60855.
- [Google Scholar]






