Translate this page into:
Protection against osteoarthritis in experimental animals by nanogold conjugated snake venom protein toxin gold nanoparticle-Naja kaouthia cytotoxin 1
Reprint requests: Dr Antony Gomes, Department of Physiology, Laboratory of Toxinology & Experimental, Pharmacodynamics, University of Calcutta, 92 A P C Road, Kolkata 700 009, West Bengal, India e-mail: agomescu@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Increased severity of osteoarthritis (OA) and adverse side effects of its treatment led to the search for alternative therapies. It was previously reported that snake venom protein toxin Naja kaouthia cytotoxin 1 (NKCT1) and gold nanoparticle (GNP) individually have potential against excremental arthritis. In this study, we analyzed the protective activity of GNP conjugated protein toxin NKCT1 (GNP-NKCT1) against experimental OA.
Methods:
Gold nanoparticle conjugation with NKCT1 (GNP-NKCT1) was done and its physiochemical properties were studied. OA was induced in male albino rats by intra-articular injection of bacterial collagenase and treatment was done with NKCT1/GNP-NKCT1/standard drug (indomethacin). Physical parameter (ankle diameter), urinary markers (hydroxyproline, glucosamine, pyridinoline, deoxypyridinoline), serum and synovial membrane pro-inflammatory markers [tumour necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), IL-17, vascular endothelial growth factor (VEGF)] and matrix metalloproteinase 1 (MMP1) were measured. Joint histopathology and scanning electron microscopy imaging of articular cartilage surface were also done.
Results:
Physical parameters, urinary markers, serum and synovial membrane pro-inflammatory makers and MMP1 were increased in arthritic rats and significantly restored after GNP-NKCT1/NKCT1 treatment. Joint histopathology and scanning electron microscopy imaging of articular cartilage surface also indicated the protective effect of GNP-NKCT1 against inflammatory response and cartilage degradation in osteoarthritic rats.
Interpretation & conclusions:
In this study restoration of the arthritic markers and bone degradation by GNP-NKCT1 treatment indicated the anti-osteoarthritic property of GNP-NKCT1. Further studies need to be done to confirm these findings.
Keywords
GNP-Naja kaouthia cytotoxin 1
gold nanoparticle
Naja kaouthia
nanoconjugation
osteoarthritis
Osteoarthritis (OA) is one of the most common forms of arthritis caused mainly due to injury, inflammation and ageing process1. The pathological lesions of OA are characterized by clustering of the extracellular matrix (ECM) with exposure of subchondral bone and subchondral bone sclerosis with osteopathy formation2. The ECM of the articular cartilage is composed of type-II collagen and proteoglycans. Pathologic condition of OA is related to degradation of ECM along with the symptoms such as pain, stiffness, swelling, warmth and creaking of the affected joints.
Besides physiotherapy and exercise, available drugs for OA mainly include non-steroidal anti-inflammatory drugs (NSAIDs such as ibuprofen, aceclofenac), disease-modifying antirheumatic drugs (DMARDs such as ethotrexate, cyclosporin A), and anticytokine therapy (infliximab)3. Gastrointestinal irritation, increase in cardiovascular risk, nephrotoxicity, loss of response with chronic use are well-known limitations of these drugs.
In the traditional systems of medicine in India, treatment of arthritis and pain with cobra snake venom was a common practice4. It was reported that Indian cobra (Naja kaouthia) venom (NKV) prevented arthritis by lowering the proinflammatory cytokines in animal model5. Further, a protein toxin Naja kaouthia cytotoxin 1 (NKCT-1) having cytotoxicity, neuro- and cardiotoxicity was isolated from NKV6. The NKCT1 was found to possess anti-inflammatory and anti-nociceptive property7. A cytotoxin molecule NN-32, isolated from Naja naja venom has also been reported to possess anti-arthritic property8. Gold has been reported as a therapeutic agent against arthritis in traditional medicine systems of India910. Research in the area of nanotechnology revealed that gold nanoparticles had anti-inflammatory property and could be used for the treatment of arthritis11. Bhowmik et al12 showed the successful conjugation of gold nanoparticle with NKCT1 (GNP-NKCT1) which increased the bioactive potential of NKCT1 against leukaemia. In our previous studies GNP-NKCT1 showed protective activity against Ehrlich ascites carcinoma and rheumatoid arthritis in experimental animal model713. The present study was an effort to investigate the activity of nanogold conjugated NKCT1 against OA in the experimental animal model.
Material & Methods
This study was conducted in the departments of Physiology and Biochemistry, University of Calcutta, Kolkata, India. The experiments were conducted according to the departmental animal ethics committee for the purpose of control and supervision of experiments on animals. All animal experiments were approved by the Institutional animal ethics committee (Ref No: 820/04/ac/CPCSEA.2010).
Purification of Naja kaouthia cytotoxin 1 (NKCT1): NKCT1 was purified from NKV by ion-exchange column chromatography and high performance liquid chromatography (HPLC). Lyophilized NKV was purchased from Calcutta Snake Park, Kolkata, India. Venom concentration was expressed in terms of dry weight/protein equivalent14. The fraction was desalted and concentrated by centricon (MWCO 3k, Millipore). Purified NKCT1 was checked for homogeneity by sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis15.
Synthesis and characterization of GNP-NKCT1: GNP-NKCT1 was prepared and characterized according to Bhowmik et al12. Size distribution of GNP-NKCT1 was confirmed through dynamic light scattering (DLS) study.
Experimental animals: Wistar male albino rats (body weight 120±10 g) were procured from the approved animal breeders and housed in standard polypropylene cages at controlled temperature (25±2°C) and relative humidity (65±5%) with 12 h light and dark cycle. The animals were provided with pellet diet, green vegetables and water ad libitum.
Anti-osteoarthritic activity
Development of experimental osteoarthritis: Experimental OA was developed by intra-articular injection of 20 μl bacterial collagenase (5 CDU, Sigma, USA) in the right knee joint. Same amount of 0.9 per cent saline was injected in the sham control knee joint16.
Treatment schedule: It has been previously reported that NKCT1 possesses anti-inflammatory property. Therefore, a comparative analysis of anti-arthritic potential of NKCT1, a clinically used standard drug (indomethacin) and GNP conjugated NKCT1 was done. In this study, 30 male albino Wistar rats were divided by simple random selection17 into the five groups (n=6). Rats were divided into the following five groups. Groups 1 and 2 were taken as negative (sham) and positive (arthritis) control, respectively. Group 3: indomethacin (SRL, India) treated (0.25 mg/100 g × 5 days alternately, po), group 4: NKCT1 treated (2 μg/100 g × 14 days, ip) and group 5: GNP-NKCT1 treated (2 μg/100 g × 14 days, ip). Treatment period was scheduled according to experimental model for OA established by van der Kraan et al16. Treatment started from the next day after OA induction. The rats in the control group received the same volume of vehicle. Urine was collected on day 14. On day 16, blood was collected and serum was separated for the analysis of biochemical parameters, using laboratory kits (Merck, India) and through ELISA kit (RayBio, USA) using an ELISA reader (BioTek, ELx800, USA).
On days 0, 2, 5, 10 and 15, ankle swellings of all animals were measured (mm) with digital caliper (Mitutoyo, Japan). Ankle diameter was measured prior to arthritis induction was used as the control diameter (day 0). Ankles of the rats were finally collected for histological studies18.
Analysis of urinary parameters and serum markers: Urinary hydroxyproline and glucosamine were measured spectrophotometrically19 (λ=555 and 540 nm, respectively), and urinary pyridinoline20 and deoxypyridinoline levels were measured by ELISA (RayBio, USA). Serum markers for OA (TNF-α, IL-1β, MMP-1, VEGF, IL-17) were measured by ELISA (R&D, USA).
Analysis of inflammatory parameters in the joint tissue: Expression of pro-inflammatory signalling molecule in synovial membrane was analyzed through Western blot21. The tissue samples were collected from sham control, arthritis control, NKCT1- and GNP-NKCT1-treated groups, homogenized in complete radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Inc., USA). The total protein was quantified with a bicinchoninic acid protein assay kit (Sigma, USA). All preparations were performed at 4°C. Synovial protein (30 μg) was subjected to a 12 per cent SDS-polyacrylamide gel and blotted onto polyvinylidene difluoride membrane. Membranes were incubated with the desired primary antibody (IL-1β, TNF-α from BD, USA) overnight at 4°C after blocking with 5 per cent skim milk in TBST (Tris buffered saline, 0.1% Tween 20) for 3 h. Next day, these were incubated with an appropriate horse radish peroxidase-conjugated secondary antibody for 1 h. After being washed, the membranes were visualized by enhanced chemiluminescence.
Histopathological studies of ankle joint: Ankle joints were collected from sham control, arthritis control, NKCT1- and GNP-NKCT1-treated groups, fixed in 10 per cent buffered formalin for 24 h, decalcified in osteomol for five days, dehydrated in graded alcohol (50, 70, 80, 90, 100%), cleared in xylene and embedded in paraffin wax (56-58°C). Sections (5 μm) were cut with rotary microtome (Weswox Optik, India), stained with haematoxylin-eosin and observed under bright field microscope (Motic BA 450, Germany) and photographs captured with Motic software (Motic Images Plus 2.0 software).
Study of articular cartilage by scanning electron microscopy: Samples were collected from sham control, arthritis control, NKCT1- and GNP-NKCT1-treated groups, prefixed with 2.5 per cent glutaraldehyde in phosphate-buffered saline (PBS), rinsed with PBS, fixed with 1 per cent osmium tetroxide in PBS for 2 h, and dehydrated in a series of ethanol, followed by critical-point drying with an HCP-2 apparatus (Hitachi, Japan), employing CO2 as the transitional fluid. The specimens mounted on stubs were coated with platinum and were examined with a scanning electron microscope (S-4500; Hitachi).
Statistical analysis: One-way analysis of variance was employed for statistical analysis using Origin pro 8 software (OriginLab, Northampton, MA, USA).
Results
GNP-NKCT1 was a light purple coloured colloidal solution, stable at room temperature (25±2°C) and pH 7.2. Surface plasmon resonance spectroscopy of NKCT1 (λmax=220 nm), GNP (λmax=530 nm) and GNP-NKCT1 (both the λmax=220 nm and λmax=528 nm) were detected by ultra violet-visible (UV-Vis) spectroscopy. Hydrodynamic size of GNP-NKCT1 determined by DLS was found to be 68-122 nm with an average size of 92 nm.
Anti-osteoarthritic activity of GNP-NKCT1
Effect of GNP-NKCT1 on physical parameter: Induction of OA resulted in a significant increase of the ankle diameter (7.23±0.84mm) in arthritis control and treatment groups on day 2 compared to sham control group 1 rats (4.13±0.13 mm). After 15 days treatment with GNP-NKCT1 ankle diameter was significantly decreased up to 4.81±0.41 mm; NKCT1 treatment significantly (P<0.05) decreased ankle diameter up to 5.13±0.65 mm compared to arthritic control group 2 rats (6.51±0.57 mm). Standard drug treatment significantly decreased ankle diameter up to 5.48±0.47 mm after day 15 (Table).

Effect of GNP-NKCT-1 on urinary parameters: After 15 days of OA induction, urinary hydroxyproline, glucosamine, pyridinoline deoxipyridinoline levels were significantly (P<0.05) increased in group 2 arthritic animals compared to group 1 sham control animals confirming the onset of OA. GNP-NKCT1 treatment significantly decreased hydroxyproline, glucosamine, pyridinoline and deoxipyridinoline level by 73.4±0.2, 74.27±0.6, 63.1±0.7 and 82.4±0.8 per cent, respectively compared to osteoarthritic control group 2 rats. NKCT1 treatment significantly (P<0.05) decreased these urinary parameters compared to osteoarthritic control group 2 rats, whereas standard drug treatment significantly decreased those urinary parameters by 63.2±0.7, 56.3±0.4, 64.32±0.5 and 68.4±0.7 per cent respectively as compared to osteoarthritic control group 2 rats (Fig. 1).
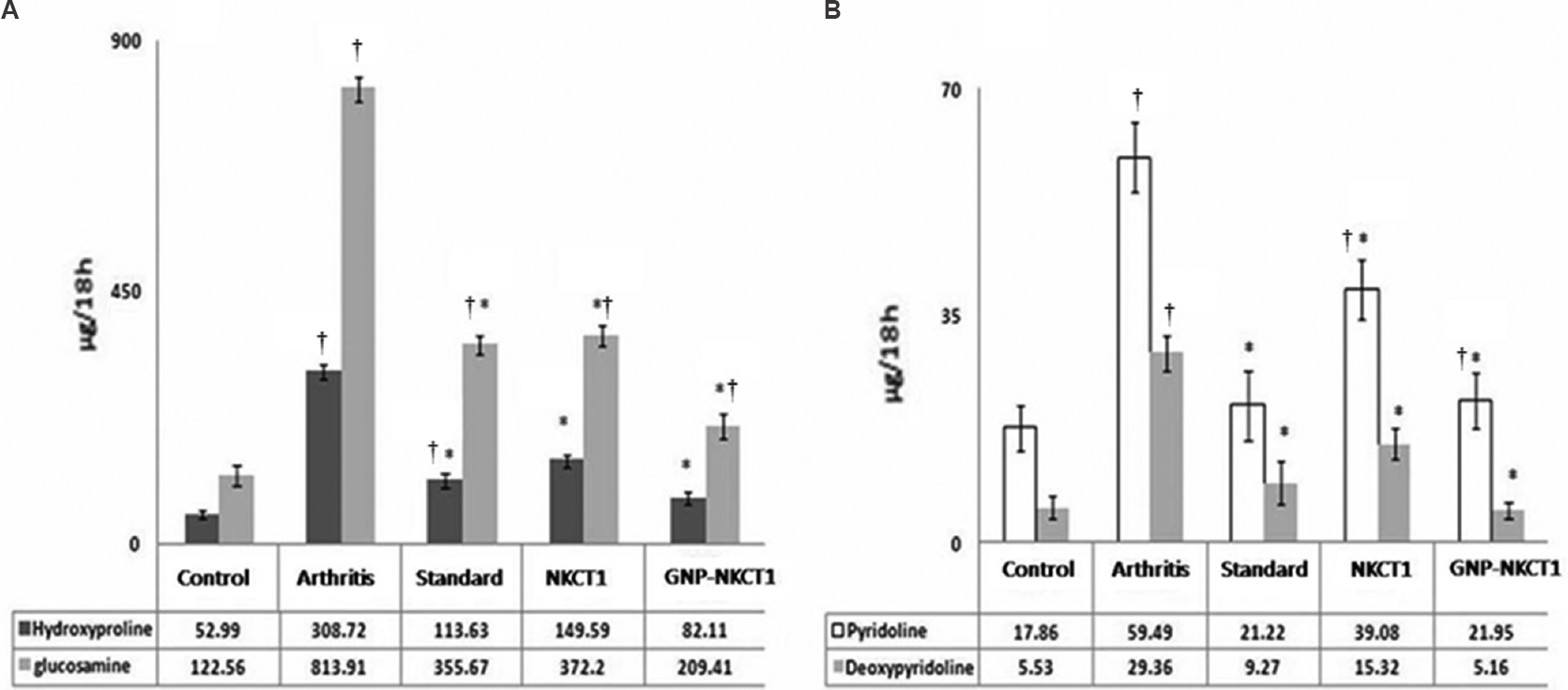
- Effect of Naja kaouthia cytotoxin 1 (NKCT1), gold nanoparticle - Naja kaouthia cytotoxin 1 (GNP-NKCT1) and standard drug on urinary hydroxyproline/glucosamine levels (A) and pyridinoline/deoxypyridolone levels (B) of collagenase induced osteoarthritis in male albino rats after day 15. Data (n=6) represent the mean±standard error of mean. *P<0.05 compared to arthritic control group. †P<0.05 compared to sham control group.
Effect of GNP-NKCT1 on serum markers: Serum pro-inflammatory cytokine levels were increased significantly in group 2 animals as compared with sham control group 1 animals. GNP-NKCT1 treatment significantly (P<0.05) decreased TNF-α, IL-1β, MMP-1, VEGF and IL-17 levels compared to osteoarthritic control group 2 rats. NKCT1 treatment also significantly (P<0.05) decreased TNF-α, IL-1β, MMP-1, VEGF and IL-17 levels compared to osteoarthritic control group 2 rats, whereas standard drug treatment significantly (P<0.05) decreased TNF-α, IL-1β, MMP-1, VEGF and IL-17 levels compared to group 2 rats (Fig. 2).

- Effect of Naja kaouthia cytotoxin 1 (NKCT1), gold nanoparticle (GNP) - NKCT1 and standard drug on, (A) interleukin-1β (IL-1β)/matrix metalloproteinase 1 (MMP-1)/vascular endothelial growth factor (VEGF) (B) tumour necrosis factor-alpha (TNF-α) and interleukin-17 levels of collagenase induced osteoarthritis in male albino rats. Data (n=6) represent the mean±standard error of mean. *P<0.05 compared to arthritic control group. †P<0.05 compared to sham control group.
Effect of GNP-NKCT1 on inflammatory markers of synovial membrane: Synovial membrane TNF-α, IL-1β expression levels were significantly increased in arthritic control group 2 rats as compared with sham control group 1 rats. Lower intensity of the bands clearly indicated that the protein expression levels of TNF-α and IL-1β in GNP-NKCT1-treated group 5 rats were decreased as compared to arthritic control group 2 and NKCT1-treated group 4 rats (Fig. 3).
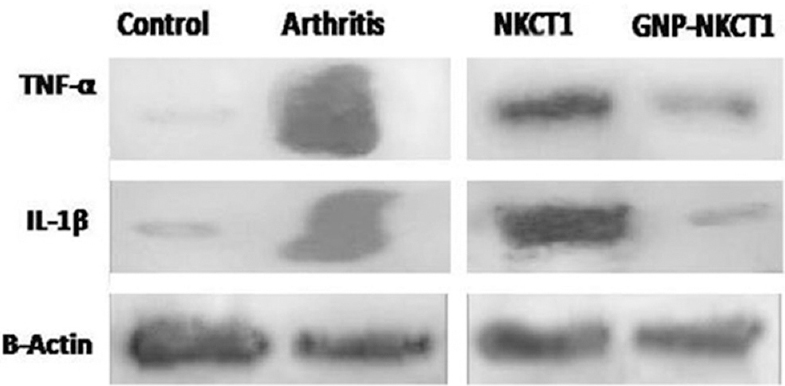
- Effect of Naja kaouthia cytotoxin 1 (NKCT1) and gold nanoparticle (GNP)-NKCT1 on the expression of tumour necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β) in synovial tissue of collagenase induced arthric rats analyzed through Western blot. β-Actin served as a loading control.
Effect of GNP-NKCT1 on joint histopathology: Arthritic control group 2 rats showed cellular infiltration within the synovial membrane that expanded and extended into the synovial space. The expansion of synovial membrane resulted in a decrease of synovial space, pannus formation and destruction of articular cartilage by secreting MMPs from inflammatory cell population. GNP-NKCT1-, and NKCT1-treated groups showed partial restoration of normal architecture of joint histology as compared with OA control group 2 rats (Fig. 4).
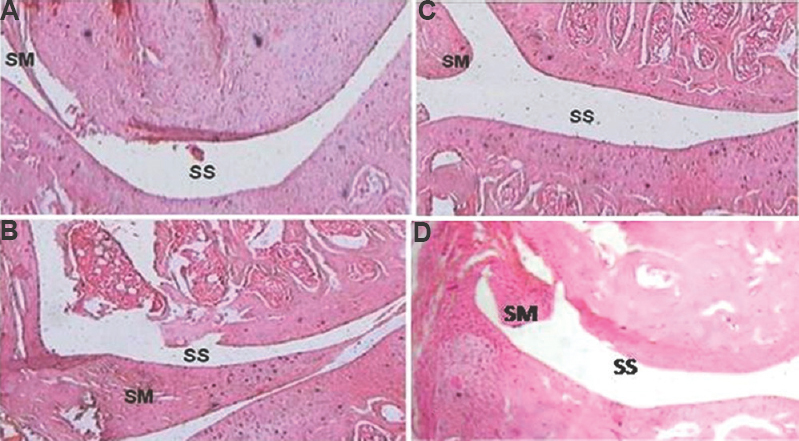
- Effect of Naja kaouthia cytotoxin 1 (NKCT1) and gold nanoparticle (GNP)-NKCT1 on joint histopathology of collagenase induced osteoarthritis in male albino rats. Sham control (A), arthritis control (B), GNP-NKCT1 treated (C), and NKCT1 treated (D). SM, synovial membrane; SS, synovial space. Stained with haematoxylin-eosin. Magnification= ×30.
Effect of GNP-NKCT1 on articular cartilage scanning electron microscopy: Articular cartilage surface was smooth in control group 1 rats based on scanning electron microscopy, whereas such smooth appearance was lost and mineral density was decreased on the joint surface in group 2 rats after induction of OA with collagenase. GNP-NKCT1, NKCT1 and indomethacin-treated groups showed partial protection against degradation of the articular cartilage (Fig. 5).
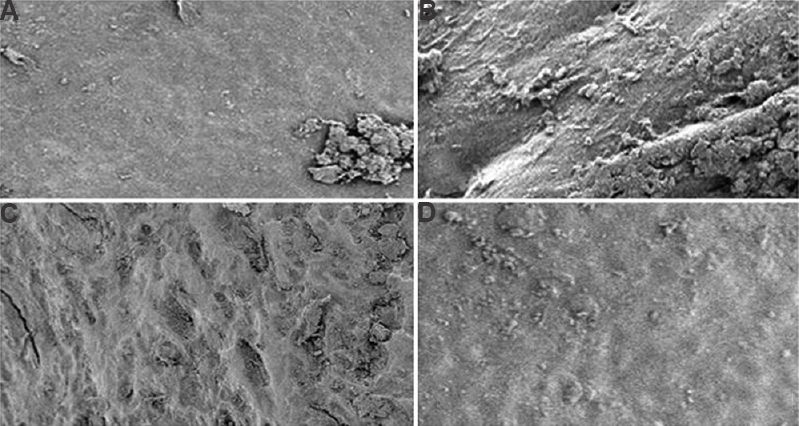
- Scanning electron micrographs of knee joint articular surface of collagenase induced osteoarthritis in male albino rats. Control (A), arthritis control (B), gold nanoparticle (GNP)-Naja kaouthia cytotoxin 1 (NKCT1) treated (C), NKCT1 treated (D). Magnification= ×350.
Discussion
Increased pro-inflammatory cytokines and infiltration of the synoviocytes are the main cause of ankle swelling during the progression of OA22. The present study revealed that GNP-NKCT1 treatment reduced the ankle volume in rats with collagenase induced arthritis. It was found that GNP-NKCT1 significantly reduced the pro-inflammatory markers IL-1, TNF-α, IL-17 and VEGF levels, which were increased after OA induction in rats. Reduction of pro-inflammatory cytokines levels by GNP-NKCT1 treatment regulated the cell invasion within the synovium and subsequently reduced the inflammation and swelling of the ankle joint.
Hydroxyproline and glucosamine are the major components of collagen and glycosaminoglycan respectively, forms the ECM and joint cartilage. Invasion of synoviocytes within the joint, releases the inflammatory cytokines which in turn increase the osteoclast and matrix MMPs activity and results in the degradation of ECM23. Hydroxyproline and glucosamine, normally excreted through urine, indicate the excess breakdown of ECM during progression of arthritis24. Pyridinoline and deoxypyridinoline are excreted unmetabolized in urine and were also recognised as the markers of the bone resorbtion and osteoclast activity during bone and joint disease25. In the present study, quantification of increased level of urinary hydroxyproline, glucosamine, pyridinoline and deoxypyridinoline, indicated the breakdown of collagen matrix during the onset of OA in arthritic control rats, which was restored after GNP-NKCT1 treatment. The protective effect of GNP-NKCT1 against ECM breakdown was further supported by its property to restore the increased MMP1 level in experimental arthritic rats.
TNF-α secreted from macrophages, has been reported to be a potent inducer of IL-1β in animal studies26. IL-1β and TNF-α act together synergistically to cause further damage to the joints in patients with arthritis. It has been reported the TNF-α stimulation of primary synovial cells results in phosphorylation of IKβ and subsequently nuclear translocation of nuclear factor-kappaB (NF-kβ)27. NF-kβ also activates the transcription of MMPs from synovial fibroblast and chemokines that recruits immune cells to the inflamed pannus27. IL-17 was also found to act synergistically with TNF-α and induced the pro-inflammatory pathways involving IL-1β, IL-6 and toll like receptor (TLR) agonists. These cytokines activate the NF-kβ, resulting in the amplification of inflammatory signalling through the transactivation of different NF-kβ responsive genes28. In the present study, it was found that GNP-NKCT1 restored the TNF-α, IL-1β, IL-17 and MMP-1 levels in the peripheral blood and TNF-α, IL-1β level in the synovial tissue.
Angiogenesis is recognized as a key event in the formation and maintenance of pannus in arthritis. Particularly, in the early stages of the disease, the newly formed vessels promote persistence of synovial inflammation by transporting the inflammatory cells to the site of synovitis in arthritis as well as supplying nutrients and oxygen for hyperplastic synovium. Initiation of angiogenesis was associated with expression of a numbers of angiogenic factors among which VEGF-A played a crucial role in neovascularization during pannus formation29. Inhibition of VEGF activity has been shown to have beneficial effects in the treatment and prevention of experimental arthritis30. The present investigation revealed that snake venom protein toxin NKCT1 and GNP-NKCT1, both had the potential to reduce the VEGF and other cytokine levels in arthritic rats. Tsai et al30 reported the reduction of angiogenesis, macrophage infiltration and pro-inflammatory cytokines in synovium of rats with collagenase induced arthritis by nanogold. Therefore, it may be assumed that GNP conjugation is likely to increase the potential of NKCT1 to reduce the VEGF level in arthritic animals31. However, further work to understand the detail molecular mechanism is warranted.
In conclusion, this study confirmed the protective activity of nanogold conjugated snake venom protein toxin, NKCT1, against OA in an animal model by limiting the inflammatory markers at the molecular level.
Acknowledgment
This work was sponsored by the Department of Biotechnology, New Delhi, India (Ref. no. BT/PR14811/NNT/28/500/2010).
Conflicts of Interest: None.
References
- The development of novel therapies for rheumatoid arthritis. Expert Opin Ther Pat. 2008;18:723-38.
- [Google Scholar]
- Snake venom as therapeutic agents: from toxin to drug development. Indian J Exp Biol. 2002;40:1353-8.
- [Google Scholar]
- Anti-arthritic activity of Indian monocellate cobra (Naja kaouthia) venom on adjuvant induced arthritis. Toxicon. 2010;55:670-3.
- [Google Scholar]
- A lethal cardiotoxic-cytotoxic protein from the Indian monocellate cobra (Naja kaouthia) venom. Toxicon. 2010;56:569-79.
- [Google Scholar]
- In vivo and in vitro toxicity of nanogold conjugated snake venom protein toxin GNP-NKCT1. Toxicol Rep. 2014;1:74-84.
- [Google Scholar]
- Anti arthritic and anti inflammatory activity of a cytotoxic protein NN-32 from Indian spectacle cobra (Naja naja) venom in male albino rats. Toxicon. 2014;90:106-10.
- [Google Scholar]
- Inflammatory response to therapeutic gold bead implantation in canine hip joint osteoarthritis. Vet Pathol. 2011;48:1118-24.
- [Google Scholar]
- Blood compatibility studies of Swarna bhasma (gold bhasma), an Ayurvedic drug. Int J Ayurveda Res. 2011;2:14-22.
- [Google Scholar]
- Antileukemic potential of PEGylated gold nanoparticle conjugated with protein toxin (NKCT1) isolated from Indian cobra (Naja kaouthia) venom. Cancer Nanotechnol. 2013;4:39-55.
- [Google Scholar]
- Influence of gold nanoparticle tagged snake venom protein toxin NKCT1 on Ehrlich ascites carcinoma (EAC) and EAC induced solid tumor bearing male albino mice. Curr Drug Deliv. 2014;11:652-64.
- [Google Scholar]
- Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970;49:99-113.
- [Google Scholar]
- Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol. 1989;135:1001-14.
- [Google Scholar]
- Introduction to the practice of statistics (5th ed). New York: Freeman; 2006. p. :219.
- Medical laboratory technology. Vol I. New Delhi: Tata McGraw Hill Publication; 1990. p. :224.
- A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27:1824-8.
- [Google Scholar]
- Effects and mechanisms of Vitamin A and Vitamin E on the levels of serum leptin and other related cytokines in rats with rheumatoid arthritis. Exp Ther Med. 2014;8:499-504.
- [Google Scholar]
- Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484-99.
- [Google Scholar]
- Anti-osteoporosis and anti-osteoarthritis activity of fresh water snail (Viviparous bengalensis) flesh extract in experimental animal model. Open J Rheumatol Autoimmune Dis. 2013;3:10-7.
- [Google Scholar]
- Urinary markers of bone resorption, pyridinoline and deoxypyridinoline, are increased in sickle cell patients with further increments during painful crisis. Am J Hematol. 2010;85:902-4.
- [Google Scholar]
- Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci U S A. 1997;94:9792-6.
- [Google Scholar]
- Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759-71.
- [Google Scholar]
- The dual function cytokine IL-33 interacts with the transcription factor NF-κB to dampen NF-κB-stimulated gene transcription. J Immunol. 2011;187:1609-16.
- [Google Scholar]
- Vascular permeability factor/endothelial growth factor: accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994;180:341-6.
- [Google Scholar]
- Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 2007;56:544-54.
- [Google Scholar]
- Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge. Nanomedicine. 2011;7:580-7.
- [Google Scholar]






