Translate this page into:
Prevalence of normoalbuminuric chronic kidney disease among individuals with type 2 diabetes mellitus from India
For correspondence: Dr Abilash Nair, Department of Endocrinology & Metabolism, Government Medical College, Thiruvananthapuram 695 011, Kerala, India e-mail: abhimck@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
A subset of diabetic individuals are known to develop progressive renal insufficiency without albuminuria, referred to as normoalbuminuric chronic kidney disease (NACKD). There is, however, a paucity of studies regarding this condition in India. So, this study, aimed to find the prevalence of normoalbuminuric renal dysfunction and its clinical associations in diabetic Indian population.
Methods:
Medical record search of patients with type 2 diabetes mellitus at a tertiary care centre was done. Based on the urinary albumin:creatinine ratio (>30 mg/g creatinine) and estimated glomerular filtration rate (e-GFR) (<60 ml/min/1.73m2), individuals were classified as having, (i) no kidney disease (NKD), (ii) chronic kidney disease (CKD), (iii) albuminuria alone (ALB), (iv) normoalbuminuric low e-GFR (NACKD) and (v) albuminuria with low e-GFR albuminuric CKD (ACKD). Furthermore, the clinical and biochemical parameters of these groups were also compared.
Results:
Data from 3534 diabetes patients with a mean age of 53.8±10.9 yr and mean duration of diabetes of 10.3±7.5 yr were available for the analysis. NACKD constituted 39.1 per cent of the patients with reduced e-GFR, NACKD was found in 2.1 per cent and ACKD in 3.4 per cent of all diabetic patients. Compared to NKD patients, was found an independent association between NACKD and higher age, male sex, lower body weight and statin intake but not with glycated haemoglobin, fasting and postprandial plasma glucose. Patients with NACKD were found to be older than those with ACKD. Retinopathy was found to be more prevalent in the ACKD patients, whereas the rates of macrovascular complications were found to be similar between the groups. The prevalence of NACKD relative to ACKD decreased in CKD stages 3b, 4 and 5.
Interpretation & conclusions:
The results of this study suggest that NACKD constituted greater than one third of patients with diabetes and decreased e-GFR, which showed a strong association with age but not with duration or severity of hyperglycaemia or the presence of retinopathy. Both NACKD and ACKD showed similar associations with macrovascular disease.
Keywords
Chronic kidney disease
diabetic kidney disease
diabetic nephropathy
e-GFR
normoalbuminuric
Diabetes mellitus accounts for around 50 per cent of cases of end-stage renal disease (ESRD) in the developed world1. Other than the progression to ESRD, diabetic kidney disease can lead to cardiovascular disease and predispose to infections and hence contribute to mortality2. Diabetic nephropathy is classically described by the clinical triad of proteinuria, hypertension and ultimately, low glomerular filtration rate (GFR)3. It is known that ESRD or doubling of serum creatinine generally occurs within 10 yr of detection in around 20 per cent of micro-albuminuric individuals and in 60 per cent of macro-albuminuric individuals4,5. Recently, the traditional concept about the natural history of diabetic kidney disease has been challenged with emerging evidence citing progressive decline in GFR without significant albuminuria in a subset of patients with type 1 or type 2 diabetes (T2D), i.e. normoalbuminuric chronic kidney disease (NACKD)3. There is, however, a paucity of data from India on the relative frequency of normoalbuminuric compared to albuminuric renal dysfunction. This study attempted to find the prevalence of normoalbuminuric renal dysfunction and its clinical association among Indian patients attending a tertiary diabetes care centre.
Material & Methods
Medical record search was done to find out the proportion of NACKD among diabetes patients attending the Indian Institute of Diabetes, Thiruvananthapuram, India, a tertiary diabetes care centre. The data were obtained from the electronic record of the clinical, biochemical and medication profiles of patients newly registered with the hospital between June 2017 to January 2020, after approval of the Human Ethics Committee of Government Medical College, Thiruvananthapuram.
Inclusion/exclusion criteria: The study included all newly registered T2D patients attending the hospital and whose records had a clinical history, weight recording, serum creatinine, urine albumin : creatinine ratio and glycated haemoglobin (HbA1c) estimation during the study period. The presence of poorly controlled hypothyroidism (serum thyroid-stimulating hormone [TSH] >10 μIU/ml)/hyperthyroidism (serum thyroxine >upper limit of normal), liver disease, chronic infections like tuberculosis and recurrent or active urinary tract infections were kept as criteria for exclusion.
Sample size: A study by Laranjinha et al6, had identified the prevalence (P) of non-albuminuric CKD in T2D patients as 46.6 per cent. The minimum required sample size was estimated to be 3250 patients using the formula:
4P×(100−P)/d2
wher, P=46.6 per cent, (100−P) = 53.4 per cent, d (desired precision) was set at 1.75 per cent. For the current study, a total of 3615 patients were screened and 81 patients were left out after subjecting to exclusion criteria. The data of 3534 patients were available for final analysis.
Study procedure: The anthropometric measurements were done by staff nurses and the clinical details, diagnoses and medications were recorded into the electronic database by the treating physicians themselves. Biochemical reports were entered into electronic records directly from the laboratory. Trained staff were utilized to fill these details in a separate pro forma for each patient, after retrieving them from the records. Demographic parameters, duration of diabetes and blood pressure were noted. History of addictions, comorbidities and treatment were also noted from records. Smoking and alcoholism were defined as any inhaled tobacco use or any ethanol ingestion within the past three months. Details of prior treatments were specifically checked for diagnosis liver disease, chronic infections, treatment with angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) and statins was also assessed. Laboratory data were collected from the patients’ last visit details, including HbA1c, fasting plasma glucose (FPG) and 2 h postprandial plasma glucose (2 h PPPG), fasting lipid profile, thyroid profile and renal function test. For those patients with a low estimated GFR (e-GFR) (Cockroft and Gault method), records were further searched for an alternative cause of CKD. Those who did not have a mention of CKD or its reasons in records were contacted to get their treatment records pertaining to kidney disease, including ultrasound abdomen and urine sediment. Details about the presence of coronary artery disease, cerebrovascular accident, peripheral arterial occlusive disease and retinopathy were obtained from hospital records. Details of fundus fluorescein angiography testing were not available in all patients.
Study parameters: All biochemical investigations were done using the same analyzers under a National Accreditation Board for Laboratories, India, certified quality control programme as part of existing hospital protocol. From the available parameters body mass index (BMI) and e-GFR (Cockcroft and Gault) were calculated for each patient.
The proportion was found out for albuminuric (urine albumin: creatinine ratio more than 30 mg/g creatinine) and normoalbuminuric fall in e-GFR (<60 ml/min/1.73 m2 using the Cockroft and Gault formula) and that of albuminuria without low e-GFR. These categories were labelled as albuminuria alone (ALB), NACKD and albuminuric CKD (ACKD), respectively. Other patients without the above renal abnormalities were labelled as no kidney disease (NKD).
Statistical analysis: Data were tabulated Microsoft Excel 2010. IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA) was used for the analysis of data. The variables were tested for their distribution and classified as normally distributed or not. Continuous variables were expressed as mean±standard deviation (normal distribution) or as median interquartile range (IQR) if not normally distributed. Categorical variables were expressed as frequencies (N) and percentages (%). Analysis of variance (with post hoc Bonferoni’s correction for subgroup comparison) was used to make the comparison of normally distributed continuous variables between the multiple groups of NKD, NACKD and ACKD. Chi-square test was used for categorical variables for finding the significance of the association. For the non-normally distributed variables Mann-Whitney U test was used. Logistic regression was employed to find out the independent association of co-varying parameters (e.g. age, duration of diabetes, weight, statin intake) with NACKD. P<0.05 was considered as significant.
Results
A total of 3615 patients satisfying the inclusion criteria were included in the study. Patients with poorly controlled hypothyroidism (TSH >10 μIU/ml) or other comorbidities mentioned in the exclusion criteria (n=81) were excluded, with 3534 patient’s data being available for final analysis. The clinical characteristics of the study participants are shown in Table I. The mean age of the study participants was 53.8±10.9 yr, the median (IQR) duration of diabetes was 7.5 (2.7-13.8) yr and the mean HbA1c of 8.8±2.3 per cent (72.7 + 9 mmol/mol). Hypertension was previously diagnosed in 1256 (35.4%) patients, but 1350 (38.2%) had a systolic BP of more than 140 mm of Hg and 114 (3.2%) patients had a diastolic BP of >90 mm of Hg.
| Study parameter | Mean±SD/median (IQR)/n (%) |
|---|---|
| Total number of diabetes patients studied | 3534 |
| Age (yr) | 53.8±10.9 |
| Males | 2246 (63.4) |
| Smoker | 412 (11.6) |
| H/o alcohol intake | 762 (21.6) |
| Known hypertension | 1256 (35.4) |
| Known dyslipidemia | 828 (23.4) |
| Duration of diabetes (yr) | 7.5 (2.7-13.8) |
| BMI (kg/m2) | 27.0±4.0 |
| BMI (>23 kg/m2) | 3042 (86.1) |
| Systolic BP (mmHg) | 131.1±16.4 |
| Diastolic BP (mmHg) | 78.4±7.8 |
| Haemoglobin (g/dl) | 13.6±1.7 |
| Total leucocyte count (cells/mm3) | 7069±1586 |
| ESR (mm/h) | 14 (7-27) |
| Serum creatinine (mg/dl) | 0.73 (0.62-0.88) |
| eGFR (ml/min/1.73 m2) | 113.8 (89.8-140.1) |
| UACR (mg/g creatinine) | 15.4 (8.4-38.1) |
| Serum thyrotropin (TSH - μIU/ml) | 2.1 (1.4-3.2) |
| Serum aspartate aminotransferase (IU/l) | 25 (20-33) |
| Serum alanine aminotransferase (IU/l) | 28 (21-41) |
| Serum total cholesterol (mg/dl) | 183.4±42.9 |
| Serum triglyceride (mg/dl) | 119 (89-169) |
| Serum high density lipoprotein (mg/dl) | 40.9±9.2 |
| Serum low density lipoprotein (mg/dl) | 114.1±38.1 |
| Glycated haemoglobin (%) | 8.8±2.3 |
| Fasting plasma glucose (mg/dl) | 190.0±75.5 |
| Post prandial plasma glucose (mg/dl) | 286.0±115.0 |
BMI, body mass index; ESR, erythrocyte sedimentation rate; TSH, thyroid stimulating hormone; SD, standard deviation, IQR, interquartile range; eGFR, estimated glomerular filtration rate; UACR, urine albumin: creatinine ratio; BP, blood pressure
Regarding renal dysfunction, 2379 (67.3%) patients had NKD, 956 patients (27.05 %) had ALB, 121 (3.4%) had ACKD and 75 (2.1%) had NACKD whereas three patients (0.1%) were found to have a non-diabetic cause of renal function decline (Fig. 1). Hence, among patients with diabetes reaching a stage 3a CKD or worse (e-GFR< 60 ml/min/1.73 m2), 38.2 per cent (75 of 196) were normoalbuminuric. The proportion of patients with normoalbuminuria across CKD stages is shown in Fig. 2.
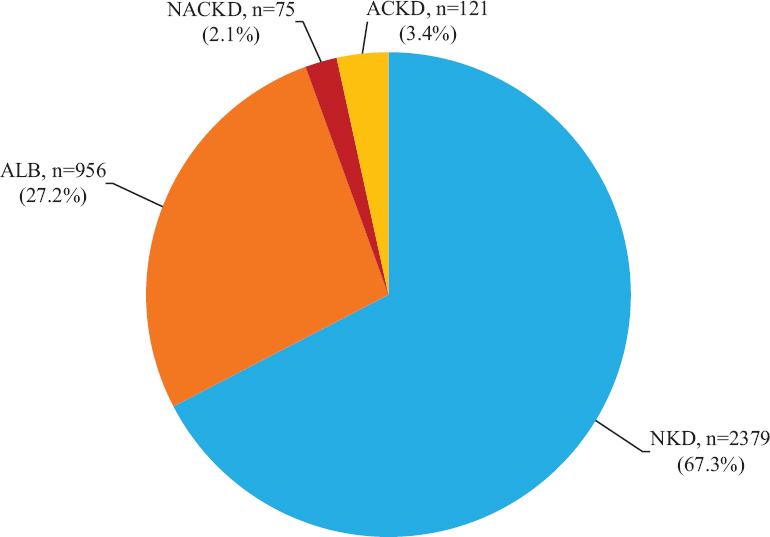
- Categories of diabetic patients according to the presence of albuminuria and/or e-GFR <60 ml/min/1.73 m2. e-GFR, estimated glomerular filtration rate
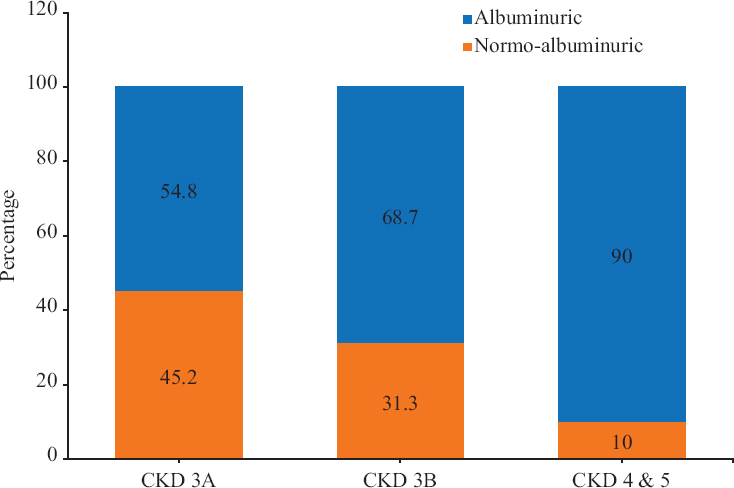
- Proportion of normoalbuminuric patients among different stages of CKD. CKD, chronic kidney disease
A comparison was made between patients with NKD and NACKD (Table II) to find the associations of NACKD with other clinical variables. Higher proportion of NACKD patients were males (70.6%) when compared to those with NKD (59.2%) (P<0.05). Similarly, subjects with a history of hypertension and dyslipidaemia, also were more likely to have NACKD. Significantly higher number of subjects with NACKD was on statins and ACEI/ARBs when compared to those with NKD. Furthermore, subjects with lower weight, lower BMI, a lower diastolic BP were found to have a significantly higher chance of NACKD. On binary logistic regression, higher age, male sex, lower body weight and statin intake were found as risk factors for NACKD. Interestingly, none of the glycaemic parameters (FBG, PPBG and HbA1c) showed an association with NACKD.
| Study parameter | Mean±SD/median (IQR) or n (%) | P (NACKD vs. NKD) | P (NACKD vs. ACKD) | ||
|---|---|---|---|---|---|
| NKD | NACKD | ACKD | |||
| Number of subjects (n) | 2379 | 75 | 121 | NA | NA |
| Age (yr) | 52.7±10.5 | 70.7±10.0 | 64.9±9.0 | <0.001** | <0.001** |
| Duration of diabetes (yr) | 6.2 (2.1-11.6) | 14.9 (5.7-21.3) | 16.8 (11.0-24.7) | <0.01*** | 0.17*** |
| Male | 1409 (59.2) | 53 (70.6) | 96 (79.3) | 0.04* | 0.16* |
| Smoker | 243 (10.2) | 10 (13.3) | 22 (18.1) | 0.37* | 0.37* |
| Alcoholism | 462 (19.4) | 11 (14.6) | 20 (16.8) | 0.30* | <0.01* |
| Hypertension | 741 (31.2) | 37 (49.3) | 92 (76) | <0.01* | <0.01* |
| Dyslipidaemia | 510 (21.5) | 30 (40.0) | 60 (50.4) | <0.01* | 0.01* |
| Systolic BP (mmHg) | 129.4±15.5 | 129.6±14.6 | 142.1±18.6 | 0.99** | <0.001** |
| Diastolic BP (mmHg) | 78.0±7.4 | 75.1±7.4 | 79.0±7.6 | <0.01** | 0.003** |
| Body weight (kg) | 71.0±12.1 | 59.5±9.9 | 66.5±11.0 | <0.01** | <0.01** |
| BMI (kg/m2) | 27.1±4.0 | 24.1±3.8 | 25.4±3.5 | <0.01** | 0.99** |
| Fasting plasma glucose (mg/dl) | 179.6±69.2 | 158.6±64.6 | 189.4±95.0 | 0.09** | 0.02** |
| Post prandial plasma glucose (mg/dl) | 267.0±109.8 | 253±96 | 305.9±124.0 | 0.99** | <0.01** |
| Glycated haemoglobin (%) | 8.5±2.3 | 8.2±2.3 | 9.0±2.2 | 0.99** | 0.21** |
| Haemoglobin (g/dl) | 13.6±1.6 | 12.8±1.7 | 11.8±2.0 | <0.01** | <0.01** |
| ESR (mm/h) | 13.0 (6.0-24.0) | 16.0 (8.0-36.0) | 36.0 (18.0-58.0) | <0.01*** | <0.01*** |
| Serum potassium (mEq/l) | 4.2±0.4 | 4.2±0.4 | 4.3±0.4 | 0.160** | 0.99** |
| Serum aspartate aminotransferase (IU/l) | 25.0 (21.0-33.0) | 23.0 (20.0-29.5) | 21.0 (20.0-27.0) | 0.99*** | 0.29*** |
| Serum alanine aminotransferase (IU/l) | 29.0 (21.0-42.0) | 23.0 (20.0-32.0) | 21.0 (20.0-28.0) | 0.99*** | 0.99*** |
| Serum cholesterol (mg/dl) | 182.8±40.9 | 163.5±42.6 | 166.4±50.3 | <0.01** | 0.99** |
| Serum high-density cholesterol (mg/dl) | 41.3±9.3 | 41.1±8.4 | 39.5±9.3 | 0.09** | 0.99** |
| Serum low-density cholesterol (mg/dl) | 114.4±36.7 | 98.0±40.2 | 99.0±44.7 | <0.01** | 0.99** |
| Serum triglycerides (mg/dl) | 110.0 (83.7-153) | 102.0 (85.5-135.5) | 117.0 (91.0-175.0) | 0.07*** | 0.03*** |
| Serum thyrotropin (μg/dl) | 2.0 (1.4-3.1) | 2.6 (1.7-4.0) | 2.6 (1.7-3.2) | 0.06*** | 0.99*** |
| Blood urea (mg/dl) | 23.0 (20.0-28.0) | 32.0 (28.0-39.5) | 47.0 (36.0-56.0) | <0.01*** | <0.01*** |
| Serum creatinine (mg/dl) | 0.7 (0.6-0.8) | 1.1 (0.9-1.2) | 1.4 (1.2-2.0) | <0.01*** | <0.01*** |
| UACR (mg/g%) | 11.1 (7.3-16.2) | 11.1 (7.8-16.4) | 153.8 (97.3-198.2) | 0.61*** | <0.01*** |
| Statin intake | 819 (34.5) | 38 (50.6) | 65 (53.7) | <0.01* | 0.67* |
| ACEI/ARB intake | 475 (20) | 34 (45.3) | 66 (54.5) | <0.01* | 0.20* |
| Retinopathy | Detalis NA | 13/49 (26.5) | 37/75 (49.3) | - | 0.01* |
| Past history of coronary artery disease | Detalis NA | 20/66 (30.0) | 34/98 (34.6) | - | 0.70* |
| Previous cerebrovascular accident | Detalis NA | 3/66 (4.5) | 8/98 (8.2) | - | 0.95* |
| History of peripheral arterial disease | Detalis NA | 1/66 (1.5) | 8/98 (8.2) | - | 0.17* |
The significance of association (P value) between the variables computed using *Chi-square test (categorical variables), **ANOVA with post-hoc Bonferoni’s correction for normally distributed continuous variables marked with, ***Mann-Whitney U test for non-normally distributed variables. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; UACR, urine albumin: creatinine ratio, NKD, no kidney disease, NACKD, normoalbuminuric chronic kidney disease, ACKD, albuminuric chronic kidney disease; NA, not available
A comparison was also made between the ACKD and NACKD groups (Table II). It was found that NACKD patients had a significantly higher age, lower duration of diabetes, lower body weight, lower systolic and diastolic blood pressures, lower fasting (FPG) and postprandial (PPPG) plasma glucose and lower HbA1c levels. Hypertension was more prevalent in ACKD patients. On binary logistic regression, among the above associations, only a higher age was identified as the risk factor. The proportion of patients with retinopathy was significantly higher in those with ACKD when compared with NACKD patients, whereas the proportion of patients having coronary artery disease, cerebrovascular accidents and peripheral vascular occlusive disease was found to be equal among both the groups.
Further analysis was performed to find the prevalence of NACKD in more severe stages of renal dysfunction and it was seen that, as the e-GFR level decreases, the proportion of patients with the normoalbuminuric variant of CKD comes down. The proportion of patients with normoalbuminuria in CKD stage 3A (eGFR 45-59 ml/min) was 45.1 per cent, whereas in patients with CKD stage 3B (eGFR 30-44 ml/min) was 31.3 per cent and that for stages 4 and 5 (eGFR <30 ml/min) was 10.0 per cent (Fig. 1).
Discussion
Till the last decade, albuminuria was considered the sine-qua-non of CKD caused by diabetes; it was believed to be a marker which shows up much before the fall in e-GFR7. However, recent studies have shown that significant proportions of patients with T2D have a fall in e-GFR before the onset of micro-albuminuria. Albuminuria and decreased GFR were identified as complementary manifestations of diabetic kidney disease and not obligatory or temporally related ones8. Decline in GFR, unlike albuminuria is progressive and spontaneous regression is unlikely. Although 30 years have passed since the first reports of NACKD in T2D mellitus3, there are scarce data from India regarding its prevalence or associations. The present study was done to determine the prevalence and clinical association of NACKD in Indian patients with T2D.
In the present study, 3534 patients with T2D having a mean age of 53.8±10.9 yr and a mean duration of diabetes of 10.3±7.5 yr, attending a tertiary diabetes care centre in South India were studied. The prevalence of NACKD in this diabetic cohort was 2.1 per cent which constituted more than one-third of all patients with CKD. Other previous studies report a contribution of 22-73 per cent by NACKD to the population of patients with compromised GFR.
A large retrospective study in Sweden, including 94,446 participants with diabetes, showed that 17 per cent of participants had renal impairment, with a high proportion of (62%) normoalbuminuric subjects9. Similarly, among the patients with T2D and eGFR <60 ml/min/1.73 m2 included in NHANES III, 30 per cent had NACKD. Another study (n = 15 773), conducted across 19 diabetes clinics in Italy, found that among patients with e-GFR less than 60 ml/min per 1.73 m2, 56.6 per cent were normoalbuminuric10. A follow up study including the patients having low eGFR in the UKPDS cohort with 1132 participants had shown that 51 per cent had no albuminuria11. Other studies have shown a lower proportion (23.7% and 28.4%) of NACKD12,13, but patients with renin-angiotensin blocker therapy were excluded from these analyses. The recent rise in the proportion of NACKD in diabetic patients was thought to be contributed by RAS inhibitor therapy, but a Japanese study14 showed that even after adjusting for RAS inhibitor therapy, 73 per cent of patients among those with low e-GFR had normoalbuminuria. In the present study also, the proportion of patients taking ACEI/ARB therapy was found to be statistically similar among NACKD and ACKD groups.
Literature search did not reveal any study from India done exclusively in a large cohort of T2D subjects to find the proportion of patients with NACKD. A study on non-communicable diseases, which included 53 diabetic patients showed a prevalence of NACKD to be around 11.3 per cent15. Another study done on 75 diabetic patients from Pune, India, showed that the proportion of NACKD among all patients with low e-GFR was 50 per cent16. The prevalence of NACKD was reportedly higher in hypertensive individuals as compared to individuals with diabetes mellitus.
In the current study, males were found to be more likely to have NACKD when compared to females, which is in contradiction to studies done elsewhere9,17. Other associations found were age, lower body weight and statin intake. Interestingly, none of the glycaemic parameters, including HbA1c showed any association with normoalbuminuric fall in e-GFR. A Swedish study found that normoalbuminuric participants had better metabolic control and lower BMI as compared to those with ACKD, similar to the findings of the current study8. In this study, patients having ACKD were found to have a longer duration of diabetes, higher HbA1c as well as other glycaemic parameters than those with NACKD, whereas higher age was the only independent association favouring NACKD.
In the present study, only 26.5 per cent of patients with NACKD had retinopathy. By contrast, 49.3 per cent of patients with ACKD had retinopathy. This is in accordance with the RIACE study18 where advanced retinopathy was twice as common in the albuminuric CKD group compared to the group with NACKD. The reduced prevalence of retinopathy in normoalbuminuric fraction might exclude microangiopathy as the major causative factor. The degree of macroangiopathy in albuminuric and non-albuminuric CKD appears similar, as evidenced by the comparable proportions of coronary artery and cerebrovascular diseases among both groups. A large prospective study6 from Portugal demonstrated that increased urinary albumin excretion and reduced eGFR are both independently associated with the risk for both cardiovascular outcomes and microvascular complications in patients with T2D. This would mean that reduced GFR is an independent risk factor for cardiovascular disease in all patients with T2D, irrespective of albuminuria concentrations. The statin use identified to be associated with NACKD could be taken as surrogate evidence for the link between coronary artery/cerebrovascular disease or dyslipidemia with NACKD.
The significance of NACKD has been doubted by some previous studies, which showed a similar fall in age-matched non-diabetic patients as seen in the present study. Furthermore, the current study could not demonstrate a relation of NACKD with any parameter related to hyperglycaemia. Moreover, the prevalence of normoalbuminuric renal dysfunction when considering a lower e-GFR cut-off of 45 ml/min/1.73 m2 and 30 ml/min/1.73 m2 comes down significantly and albuminuria becomes more frequent as the e-GFR declines. Similar observations have been made by Singh et al19 who found that the proportion of patients with proteinuria increased as eGFR decreased and almost all participants with eGFR <45 ml/min/1.73 m2 had evidence of dipstick proteinuria. From these observations, it could be hypothesized that, although the lesser degrees of renal dysfunction may be normoalbuminuric, albuminuria is essential for the development of severe stages of CKD in diabetes. Alternatively, a fall in e-GFR to low values is often accompanied by albuminuria due to secondary glomerular dysfunction irrespective of the cause or mode of initial e-GFR fall. The progression of renal dysfunction from the initial moderate normoalbuminuric fall in e-GFR in patients with diabetes is an aspect which is not well studied in a prospective fashion. Conflicting reports on accelerated fall in e-GFR as well as those showing lack of it exist20-22. Koye et al13 in their study observed that the individuals with non-albuminuric variant of diabetic kidney disease had a lower risk of rapid decline of GFR and progression to ESKD compared to those with albuminuria. The diagnosis of diabetic kidney disease could be delayed in a significant proportion of subjects if albuminuria alone is assessed without GFR for screening and monitoring renal dysfunction in diabetes mellitus.
The strength of the study is its large sample size. As per the authors’ knowledge this may be the first large study reporting the relative prevalence of normoalbuminuric fall in eGFR in Indian patients with T2D compared to the albuminuric subjects. Limitations of this study include an absence of detailed investigations or follow up for patients who had NACKD, to rule out other causes of renal dysfunction. These were due to the retrospective nature of the study. We were also not able to repeat the urinary sampling in albuminuric subjects. Being a study from a tertiary care centre there could also be a referral bias whereby the higher proportion of subjects with severe hyperglycaemia, longer duration of diabetes and hence the complications of diabetes could also have been included in the study. However, since the prevalence of NACKD was not found to be related to the duration of diabetes or degree of hyperglycaemia, this bias is unlikely to affect the conclusions of this study.
Overall, NACKD accounts for more than one third of subjects with CKD among South Indian patients with T2D. The proportion of NACKD decreases in more severe stages of CKD. The normoalbuminuric decline in e-GFR was associated with higher age, male sex, lower body weight but not with the duration or severity of hyperglycaemia. NACKD group has a similar proportion of patients with macrovascular complications as ACKD, but fewer of them have retinopathy in contrast to ACKD. Longitudinal studies are hence required to delineate the progression of NACKD, if any, and its long-term implications to cardiovascular health and mortality in patients with T2D mellitus.
Acknowledgment:
Authors acknowledge Dr Anish T.S., Associate Professor, Department of Community Medicine, Government Medical College, Thiruvananthapuram, for supervision of statistical calculations and conclusions. Authors acknowledge Shri Sudi Sisupalan (Dip. Nursing), Ms Arya Suresh, Keerthi UK and Ms Priyadarsana L (Research Assistants at the Department of Endocrinology, Govt. Medical College, Thiruvananthapuram) and Shri Sajith P S (System Administrator, at Indian Institute of Diabetes, Thiruvananthapuram) for data acquisition and analysis.
Financial support & sponsorship: The study was funded by the Research Society for the Study of Diabetes in India, Kerala Chapter, through a research grant provided to author JC.
Conflicts of Interest: None.
References
- Diabetic kidney disease:A report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-33.
- [Google Scholar]
- Complications of diabetes mellitus. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg H, eds. Williams textbook of endocrinology (13th ed). Philadelphia, PA: Elsevier; 2016. p. :1516.
- [Google Scholar]
- Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet. 1998;352:213-9.
- [Google Scholar]
- Progression of diabetic kidney disease in the absence of albuminuria. Diabetes Care. 2019;42:1842-4.
- [Google Scholar]
- Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig. 2017;8:6-18.
- [Google Scholar]
- Diabetic kidney disease:Is there a non-albuminuric phenotype in type 2 diabetic patients? Nefrologia. 2016;36:503-9.
- [Google Scholar]
- Am J Kidney Dis. 2007;49((2 Suppl 2)):S12-154.
- Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813-21.
- [Google Scholar]
- Ongoing treatment with renin-angiotensin-aldosterone-blocking agents does not predict normoalbuminuric renal impairment in a general type 2 diabetes population. J Diabetes Complications. 2013;27:229-34.
- [Google Scholar]
- Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011;29:1802-9.
- [Google Scholar]
- Risk factors for renal dysfunction in type 2 diabetes:U. K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832-9.
- [Google Scholar]
- Prevalent rate of nonalbuminuric renal insufficiency and its association with cardiovascular disease event in Korean type 2 diabetes. Endocrinol Metab (Seoul). 2016;31:577-85.
- [Google Scholar]
- Risk of progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes:The CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2018;72:653-61.
- [Google Scholar]
- Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes:The Japan Diabetes Clinical Data Management study (JDDM15) Nephrol Dial Transplant. 2009;24:1212-9.
- [Google Scholar]
- Nonalbuminuric chronic kidney disease:A dominant presentation in noncommunicable disease population of rural central India. J Family Med Prim Care. 2018;7:442-6.
- [Google Scholar]
- Correlation of microalbuminuria with estimated GFR (eGFR) by Cockcroft-Gault and MDRD formula in type 2 diabetics and hypertensives. Indian J Clin Biochem. 2015;30:271-4.
- [Google Scholar]
- Normoalbuminuric diabetic kidney disease in the U. S. population. J Diabetes Complications. 2013;27:123-7.
- [Google Scholar]
- Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes:The Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2012;35:2317-23.
- [Google Scholar]
- Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in North India using Cockcroft-Gault and Modification of Diet in Renal Disease equation:An observational, cross-sectional study. BMC Nephrol. 2009;10:4.
- [Google Scholar]
- Longitudinal change in estimated GFR among CKD patients:A 10-year follow-up study of an integrated kidney disease care program in Taiwan. PLoS One. 2017;12:e0173843.
- [Google Scholar]
- Progressive renal decline:The new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2015;38:954-62.
- [Google Scholar]
- Progressive decline in estimated glomerular filtration rate in patients with diabetes after moderate loss in kidney function-even without albuminuria. Diabetes Care. 2019;42:1886-94.
- [Google Scholar]






