Translate this page into:
Presence, patterns & predictors of hypocortisolism in patients with HIV infection in India
For correspondence: Dr. Deep Dutta, Department of Endocrinology, Post Graduate Institute of Medical Education & Research & Dr Ram Manohar Lohia Hospital, 1 Baba Kharak Singh Marg, New Delhi 110 001, India e-mail: deepdutta2000@yahoo.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Adrenal insufficiency (AI) is rarely diagnosed in patients with HIV infection, in spite of autopsy studies showing very high rates of adrenal involvement. This study was aimed to determine the presence, patterns and predictors of AI in patients with HIV infection.
Methods:
Consecutive HIV patients, 18-70 yr age, without any severe co-morbid state, having at least one-year follow up at the antiretroviral therapy clinic, underwent clinical assessment and hormone assays.
Results:
From initially screened 527 patients, 359 patients having good immune function were analyzed. Basal morning cortisol <6 μg/dl (<165 nmol/l; Group 1), 6-11 μg/dl (165-300 nmol/l; Group 2), 11-18 μg/dl (300-500 nmol/l; Group 3) and ≥18 μg/dl (500 nmol/l; Group 4) were observed in 13, 71, 199 and 76 patients, respectively. Adrenocorticotropic hormone (ACTH) stimulation test revealed 87 patients (24.23%) to have AI. AI in groups 1-4 was 100, 56.34, 17.09 and 0 per cent, respectively. AI patients were more likely to be females (P< 0.05), having longer disease duration (P< 0.05), immune reconstitution inflammatory syndrome, hyperkalaemia (P< 0.01), lower fasting glucose (P< 0.01), dehydroepiandrosterone sulphate (DHEAS) and vitamin D. Regression analysis revealed morning cortisol and DHEAS to be best predictors of AI (P=0.004 and 0.028, respectively).
Interpretation & conclusions:
AI is a significant problem in HIV-infected individuals, observed in nearly a quarter of patients. Diagnosis warrants high index of suspicion and low threshold for screening, especially in those having low DHEAS and hyperkalaemia. Morning cortisol is a reasonable screening test, with ACTH stimulation warranted to confirm diagnosis, especially in patients with morning cortisol <11 μg/dl (300 nmol/l).
Keywords
Acton prolongatum
adrenal insufficiency
adrenocorticotropic hormone
dehydroepiandrosterone-sulphate
HIV
hypoadrenalism
synacthen
vitamin D
The pattern of HIV infection in India is that of concentrated epidemic12. The prevalence rates of HIV infection in India in people with injection drug abuse, female sex workers, men who have sex with men were 18.1, 16.5 and 7 per cent, respectively12. Unfortunately, these individuals often constitute the fringe sections of the society and often do not have timely access to appropriate healthcare. Endocrine abnormalities are increasingly been reported in patients with HIV infection, a likely result of improved clinical outcomes and increased survival due to highly active antiretroviral therapy (HAART)34. We have reported thyroid dysfunction to be very common in patients with HIV infection with subclinical hypothyroidism being the predominant form observed in 14.76 per cent patients3. We have also reported hypogonadism to be a significant problem in HIV-infected men and women in India, effecting 39 and 29 per cent patients, respectively4.
Adrenocortical dysfunction is believed to be one of the common endocrinopathies in HIV56. Autopsy studies have demonstrated adrenal involvement in 40-90 per cent of HIV-infected patients7. Studies from Africa have consistently demonstrated a high prevalence of adrenal insufficiency (AI) in HIV-infected patients ranging from 27.5 to 34.5 per cent89. However, AI/hypocortisolism is rarely diagnosed in routine clinical practice in patients with HIV infection in India. Non-specific clinical features (anorexia, nausea, weight loss, fatigue and hypotension), which are very common in patients with HIV infection, along with subclinical nature of the disease may explain this. Accurate and appropriate diagnosis of hypocortisolism is imperative, as both lack of diagnosis and treatment of hypocortisolism, as well as unnecessary use of glucocorticoids is associated with increased morbidity and mortality. The recent endocrine society clinical practice guideline recommends low threshold for screening for AI10. Screening for AI has been recommended in all patients with suggestive symptoms and signs as well as in acutely ill patients10. Further, no data are available on the burden of hypocortisolism in patients with HIV infection from India. Hence, this study was aimed to determine the presence, patterns and predictors of hypocortisolism/AI in patients with HIV infection in India.
Material & Methods
Antiretroviral therapy (ART) clinic at Post-graduate Institute of Medical Education and Research and Dr Ram Manohar Lohia Hospital, New Delhi, India, an apex referral centre established by the National AIDS Control Organization, India and the World Health Organization, provides for all the necessary investigations, medications (including HAART), counselling and education to all patients with HIV infection. Consecutive ambulatory patients, 18-70 yr age, with serologically documented HIV infection, in stable clinical condition without any acute, severe illness, attending the ART clinic of the hospital during August 2014 to December 2015 were considered. Severely ill patients with multiple co-morbid states, who would warrant hospital admission, were excluded. Patients with previous history of steroid supplementation and those with vitamin D and/or calcium supplementation in the last six months were also excluded. Patient records were reviewed and patients having clinical data of at least one year of follow up were further evaluated. Patients with available CD4 cell counts at diagnosis and at first follow up (6-12 months after diagnosis) were included in the study. The study protocol was explained to the considered patients and only those who gave informed written consent were included in this study. The institutional ethics committee approved the study protocol.
Sample collection: All patients underwent detailed clinical assessment. The patients were called the subsequent day in fasting state for blood sampling. Blood samples (5 ml) were collected in plain and ethylenediaminetetraacetic acid (EDTA) vacutainers (Becton Dickinson, USA) between 8 and 9 am in the morning. Serum was separated from blood collected in plain vacutainer and processed immediately for routine biochemical analysis, and one aliquot of serum was stored at −20°C. EDTA sample processed for haematological analysis.
Hormone and biochemical analysis: Chemiluminescent microparticle immunoassay (VITROS® ECiQ Immunodiagnostic System, Johnson & Johnson, USA) was used for the estimation of morning cortisol, thyroid hormones and 25-hydroxy-vitamin-D [25(OH)D]. Cortisol assay had analytical sensitivity of 0.10 μg/dl (2.83 nmol/l), analytical range of 4.46-22.7 μg/dl (123-626 nmol/l) with intra- and inter-assay coefficient of variation (CV) of 2.2 and 4.7 per cent, respectively. Serum 25(OH)D assay had analytical sensitivity of 8.0 ng/ml, analytical range of 8-150 ng/ml, intra- and inter-assay CV of 3.4 and 5.5 per cent, respectively. Free tri-iodo-thyronine (FT3) assay had analytical sensitivity of 0.50 pg/ml, analytical range of 0.50-22.8 pg/ml, intra- and inter-assay CV of 2.2 and 6.3 per cent, respectively. Free tetra-iodo-thyronine (FT4) assay had analytical sensitivity of 0.07 ng/dl, analytical range of 0.07-6.99 ng/dl with intra- and inter-assay CV of 2.4 and 5.8 per cent, respectively. Thyroid-stimulating hormone (TSH) assay had analytical sensitivity of 0.015 mIU/l, analytical range of 0.015-100 mIU/l with intra- and inter-assay CV of 3.3 and 7.2 per cent, respectively. CD4 cell count was performed using flow cytometry (Becton Dickinson Immunocytochemistry Systems, San Jose, CA, USA). Serum calcium, phosphate, alkaline phosphate and renal function tests were done using clinical chemistry autoanalyzer based on dry chemistry micro-slide technology (VITROS® 350 chemistry system, Johnson & Johnson, USA).
Patients with morning cortisol ≥18 μg/dl (500 nmol/l) were defined to have normal adrenal function11. All patients were called the following day for adrenocorticotropic hormone (ACTH) stimulation test for the assessment of the adrenal reserve. Twenty five units of ACTH (Acton Prolongatum®, Ferring, Saint-Prex, Switzerland; which is equivalent to 250 mcg of Synacthen®) were injected intramuscularly by 40 IU insulin syringe (up to 16 mark), and blood sample was collected after 60 min for the estimation of post-ACTH stimulation cortisol11. Patients with post-ACTH cortisol ≥18 μg/dl (500 nmol/l) were defined to have normal adrenal reserve. Patients with post-ACTH cortisol <18 μg/dl (500 nmol/l) were defined to have AI (hypocortisolism).
Immune reconstitution inflammatory syndrome (IRIS) has been defined as an increased CD4 count above 200 cells/μl in patients who previously had <100-200 cells/μl of CD4 counts1213. Hence, patients in our study with baseline CD4 counts <200 cells/μl, which increased to >200 cells/μl at the first follow up following initiation of HAART were defined to have IRIS.
Sample size calculation: The prevalence of AI among HIV patients in India is not known. However, studies done in South Africa and Nigeria have shown 27.5 and 34.5 per cent of HIV positive patients had AI, respectively89. Keeping a power of 80 per cent and Type-I error at 5 per cent, a sample size of 246 patients was required in our study for accurate assessment of hypoadrenalism.
Statistical analysis: Normality of the distribution of variables was checked using the Kolmogorov-Smirnov test. All normally distributed variables have been elaborated as mean±standard deviation. All non-normally distributed variables have been mentioned as median (range). Range is a measure of the deviation of the variable and was calculated as the difference between the highest and the lowest value of the variable. Independent t test and Wilcoxon rank-sum test were done for normally distributed and skewed variables, respectively. Chi-square test was used for categorical variables. Multiple logistic regression analyses were done to determine variables that independently influenced the occurrence of hypoadrenalism after adjusting for factors in different models. Statistical Package for the Social Sciences (SPSS) version 20 (Chicago, IL, USA) was used for data analysis.
Results
Five hundred and twenty seven consecutive patients were screened, of whom 359 patients (225 males and 134 females) who fulfilled the inclusion and exclusion criteria and gave informed written consent were included in this study (Figure). The mean duration of HIV infection was 61.44±39.42 months with 88.58 per cent (318/359 patients) receiving HAART and 40.67 per cent (145/359 patients) (40.38%) having a history of tuberculosis (Table I). Three hundred and twenty two (89.69%) patients had serum 25(OH)D <30 ng/ml with mean levels of 20.23±9.13 ng/ml. At the time of hormonal analysis, 9.75 (35/359), 58.50 (210/359) and 31.75 per cent (114/359) patients had CD4 count <200 μl, 200-500 μl and >500 μl, respectively.
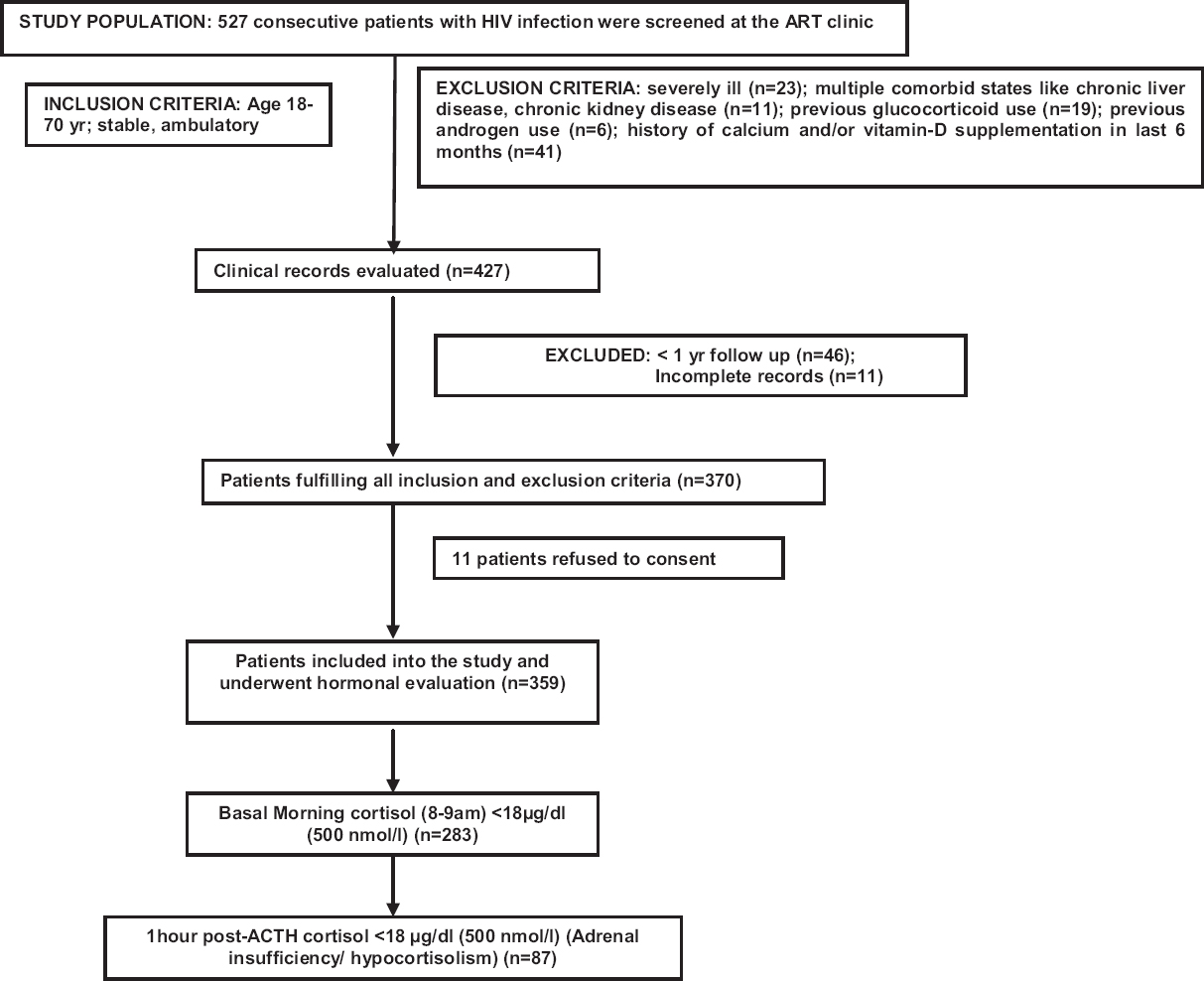
- Flowchart elaborating the study protocol and flow of patients.
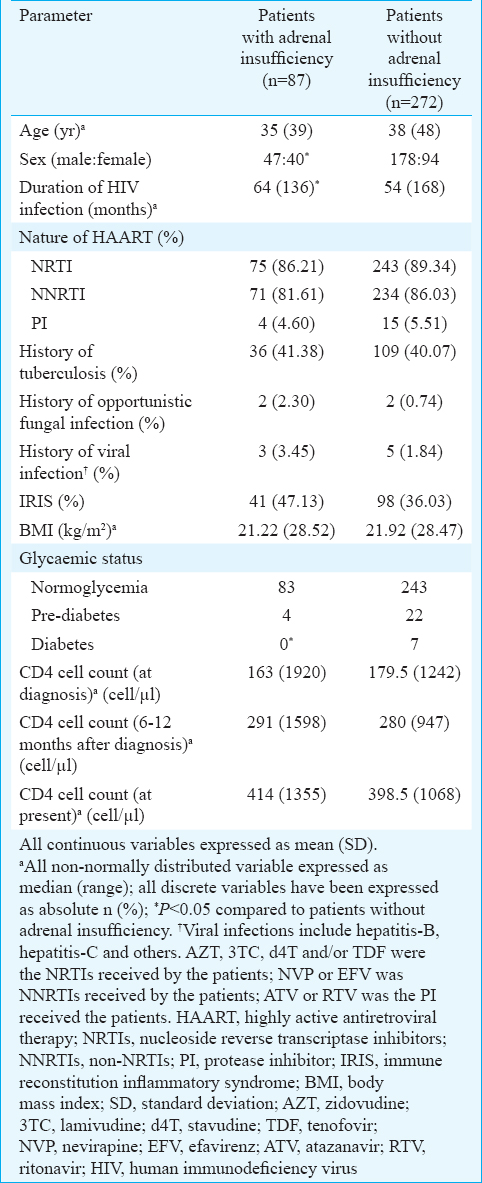
A total of 283 patients had morning cortisol <18 μg/dl (500 nmol/l). Of these, 13 patients (3.62%) had morning cortisol <6 μg/dl (<165 nmol/l) (Group 1), 71 (19.24%) had morning cortisol between 6 and 11 μg/dl (165-300 nmol/l) (Group 2) and 199 (55.43%) had morning cortisol between 11 and 18 μg/dl (300-500 nmol/l) (Group 3). Seventy six patients (21.17%) had morning cortisol ≥18 μg/dl (500 nmol/l) (Group 4). ACTH stimulation test revealed 87 patients (24.23%) to have one hour post-ACTH cortisol to be <18 μg/dl (<500 nmol/l) who were diagnosed to have AI (hypocortisolism). The increments in serum cortisol levels post-ACTH stimulation as compared to morning basal cortisol (Δ cortisol) [median (range)] in Groups 1, 2, 3 and 4 were 118 (288.5), 209 (474.9), 194 (1106) and 224 (549) nmol/l, respectively, which was significantly different (P< 0.05). The presence of AI (hypocortisolism) in patients with baseline morning cortisol <6 μg/dl (165 nmol/l), 6-11 (165-300 nmol/l), 11-18 (300-500 nmol/l) and ≥18 μg/dl (500 nmol/l) was 100 per cent (13/13 patients), 56.34 per cent (40/71 patients), 17.09 per cent (34/199 patients) and 0 per cent (0/76 patients), respectively (Table II).
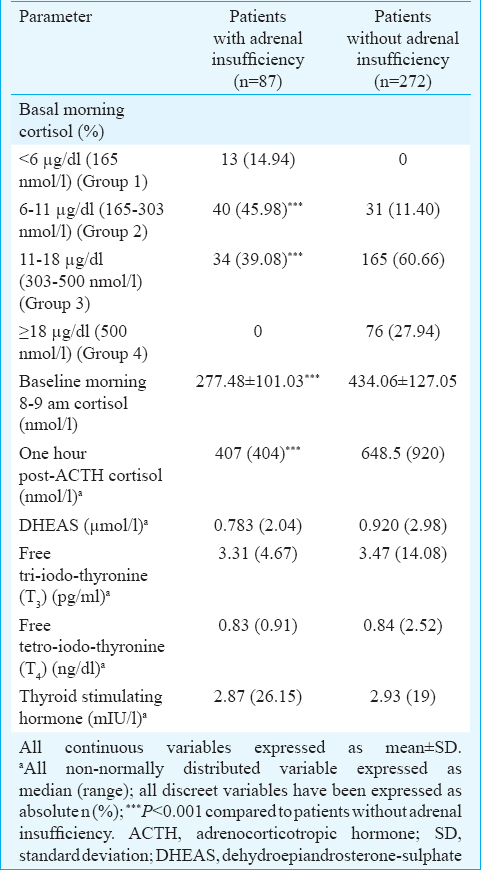
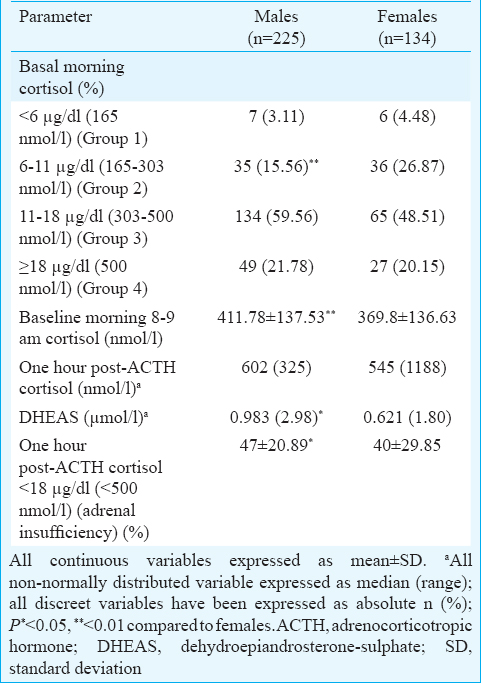
Both basal morning cortisol and dehydroepiandrosterone sulphate (DHEAS) were significantly lower in females as compared to males (P< 0.01) (Table III). In our study, patients with AI had significantly longer disease duration (P< 0.05). Diabetes and prediabetes were observed in seven (1.95%) and 26 (7.24%) patients (Table I). The occurrence of diabetes was significantly lower in patients with AI (Table I). Patients with AI had significantly higher serum potassium (P< 0.05) and lower fasting blood glucose (P=0.01), (Table IV). Two and six patients with AI had evidence of hypoglycaemia and hyperkalaemia, respectively (Table IV). Majority of the patients with AI had morning cortisol between 6 and 11 μg/dl (165-300 nmol/l) (Table II).

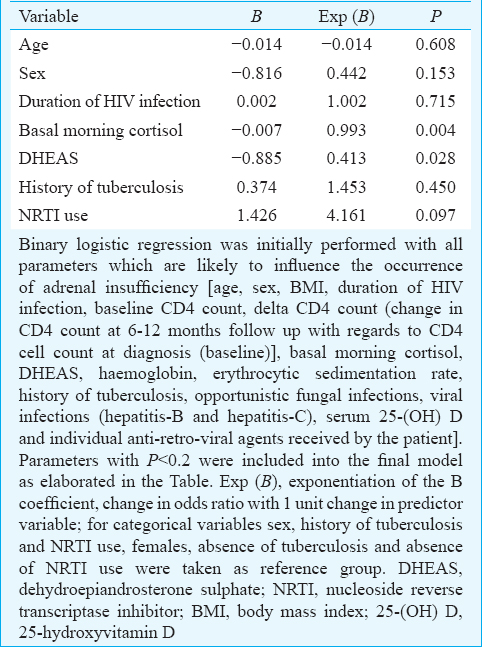
Basal and post-ACTH stimulation cortisol levels had no correlation with CD4 counts. One-hour post-ACTH stimulation but not basal cortisol had significant positive correlation with DHEAS (σ=0.213; P=0.025). Binary logistic regression analysis revealed that basal morning cortisol and serum DHEAS to be the two best independent predictors of AI among patients with HIV infection (P=0.004 and 0.028, respectively) (Table V).
Discussion
The entire spectrum of hypothalamic-pituitary-adrenal (HPA) axis dysfunction has been reported in HIV ranging from normal to high basal cortisol levels with blunt response to ACTH, a state of glucocorticoid resistance characterized by features of AI with high circulating levels of glucocorticoids to frank hypocortisolism/ AI14. Hypocortisolism usually occurs only after >80 per cent of the adrenal gland been destroyed15.
High baseline cortisol >18 μg/dl (500 nmol/l) was observed in 21.17 per cent patients in our study who had an intact adrenal response to ACTH stimulation. One group has reported hypercortisolaemia in HIV with blunted ACTH and cortisol response to stress and corticotropin-releasing hormone, especially in patients with advanced disease16. Increased systemic inflammation (increased circulating levels of cytokines) is believed to stimulate adrenal glucocorticoid synthesis1617. Increased circulating cortisol binding globulin levels may also contribute to the hypercortisolaemia in HIV infection18. Hypercortisolism was observed in patients with advanced disease and increased viraemia19. HIV envelope protein gp-120 has been linked to induction of HPA axis hyperactivity19. Stable clinical condition with good immune function (90.25% patients had CD4 cell count >200 cells/μl) may explain the intact adrenal response to ACTH stimulation in patients with basal hypercortisolaemia observed in our study. HIV viral load was not assessed and is a limitation of this study. Lack of estimation of ACTH and levels of inflammatory cytokines are also a limitation.
AI was seen in 24.23 per cent patients, which was comparable to previous reports from other parts of the globe. Studies from Brazil, Uganda, South Africa and Nigeria have reported the prevalence of AI ranging from 19 to 34.5 per cent892021. In a small study of critically ill AIDS patients with advanced disease from southern India (n=50), AI was diagnosed in 74 per cent patients using ACTH stimulation test22, which was much higher than that in this report. However, it must be highlighted that patients in our study were clinically stable, asymptomatic with good immune function. It is important to highlight that none of the patients diagnosed with AI in this study had hyperpigmentation or postural hypotension. However, patients with AI had significantly higher serum potassium and lower fasting blood glucose. Plasma ACTH was not measured in this study. Hence, it was not possible to differentiate primary from secondary AI in this study, which is a limitation of this study.
The presence of vitamin-D insufficiency [25(OH)D <30 ng/ml] among HIV-infected patients in our study was 89.69 per cent, which was higher than that observed in the general Indian population (70-75%)2324. Several pleotropic functions have been attributed to vitamin-D including its impact on immune function, autoimmunity, insulin resistance and cardiovascular function2526. Vitamin D deficiency has been linked with more rapid decline in CD4 cell count, higher occurrence of osteoporosis, cardiovascular disease, diabetes, autoimmune disease and cancer in HIV-infected patients2728. Vitamin-D receptor gene polymorphism has been linked to increased occurrence of AI29. Hence, further studies are needed to evaluate the link between vitamin D and hypocortisolism.
Certain studies have suggested serum DHEAS to have an important role in predicting clinical outcomes in HIV3031. A reduced DHEAS/cortisol ratio has been reported to be associated with a deterioration of immune status characterized by a shift from Th1- to a Th2-driven immune response30. Reduced DHEAS levels, accompanied by increased cortisol levels, were observed in patients with AIDS wasting syndrome31. This is believed to be a result of shift of steroid metabolism from adrenal androgens to glucocorticoids in patients with advanced HIV infection31. A decreased adrenal 17,20-lyase activity may explain this phenomenon3031. In our study, patients with AI had lower DHEAS levels and low serum DHEAS was an independent predictor of increased risk for AI in patients with HIV infection. The occurrence of dysglycaemia among patients with HIV infection in our study [diabetes (1.95%) and prediabetes (7.24%)] was not increased as compared to the occurrence rates in the general population in India3233. In large cross-sectional studies, the prevalence rates of diabetes among HIV-infected patients from Cameroon, Guinea-Bissau and Italy were reported to be 3.8 per cent (n=500), 5.8 per cent (n=953) and 4.5 per cent (n=755), respectively343536. There are conflicting reports with a few, but not all studies reporting increased prevalence of diabetes in HIV infected patients34353637. A study from Spain documented lower insulin resistance and lower incidence of diabetes in HIV-infected patients on ART37. In our study, the decreased occurrence of diabetes among HIV-infected patients with AI can be explained by the physiologic role of cortisol in increasing blood glucose through increased glycogenolysis and gluconeogenesis38. Low cortisol levels in AI is known to have a favourable impact on blood glucose values, which also explains the increased occurrence of hypoglycaemia in these patients38.
In conclusion, AI is a significant problem in HIV-infected patients in India with stable clinical and immune function, observed in nearly a quarter of the patients. Diagnosis of AI in HIV requires a high index of suspicion, as the typical symptoms associated with AI are usually absent. A low threshold of screening is advisable, especially in patients with low serum DHEAS and hyperkalaemia. Morning basal cortisol is a good screening test, with ACTH stimulation warranted to confirm diagnosis, especially in patients with morning cortisol <11 μg/dl (300 nmol/l). Low basal morning cortisol and DHEAS were the two predictors of AI. All patients with morning cortisol <6 μg/dl (165 mmol/l) and more than half of the patients (61%) with morning cortisol <11 μg/dl (300 nmol/l) had AI.
Acknowledgment
The authors acknowledge the participants of the study that made this study possible. Assistance of the staff of the ART clinic, department of Endocrinology and laboratory technicians of the Nursing Home laboratory of the department of Biochemistry, PGIMER & RML Hospital, New Delhi, is acknowledged.
Financial support & sponsorship: This study was financially supported by Post Graduate Institute of Medical Education & Research, & Dr Ram Manohar Lohia Hospital, New Delhi, India, as intramural research grant
Conflicts of Interest: None.
References
- HIV prevention & treatment - Reasons to rejoice & remain vigilant. Indian J Med Res. 2015;142:633-6.
- [Google Scholar]
- HIV in Indian MSM: Reasons for a concentrated epidemic & strategies for prevention. Indian J Med Res. 2011;134:920-9.
- [Google Scholar]
- Prevalence and predictors of thyroid dysfunction in patients with HIV infection and acquired immunodeficiency syndrome: An Indian perspective. J Thyroid Res. 2015;2015:517173.
- [Google Scholar]
- Occurrence, patterns and predictors of hypogonadism in patients with HIV infection in India. Indian J Med Res. 2017;145:804-14.
- [Google Scholar]
- Adrenal insufficiency in HIV infection: A review and recommendations. Am J Med Sci. 2001;321:137-44.
- [Google Scholar]
- Acquired immunodeficiency syndrome. Clinicopathologic study of 56 autopsies. Arch Pathol Lab Med. 1985;109:727-34.
- [Google Scholar]
- Basal cortisol levels and correlates of hypoadrenalism in patients with human immunodeficiency virus infection. Med Princ Pract. 2011;20:525-9.
- [Google Scholar]
- Adrenocortical function in Nigerians with human immunodeficiency virus infection. Ghana Med J. 2013;47:171-7.
- [Google Scholar]
- Diagnosis and treatment of primary adrenal insufficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364-89.
- [Google Scholar]
- Intramuscular ACTH stimulation test for assessment of adrenal function. J Assoc Physicians India. 2013;61:320-4.
- [Google Scholar]
- How best to approach endocrine evaluation in patients with HIV in the era of combined antiretroviral therapy? Clin Endocrinol (Oxf). 2013;79:310-3.
- [Google Scholar]
- Association between antiretrovirals and thyroid diseases: A cross-sectional study. Arch Endocrinol Metab. 2015;59:116-22.
- [Google Scholar]
- Dysfunction of the hypothalamic-pituitary-adrenal axis in HIV infection and disease. Hormones (Athens). 2008;7:205-16.
- [Google Scholar]
- Adrenal function in the human immunodeficiency virus-infected patient. Arch Intern Med. 2002;162:1095-8.
- [Google Scholar]
- Adrenocortical function in acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1987;65:482-7.
- [Google Scholar]
- Circadian variations in plasma levels of hypophyseal, adrenocortical and testicular hormones in men infected with human immunodeficiency virus. J Clin Endocrinol Metab. 1990;70:572-7.
- [Google Scholar]
- Thyroid and adrenal function in HIV-infected outpatients. Eur J Med Res. 1997;2:220-6.
- [Google Scholar]
- Stimulating effect of HIV-1 coat protein gp120 on corticotropin-releasing hormone and arginine vasopressin in the rat hypothalamus: Involvement of nitric oxide. Exp Neurol. 2000;166:376-84.
- [Google Scholar]
- Functional adrenal insufficiency among critically ill patients with human immunodeficiency virus in a resource-limited setting. Afr Health Sci. 2007;7:101-7.
- [Google Scholar]
- Low-dose adrenocorticotropin test in patients with the acquired immunodeficiency syndrome. Braz J Infect Dis. 2001;5:53-9.
- [Google Scholar]
- Low dose adrenocorticotropic hormone test and adrenal insufficiency in critically ill acquired immunodeficiency syndrome patients. Indian J Endocrinol Metab. 2012;16:389-94.
- [Google Scholar]
- Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: An open label randomized prospective study from Eastern India. Diabetes Res Clin Pract. 2014;103:e18-23.
- [Google Scholar]
- Serum vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J Med Res. 2013;138:853-60.
- [Google Scholar]
- Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362-71.
- [Google Scholar]
- Urinary albumin: Creatinine ratio predicts prediabetes progression to diabetes and reversal to normoglycemia: Role of associated insulin resistance, inflammatory cytokines and low vitamin D. J Diabetes. 2014;6:316-22.
- [Google Scholar]
- Vitamin D deficiency may be associated with a more rapid decline in CD4 cell count to <350 cells/μL in untreated HIV-infected adults. Curr HIV Res. 2015;13:517-23.
- [Google Scholar]
- Vitamin D deficiency in HIV infection: Not only a bone disorder. Biomed Res Int 2015 2015:735615.
- [Google Scholar]
- Vitamin D receptor genotype is associated with Addison's disease. Eur J Endocrinol. 2002;147:635-40.
- [Google Scholar]
- A possible role for the cortisol/anticortisols imbalance in the progression of human immunodeficiency virus. Psychoneuroendocrinology. 1997;22(Suppl 1):S27-31.
- [Google Scholar]
- Mechanisms of androgen deficiency in human immunodeficiency virus-infected women with the wasting syndrome. J Clin Endocrinol Metab. 2001;86:4120-6.
- [Google Scholar]
- Intervening at prediabetes stage is critical to controlling the diabetes epidemic among Asian Indians. Indian J Med Res. 2016;143:401-4.
- [Google Scholar]
- Comment on Anjana et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai urban rural epidemiology study (CURES) Diabetes Care. 2015;38:1441-8. Diabetes Care 2015; 38: e146
- [Google Scholar]
- Prediabetes and diabetes among HIV-infected adults in Cameroon. Diabetes Metab Res Rev. 2016;32:544-9.
- [Google Scholar]
- Diabetes mellitus and impaired fasting glucose in ART-naïve patients with HIV-1, HIV-2 and HIV-1/2 dual infection in Guinea-Bissau: A cross-sectional study. Trans R Soc Trop Med Hyg. 2016;110:219-27.
- [Google Scholar]
- Prevalence of diabetes mellitus, hyperinsulinaemia and metabolic syndrome among 755 adult patients with HIV-1 infection. Int J STD AIDS. 2011;22:43-5.
- [Google Scholar]
- Prevalence of insulin resistance and risk of diabetes mellitus in HIV-infected patients receiving current antiretroviral drugs. Eur J Endocrinol. 2014;171:545-54.
- [Google Scholar]
- Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3:216-26.
- [Google Scholar]






