Translate this page into:
Presence of virulence determinants amongst Staphylococcus aureus isolates from nasal colonization, superficial & invasive infections
*For correspondence: drpallabray@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Staphylococcus aureus is a highly versatile and adaptable microorganism, existing either as a commensal in the anterior nares of about 30 per cent of the human population, or as a pathogen implicated in skin, soft tissue, respiratory system, bone, joints and endovascular disorders1. S. aureus infections vary greatly in severity and range from superficial or localized, deep-seated or invasive to toxemic syndromes1. The genotypic attributes governing the occurrence of S. aureus as a commensal, and its transition to a pathogen involved in both superficial and invasive infections remain unclear. Our previous studies have revealed a dichotomy in S. aureus isolates from localized and deep-seated infections23. We, therefore, sought to study the differences in genetic repertoire of S. aureus isolates from nasal colonization, superficial infections and invasive infections, with respect to 11 major virulence determinants.
During 2004-2006, clinical isolates of S. aureus (n=69) obtained in bacteriology laboratory, Post-graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India from consecutive cases with invasive (n=35) and superficial (n=34) infections were used in this study. These isolates were selected from 500 consecutive isolates on the basis of pulsed-field gel electrophoresis (PFGE), wherein only one isolate belonging to each PFGE type was included to prevent over-representation of any gene owing to the effect of clonal similarity (data not shown). Invasive cases were defined as cases with clinical evidence of sepsis or deep-seated infection, with isolation of S. aureus in blood or an aspirate from a normally sterile site. Cases with evidence of prior trauma or penetrating injury and conditions such as diabetes mellitus, haematological malignancies and immunosuppression were excluded. The cases with superficial infections included infections limited to skin and/or subcutaneous tissue, without any clinical evidence of invasion into deeper tissues. Twenty five S. aureus isolates from the anterior nares of healthy volunteers of the institute without any history of an infective episode or antibiotic uptake in the last two weeks were also included. The study protocol was approved by the Institute's Ethics Committee.
All the 94 isolates were screened for the presence of 10 virulence genes [fibronectin-binding protein A (fnbA), collagen-binding protein (cna), serine-aspartate repeat protein (sdrE), staphylococcal enterotoxins (sea, sem, seo and sej), exfoliative toxin A (eta), intercellular adhesin (icaA) and gamma hemolysin (hlg)], and a major virulence regulator in S. aureus (accessory gene regulator; agr subgroup I-IV). The genes were detected by PCR according to the methods described previously, employing genomic DNA (for all genes except sej) or plasmid DNA (for sej)456. To determine the significance of association between various virulence genes and origin type of particular isolate (superficial, invasive or nasal), the data were tested statistically by univariate analysis using odds ratio and Fisher's exact test using SPSS software (SPSS Inc., USA). Further, a multivariate analysis was performed by logistic regression to confirm if the genes which were significantly associated with a group (on univariate analysis) were both independent of each other and also independently associated with the anatomic site of isolation. The statistical significance was calculated with 95% confidence intervals.
Comparison of virulence genes in S. aureus isolates from invasive infections with those from nasal colonization (Tables I and II) revealed a significant association of cna, icaA and sej with invasive isolates [icaA, P<0.001 and cna and sej, P<0.01 (univariate analysis); icaA, P<0.001 and sej, P<0.01 (multivariate analysis)]. cna encodes for collagen-binding protein which mediates attachment of staphylococci to cartilage, and is an important virulence factor in osteomyelitis, arthritis, sepsis, endocarditis and keratitis6. Most of the invasive isolates in this study (62.8%) were also from similar infections, with 62.9 per cent of these being positive for cna. sej is a plasmid-borne genetic determinant encoding for a pyrogenic, super-antigenic enterotoxin7. While nearly 57 per cent of the invasive isolates in this study were positive for sej, only 20 per cent of the nasal colonizers harboured this gene. Likewise, icaA, encoding for the polysaccharide intercellular adhesion (PIA) required during biofilm formation89, was present at a considerably higher proportion in invasive isolates (74%) than in nasal colonizers (20%). Previous studies have also reported a significant association of cna, ica and sej, as well as fnbA, sdrE, hlg and eta, in invasive compared with carriage isolates6789. However, O’Donnell et al10 reported no significant difference in the distribution of ica and cna amongst invasive and colonizing MRSA.
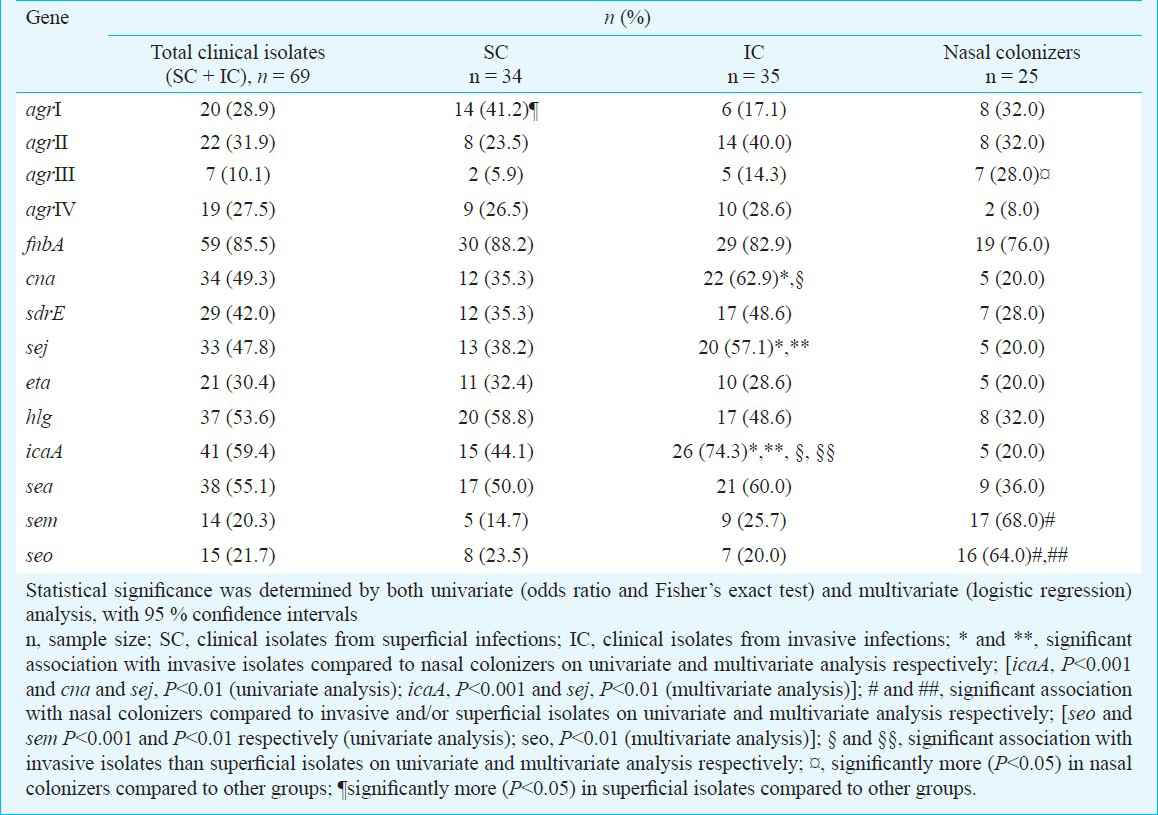
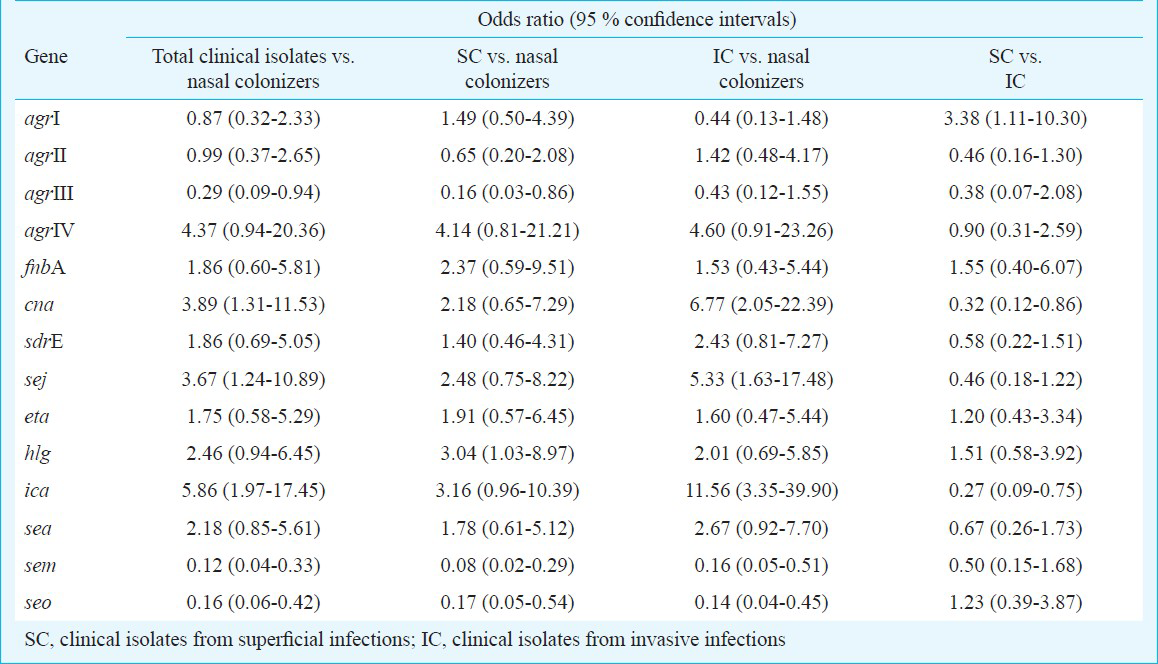
None of the virulence determinants tested showed a significant association with superficial isolates compared to nasal colonizers (Tables I and II). sem and seo were significantly more prevalent in colonizing isolates than invasive and/or superficial isolates [seo and sem, P<0.001 and P<0.01, respectively (univariate analysis); seo, P<0.01 (multivariate analysis)] (Table I). These genes are encoded by egc, a locus encoded by a mobile genetic element which may augment the carriage potential of S. aureus by inactivation of a crucial sequence or loss of another mobile genetic element carrying virulence gene during its insertion11. Several previous studies have also reported the predominance of egc locus in carriage isolates5111213141516.
Comparison of S. aureus isolates from invasive and superficial infections (Tables I and II) demonstrated a significantly greater presence of cna and icaA in invasive isolates on univariate analysis (P<0.05), and of icaA on multivariate analysis (P<0.05). sej was also more frequently present in invasive isolates but the association was not significant. In the superficial isolates, the presence of eta and hlg was higher compared to invasive ones, though not significant.
Amongst the agr subgroups (Tables I and II), the presence of agr III and agr I type was significantly greater in isolates from nasal colonization and superficial infections, respectively (P<0.05 on univariate and multivariate analysis).
The isolates from each group were also evaluated for the total number of virulence genes present (Figure). While most of the superficial isolates (74.28%) harboured 2-4 genes, majority of the invasive isolates (88.57%) harboured 3-6 genes. None of the nasal colonizers contained more than four virulence genes. Nearly two-thirds (68.58%) of the invasive isolates contained all the three or at least two of the genes out of icaA, cna and sej. In contrast, two-third (68.57%) of the superficial isolates harboured either one or none of the three genes. Also, 56 per cent of the nasal colonizers did not harbour icaA, cna or sej; 28 and 16 per cent of these isolates carried one and two of the three genes, respectively, and none of these isolates were positive for all the three genes.
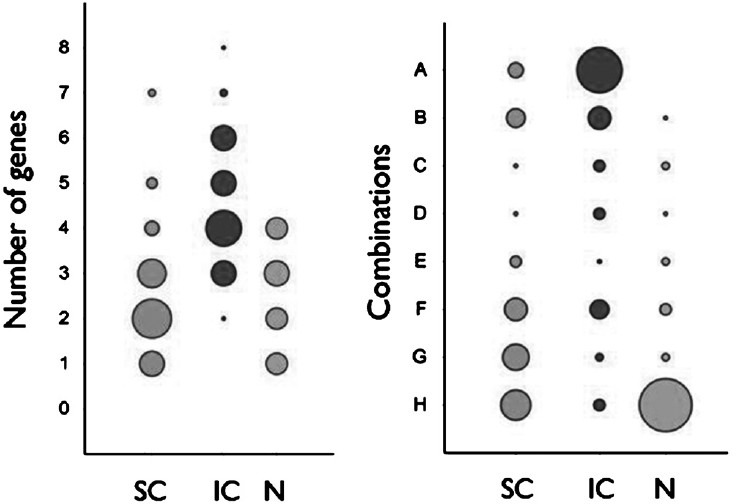
- Distribution of S. aureus isolates from nasal colonization (n = 25), superficial infections (n = 34) and invasive infections (n = 35) according to the number of virulence genes present, and different combinations of icaA, cna and sej. The diameter of the circles represents the number of isolates. A, cna +, ica +, sej +; B, cna +, ica +, sej -; C, cna +, ica -, sej +; D, cna -, ica +, sej +; E, cna +, ica -, sej -; F, cna -, ica +, sej -; G, cna -, ica -, sej +; H, cna -, ica -, sej -. SC, clinical isolates from superficial infections; IC, clinical isolates from invasive infections; N, nasal colonizers.
In conclusion, the present study suggests a considerable difference in the virulence potential of colonizing vs. superficial vs. invasive S. aureus isolates. Majority of the invasive isolates were found to harbour a greater number of virulence genes compared to the superficial or carriage isolates. Of the 11 major virulence markers tested, icaA, cna and sej were significantly associated with the invasive isolates, and detection of these genes may be a likely indicator of S. aureus invasiveness in a clinical setting. Isolates from superficial infections predominantly exhibited an agr I background, without a specific association with any of the virulence factors tested. In contrast, nasal colonizers showed agr III background and carried sem and seo more frequently. The presence of sem and seo may thus be suggestive of an isolate's carriage potential.
Acknowledgment
This work was supported by funding from PGIMER, Chandigarh, India in the form of Ph.D. research fellowship to the first author (MB). We thank Dr Richard Novick and Network on Antimicrobial Resistance in S. aureus (NARSA) for providing the positive control strains used in this study.
References
- Analysis of genetic diversity among Staphylococcus aureus isolates from patients with deep-seated and superficial staphylococcal infections using pulsed-field gel electrophoresis. Scand J Infect Dis. 2006;38:418-26.
- [Google Scholar]
- A comparative analysis of antibody repertoire against Staphylococcus aureus antigens in patients with deep-seated versus superficial staphylococcal infections. Int J Med Sci. 2005;2:129-36.
- [Google Scholar]
- Biotyping of enterotoxigenic Staphylococcus aureus by enterotoxin gene cluster (egc) polymorphism and spa typing analyses. Appl Environ Microbiol. 2006;72:6117-23.
- [Google Scholar]
- Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002;70:631-41.
- [Google Scholar]
- Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun. 2002;70:4987-96.
- [Google Scholar]
- Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J Clin Microbiol. 2003;41:1434-9.
- [Google Scholar]
- The intercellular adhesin locus ica is present in clinical isolates of Staphylococcus aureus from bacteremic patients with infected and uninfected prosthetic joints. Med Microbiol Immunol. 2001;189:127-31.
- [Google Scholar]
- Prevalence of virulence genes among invasive and colonising Staphylococcus aureus isolates. J Hosp Infect. 2011;77:89-91.
- [Google Scholar]
- Distribution of virulence genes among colonising and invasive isolates of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2008;14:625-6.
- [Google Scholar]
- Distribution of enterotoxin genes among carriage- and infection-associated isolates of Staphylococcus aureus. Lett Appl Microbiol. 2007;45:681-5.
- [Google Scholar]
- Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin Infect Dis. 2005;41:771-7.
- [Google Scholar]
- Clonal distribution and differential occurrence of the enterotoxin gene cluster, egc, in carriage-versus bacteremia-associated isolates of Staphylococcus aureus. J Clin Microbiol. 2006;44:1555-7.
- [Google Scholar]
- The profiles of enterotoxin genes in Staphylococcus aureus from nasal carriers. Lett Appl Microbiol. 2006;42:315-20.
- [Google Scholar]
- The distribution of enterotoxin and enterotoxin-like genes in Staphylococcus aureus strains isolated from nasal carriers and food samples. Int J Food Microbiol. 2007;117:319-23.
- [Google Scholar]
- Enterotoxin genes occurance among S. aureus strains isolated from inpatients and carriers. Med Dosw Mikrobiol. 2006;58:275-81.
- [Google Scholar]





