Translate this page into:
Prescription pattern & adverse drug reactions of prokinetics
For correspondence: Dr Yashashri C. Shetty, Department of Pharmacology & Therapeutics, 1st Floor, Main College Building, Seth GS Medical College & KEM Hospital, Parel, Mumbai 400 012, Maharashtra, India e-mail: yashashrirajit@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Prokinetics are extensively prescribed leading to several adverse events (AEs). The aim of this study was to assess the prescription pattern in patients receiving prokinetics, and characteristics of adverse drug reactions (ADRs) in an outpatient department set up in a tertiary care hospital in western India.
Methods:
Patients attending outpatient departments of a tertiary care hospital and who had received prokinetic agent for at least seven days over the last one month were enrolled. Causality assessment of AEs was done and assessed for severity, preventability, seriousness and predictability.
Results:
A total of 304 patients [161 males (52.96%); 143 females (47.04%)] were enrolled. Most prescriptions (299/304, 98%) included domperidone, most commonly prescribed as fixed-dose combination (FDC) with pantoprazole (274/304, 90%). Prokinetic dose was not mentioned in 251/304 (83%) prescriptions, and 18/304 (6%) did not mention frequency. Of the 378 AEs reported from 179 patients (47.35%), 306 (81%) were mild, all non-serious; 272 (72%) not preventable and 291 (77%) predictable in nature. Decreased appetite (n=31, 8.2%) and fatigue (n=27,7.14%) were most commonly reported. Causality assessment by the World Health Organization-Uppsala Monitoring Centre scale showed that 180 AEs were related to suspected drug (17 probable and 163 possible ADRs). Significant correlation was observed for AEs with increasing number of drugs per prescription (Spearman's R=+0.8, P=0.05) and with increasing therapy duration (Spearman's R=+1.00, P<0.001).
Interpretation & conclusions:
Our findings showed that prokinetics were often prescribed as FDCs, with incomplete prescriptions. Domperidone was found to be associated with multiple AEs. It is suggested that regular prescription monitoring should be done in hospitals to encourage rational use of drugs.
Keywords
Domperidone
gastric acid suppression
hypomotility
levosulpiride
prescription audit
proton pump inhibitor
Gastrointestinal (GI) motility may be impaired in many disorders such as functional dyspepsia, gastro-oesophageal reflux disease, gastroparesis (idiopathic or diabetic) and chronic idiopathic constipation1. There is considerable evidence to suggest an association between motility disorder and symptom production in functional dyspepsia23. The management of patients with GI hypomotility usually includes administration of prokinetic agents1. The various prokinetic agents used clinically are mainly the dopamine antagonists (metoclopramide, domperidone, levosulpiride and itopride) and the serotonin (5-HT) receptor agonists (5HT4 agonists such as cisapride and mosapride)4.
Though the efficacy of all the prokinetic agents for the treatment of GI hypomotility disorders is a known fact, these agents are associated with many adverse effects. The main side effects of metoclopramide include extrapyramidal symptoms such as dystonia, akathisia, parkinsonism-like symptoms and tardive dyskinesia. These appear to occur more commonly in children and young adults and at higher doses. Metoclopramide also can cause galactorrhoea by blocking the inhibitory effect of dopamine on prolactin release, but this adverse effect is relatively infrequent, albeit of major concern to females4. Levosulpiride is a therapeutic option in the management of functional dyspepsia on the basis of dopaminergic pathways controlling GI motility5. On the other hand, the serotonergic component of levosulpiride may enhance its therapeutic efficacy in functional dyspepsia6. However, it is associated with various side effects such as extrapyramidal symptoms, sedation, drowsiness, postural hypotension and increased level of prolactin associated with galactorrhoea and breast engorgement7. As domperidone does not cross blood-brain barrier, it does not cause any extrapyramidal adverse effects. However, since the pituitary gland lies outside the blood-brain barrier, it causes increase in prolactin levels leading to galactorrhoea and breast engorgement4. Itopride is well tolerated with a few minor adverse drug reactions (ADRs) such as diarrhoea, headache and abdominal pain8. Cisapride, due to QT segment prolongation, increases the risk of arrhythmia and risk of sudden death9.
Thus, prokinetic agents, though effective in hypomotility conditions, are associated with multiple adverse effects. Many times, their use has been rampant without a valid indication as many are available easily without prescription. Thus, the present study was carried out to assess the prescription pattern, find the rate of occurrence of associated adverse events (AEs), determine their causality and analyze their severity, seriousness, preventability and predictability in patients receiving any prokinetic agent from the outpatient departments (OPDs) of a tertiary care teaching hospital in western India.
Material & Methods
This present observational study was initiated in the department of Pharmacology & Therapeutics, Seth GS Medical College and KEM Hospital Mumbai, India, after approval from the Institutional Ethics Committee (EC/OA-53/2015). Written informed consents from patients or legally acceptable representatives were obtained. Adult patients (18-65 yr of age), of either gender, attending medical gastroenterology and ear-nose-throat (ENT) OPDs of the hospital and received any prokinetic agent for at least a period of seven consecutive days in the past one month, were enrolled. The study duration was pre-specified to be six months (January-June 2016). Data were analyzed in the following two months (July-August 2016). A duration specific convenience sampling method was adopted. A pre-designed case record form was used to collect relevant data, which included demographic details, prescription details pertaining to drug name, dose, route, frequency, duration and indication of use (all for both the prokinetic agents and concomitant medicines), working diagnosis and information regarding any AE. Patients' detailed history about both disease and drug therapy was noted carefully from previous medical records, and information regarding possible adverse effects was collected from the patients. If the previous medical records were not available with the patients, they were excluded from the study. From these data, causality assessment was done using both World Health Organization-Uppsala Monitoring Centre (WHO-UMC) Scale10 and Naranjo algorithm11. All the AEs were further assessed and classified according to severity (modified Hartwig-Siegel scale12), preventability (Schumock-Thornton criteria13), seriousness14, predictability, pattern and involvement of organ system [WHO-Adverse Reactions Terminology (ART) organ system classification code15].
Statistical analysis: Data were analyzed using descriptive statistics by SPSS v 21.0 (IBM Corporation, Armonk, NY, USA). Chi-square test and Fisher's exact test were applied to categorical data and Spearman's correlation was applied to continuous data.
Results
A total of 304 patients [161 males (52.96%) and 143 females (47.04%)] were included in the study. The mean age of the enrolled patients was 39 ± 12.2 yr. Of the 304 prescriptions analyzed, 298 (98%) included domperidone, and most commonly, it was prescribed as a fixed-dose combination (FDC) with pantoprazole (274/304, 90%). Levosulpiride was also prescribed as another prokinetic agent in combination with pantoprazole (Fig. 1). Dose of the prokinetic agent was not mentioned in 251 (83%) prescriptions. Where mentioned, the strength of domperidone was 30 mg along with 40 mg of pantoprazole as FDC. Most of these (239 of 304, 79%) were prescribed as once daily dosing, a few were prescribed as twice (44 of 304, 14%) or thrice daily (3 of 304, 1%) as well; whereas six per cent (18 of 304) prescriptions did not mention the frequency. In most of the cases, prokinetics were prescribed for up to seven days (69%, 211 of 304) with a maximum of 45 days in one patient (a case of liver cirrhosis with regurgitation). Four prescriptions lacked any mention of duration.
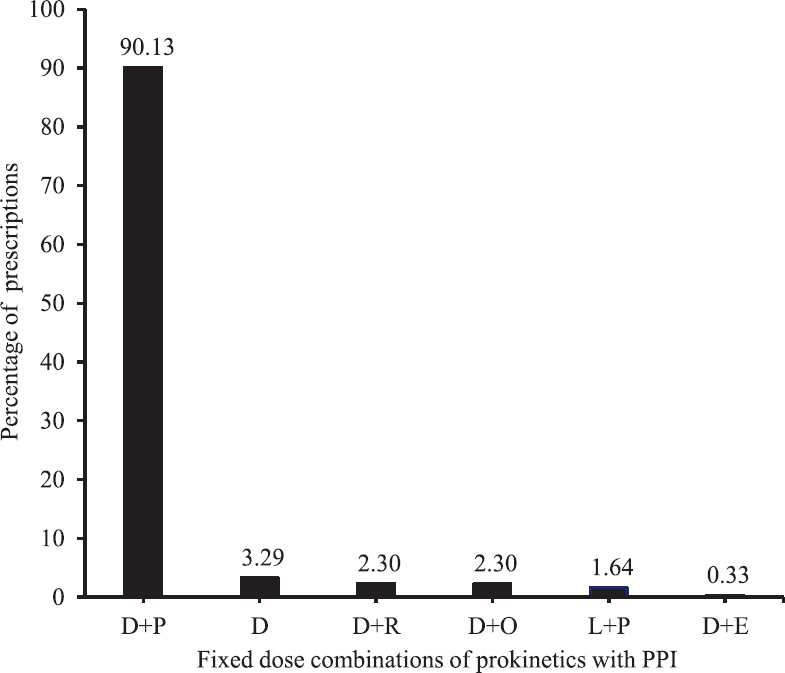
- Number of prescriptions with different prokinetic agents, single or in combination (n=304). D, domperidone; P, pantoprazole; R, rabeprazole; O, omeprazole; L, levosulpiride; E, esomeprazole; PPI, proton pump inhibitor.
The prescriptions were further analyzed to find the common drugs being prescribed along with prokinetics (Fig. 2). Most common was amoxicillin+clavulanic acid (127 of 304, i.e. 42% of total prescriptions) followed by levocetirizine (119 of 304, i.e. 39%) and non-steroidal anti-inflammatory drugs combinations (ibuprofen or diclofenac combined with paracetamol; total 107 of 304, i.e. 35%). The total number of drugs prescribed in 304 patients was 690. When prescribing pattern of these concomitant medications was analyzed, incompleteness was found in terms of dose not mentioned in 68 per cent (472 of 690), frequency not mentioned in 5.5 per cent (38 of 690) and duration not mentioned in 9.5 per cent (66 of 690) of total drug prescriptions. Half of the prescriptions contained three drugs (152 of 304, 50%), followed by four drugs (70 of 304, 23%), two drugs (50 of 304, 16%) and five drugs (29 of 304, 9%). One prescription contained only one drug (domperidone+pantoprazole FDC) and two prescriptions contained six drugs (both were cases of chronic suppurative otitis media; antibiotic/analgesic/anti-inflammatory/antihistaminic and multivitamin were co-prescribed).
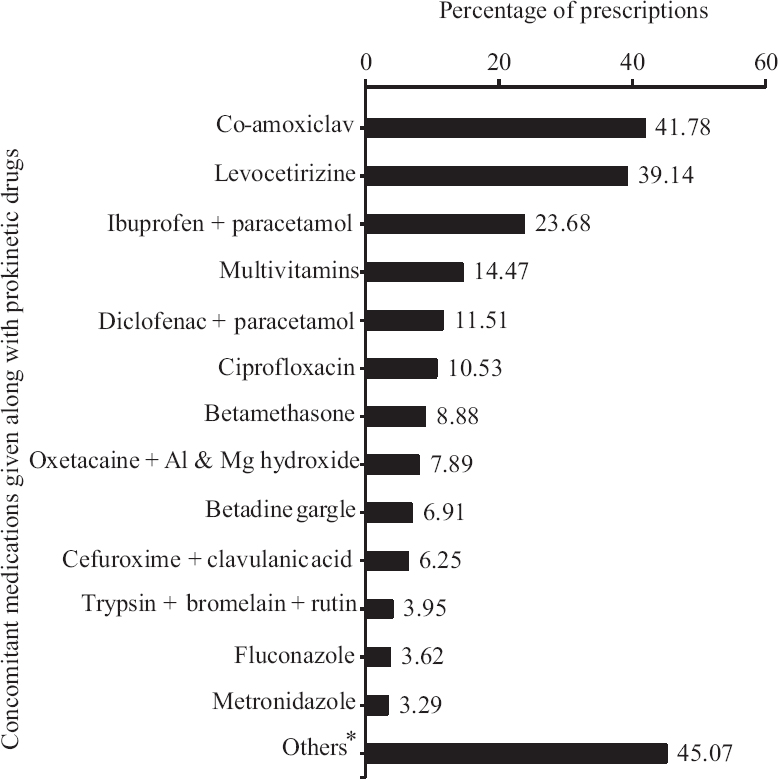
- Percentage of prescriptions with concomitant medications. *Others include cefixime, betadine gargle, ondansetron, probiotics, doxofylline, iron+folic acid, clotrimazole ointment, clonazepam, proton pump inhibitors, ranitidine, betahistine, mebendazole, metoprolol, cetirizine, chlorpheniramine maleate, chlorhexidine mouthwash, mucaine gel, salbutamol metered dose inhaler, oxymetazoline nasal drops, hyoscine, furosemide, levofloxacin, anti-tuberculosis drugs, doxycycline, mupirocin ointment, aspirin, calcium, pyridoxine, phenytoin, rifaximin.
Of the total 304 patients, at least one AE was noted in179 patients (58.8%). A total of 378 AEs were reported from these 179 patients (90 males), which were considered for causality assessment and further analysis. When occurrence of AE was compared amongst different age groups and genders, no significant difference was observed. However, a significant positive correlation was observed for occurrence of AEs in terms of increasing number of drugs per prescription (Spearman's Rho correlation coefficient, R=+0.8 and two-tailed P=0.05) and with increasing duration of therapy (Spearman's Rho correlation coefficient, R=+1.00, P<0.001). It was found that the occurrence of AEs was significantly higher in those patients receiving three or more drugs concomitantly than those receiving one or two (P<0.001). Sixty six prescriptions were found to have multiple ADRs.
Causality assessment by WHO-UMC scale revealed 180 AEs to be causally related to the suspected drug (17 probable and 163 possible ADRs). Assessment by Naranjo scale revealed similar results with 177 AEs being causally related to the suspected drug (17 probable and 160 possible ADRs). Rest of the AEs (198 by WHO-UMC and 201 by Naranjo scale) were designated as unlikely/doubtful after causality assessment (Table I). Both the scales were compared to find out the strength of agreement between them using Cohen's kappa statistical measurement and were found to be with strong agreement (98.4%) and a kappa value of 0.971 (standard error=0.012, 95% confidence interval=0.947-0.994). Severity assessment by modified Hartwig-Siegel scale revealed that 81 per cent (306 of 378) of the recorded AEs were mild and rest (72 of 378, 19%) were moderate in nature. None of the documented AEs was found to be severe or serious in nature. Preventability assessment by Schumock-Thornton criteria revealed that 72 per cent AEs (272 of 378) were not preventable, while the remaining 28 per cent (106 of 378) were preventable in nature, of which four AEs were definitely preventable and 102 AEs were probably preventable. Most of the AEs (291 of 378, 77%) were found to be predictable (type A or augmented) in nature; rest were non-predictable (type B or bizarre). Analysis of these AEs revealed that decreased appetite was most commonly associated with prokinetic use (31 of 378, 8%) followed by fatigue, throat pain/irritation, sedation/drowsiness, headache, diarrhoea, oral ulcers and ear discharge (Table II). Menstrual irregularity (usually long cycles) was reported in nine women, and five women reported breast tenderness; all were in patients receiving domperidone. All the five patients who received levosulpiride experienced AEs in the form of fatigue, headache and abdominal fullness. When AEs were analyzed according to the WHO-ART organ system classification code, GI system (WHO-ART code: 0600) was the most commonly affected organ system (129 of 378, 34%), followed by general body as a whole (code: 1810, 84 of 378, 22%) and neurological system (code: 0400, 56 of 378, 15%) (Fig. 3).
| Description | WHO-UMC scale10 (n=378) | Naranjo algorithm11 (n=378) |
|---|---|---|
| Certain/definite | 0 | 0 |
| Probable (%) | 17 (4.49) | 17 (4.49) |
| Possible (%) | 163 (43.12) | 160 (42.32) |
| Unlikely/doubtful (%) | 198 (52.38) | 201 (53.17) |
| Conditional/unclassified | 0 | NA |
| Un-assessable/unclassifiable | 0 | NA |
WHO-UMC, World Health Organization-Uppsala Monitoring Centre; NA, not applicable
| Adverse event | n (%) |
|---|---|
| Decreased appetite | 31 (8.2) |
| Fatigue | 27 (7.14) |
| Throat pain | 27 (7.14) |
| Sedation/drowsiness | 25 (6.61) |
| Headache | 22 (5.82) |
| Diarrhoea | 20 (5.29) |
| Ulcers (oral/palatal/lower lip) | 18 (4.76) |
| Ear discharge | 18 (4.76) |
| Throat irritation | 18 (4.76) |
| Nausea | 15 (3.96) |
| Abdominal pain | 14 (3.70) |
| Constipation | 12 (3.17) |
| Dizziness | 11 (2.91) |
| Dysphagia | 11 (2.91) |
| Running nose | 11 (2.91) |
| Menstrual irregularity | 9 (2.38) |
| Regurgitation | 8 (2.11) |
| Body ache | 7 (1.85) |
| Hoarseness of voice | 7 (1.85) |
| Ear ache | 6 (1.58) |
| Breast tenderness | 5 (1.32) |
| Others | 56 (14.82) |
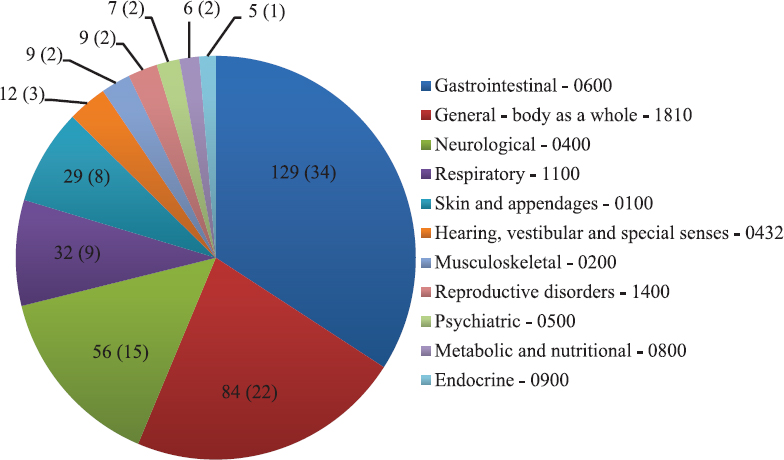
- Distribution of adverse events according to the World Health Organization-Adverse Reactions Terminology Organ System Classification Code15 (n=378). Absolute number of adverse events is provided in the pie chart followed by percentage in parenthesis.
Discussion
The WHO defined rational use of drugs as patients receiving medications appropriate to their clinical needs, in doses that meet their individual requirements, for an adequate period of time and at the lowest cost to them and their community16. The WHO and the International Network of Rational Use of Drugs have developed a set of drug prescribing indicators to be used as measures of prescribing performance in healthcare settings17. The evaluation of quality of medical care provided in a particular set-up is called medical audit and prescription audit is a part of it, which seeks to monitor, evaluate and if necessary suggest modifications in the prescribing practices of medical practitioners, and thus considered as a useful approach to achieve the objective of improving the quality of patient care1819.
Prescription audits in different setups have many times showed the lack of completeness, legibility and rationality. Studies192021 have found flaws in prescribing while analyzing the pattern of the same in their institutions. Incompleteness in prescribing prokinetic agents and concomitant medications were observed in our study. Dose of the prokinetic agent was not mentioned in 83 per cent of the prescriptions, six per cent prescriptions did not mention the frequency and three per cent prescriptions lacked any mention of proposed duration of therapy. As a result, the prescribed daily dose versus the defined daily dose could not be calculated and compared. Similarly, when prescription pattern of concomitantly administered medications were analyzed, incompleteness was found in terms of dose not mentioned in 68 per cent, frequency not mentioned in 5.5 per cent and duration not mentioned in 9.5 per cent prescriptions.
Although symptom relief rates were found to be significantly higher with levosulpiride group as compared to domperidone and metoclopramide22, yet in our study, domperidone was the most commonly prescribed prokinetic and was usually prescribed as a FDC with pantoprazole. This was probably because domperidone is less expensive as compared to other prokinetic drugs and is freely available from hospital formulary. John et al23 found metoclopramide as the most frequently utilized prokinetic agent in critically ill patients from a tertiary care hospital in south India, though in our study none of the patients received metoclopramide. Increased use of domperidone over other prokinetics in paediatric age group was observed by Mt-Isa et al24.
Approximately 59 per cent of the patients who received prokinetic agents showed one or more AEs, which were subjected to causality assessment and further analysis. It was decided to use both WHO-UMC scale and Naranjo algorithm as there is no gold standard for causality assessment, and therefore, one scale cannot be preferred over the other. Both of these are widely used causality assessment tools, but none has been validated so far to give acceptable reproducible results25. The scales showed 'very good' strength of agreement (98.4%) in assessing causality by Cohen's kappa statistical measurement (kappa value of 0.971). Belhekar et al25 reported poor agreement between the two scales while Mittal and Gupta26 reported a moderate to good agreement. Such variations in different settings are expected as skill of assessing the causality may vary based on knowledge, experience and interpretation of the personnel assessing the causality.
Most of the AEs were mild (81%) and non-serious (100%) in nature. Of the 89 women in whom AEs were detected, nine reported menstrual irregularity (usually delayed menstruation) and five reported breast tenderness. Galactorrhoea has also been reported as AE of domperidone2728.
Nearly one-third (28%) of the AEs were preventable in nature. For example, in a few cases, appropriate history-taking and the absence of a definite indication would have prevented the irrational administration of the prokinetic agent and the subsequent ADRs. Preventable ADRs are a major burden on the healthcare system, so more careful and vigilant choice and administration of prokinetic agent are needed on the part of the physicians. Majority of the AEs (77%) were found to be predictable or Type A (augmented) reactions in nature, thought to be an extension of the pharmacological profile of the drugs. It has been stated that in general, for type B reactions, the drug needs to be discontinued29, but in the present study, the offending drugs were not withdrawn even in the cases of type B reactions as those AEs were mild and non-serious in nature.
The present study had some limitations. It was based on data from only two OPDs; hence, the findings cannot be generalized to other setups and there is a need to extend it to the remaining clinical departments. It was a cross-sectional study, and hence, a follow up study needs to be undertaken to capture delayed AEs. Prescriptions were mostly restricted as per the availability of the drug in the hospital; further analysis needs to be extended to other prokinetics. A chance of recall bias was there which should be kept in mind.
In conclusion, our findings showed that the prokinetic drugs were rampantly prescribed drugs in FDCs and with incomplete prescriptions, and many of the receivers experienced multiple AEs. There should be regular prescription audits and physicians should be encouraged about rational use of drugs. Further, there should be a highly efficient pharmacovigilance programme in place along with patient awareness activities to prevent and report the AEs.
Financial support & sponsorship: Authors acknowledge the Diamond Jubilee Society Trust, Seth GS Medical College and KEM Hospital, Mumbai, for funding the study.
Conflicts of Interest: None.
References
- Prokinetic agents: Current aspects with focus on cisapride. Ann Gastroenterol. 2015;13:269-89.
- [Google Scholar]
- Effectiveness and safety of levosulpiride in the treatment of dysmotility-like functional dyspepsia. Ther Clin Risk Manag. 2007;3:149-55.
- [Google Scholar]
- Dyspeptic symptoms in primary care. An observational study in general practice. Eur J Gastroenterol Hepatol. 2002;14:985-90.
- [Google Scholar]
- Brunton L, Chabner B, Knollmann B, eds. Goodman & Gilman's the pharmacological basis of therapeutics (13th ed). New York: McGraw Hill; 2018.
- Effect of acute and chronic levosulpiride administration on gastric tone and perception in functional dyspepsia. Aliment Pharmacol Ther. 2002;16:613-22.
- [Google Scholar]
- Review article: Clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004;19:379-90.
- [Google Scholar]
- Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011;12:147-56.
- [Google Scholar]
- The use of the WHO-UMC system for Standardized Case Causality Assessment. Available from: http://www.who.int/medicines/areas/quality-safety-efficacy/WHOcausality assessmentpdf
- A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-45.
- [Google Scholar]
- Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229-32.
- [Google Scholar]
- Indian Pharmacopoeia Commission. Suspected Adverse Drug Reaction Reporting form: For Voluntary reporting of Adverse Drug Reactions by healthcare professionals. Ghaziabad: Ministry of Health & Family Welfare, Government of India; Available from: http://www.ijp-online.com/documents/AdverseReaction.pdf
- The WHO Adverse Reaction Terminology: WHO-ART. Available from: https://www.who-umc.org/vigibase/services/learn-more-about-who-art/
- World Health Organization. How to investigate drug use in health facilities: Selected drug use indicators. (EDM research series no. 007). Geneva: WHO; 1993.
- 2005. Rational use of medicines by prescribers and patients. Geneva: WHO; Available from: https://apps.who.int/iris/bitstream/handle/10665/20236/B115_40-en.pdf?sequence=1&isAllowed=y
- Reviewing audit: Barriers and facilitating factors for effective clinical audit. Qual Health Care. 2000;9:23-36.
- [Google Scholar]
- Prescription audit in outpatient department of multispecialty hospital in Western India: An observational study. Int J Clin Trials. 2015;2:14-9.
- [Google Scholar]
- A study of prescription auditing in a tertiary care teaching hospital of Eastern India. J Drug Deliv Ther. 2014;4:140-9.
- [Google Scholar]
- Prescription audit study in a tertiary care hospital using the anatomical therapeutic chemical and defined daily dose classification concept. Int J Basic Clin Pharmacol. 2014;3:889-901.
- [Google Scholar]
- Efficacy and tolerability of levosulipride, domperidone and metoclopramide in patients with non-ulcer functional dyspepsia: A comparative analysis. J Clin Diagn Res. 2015;9:FC09-12.
- [Google Scholar]
- Utilization profile of gastrointestinal medications among the critically Ill patients of a tertiary care hospital. Jordan J Pharm Sci. 2013;6:299-307.
- [Google Scholar]
- Prokinetics prescribing in paediatrics: Evidence on cisapride, domperidone, and metoclopramide. J Pediatr Gastroenterol Nutr. 2015;60:508-14.
- [Google Scholar]
- A study of agreement between the Naranjo algorithm and WHO-UMC criteria for causality assessment of adverse drug reactions. Indian J Pharmacol. 2014;46:117-20.
- [Google Scholar]
- Comparison of agreement and rational uses of the WHO and Naranjo adverse event causality assessment tools. J Pharmacol Pharmacother. 2015;6:91-3.
- [Google Scholar]
- Galactorrhea-a rare side effect of domperidone. J Indian Acad Clin Med. 2011;12:225-6.
- [Google Scholar]
- Domperidone induced galactorrhea: An unusual presentation of a common drug. Indian J Pharmacol. 2013;45:307-8.
- [Google Scholar]
- Anticipating, investigating and managing the adverse effects of drugs. Clin Med (Lond). 2005;5:23-6.
- [Google Scholar]






