Translate this page into:
Preclinical safety & toxicity evaluation of pooled, allogeneic human bone marrow-derived mesenchymal stromal cells
Reprint requests: Dr Anish Sen Majumdar, Stempeutics Research Pvt. Ltd., Akshay Tech Park, Whitefield, Bengaluru 560 066, Karnataka, India e-mail: anish.majumdar@stempeutics.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Administration of ex vivo-expanded human bone marrow-derived mesenchymal stromal cells (hBMMSC) obtained from single donors has shown therapeutic benefits in both preclinical and clinical studies. In this study, the safety, toxicity and biodistribution profiles of a pooled hBMMSC population, produced from three healthy donors were assessed in rodent and non-rodents.
Methods:
The pooled hBMMSC population was characterized by their expression of various cell surface markers, differentiation potential and immunomodulatory activity. To establish in vivo safety of the pooled cells, these were administered by various injection routes into rodents and non-rodents to determine overall toxicity, biodistribution and tumorigenic potential in a series of preclinical studies.
Results:
Single injections of hBMMSC at various doses through intravenous or intramuscular routes did not cause toxicity in rats and rabbits. In addition, repeat administration of hBMMSC was also well tolerated by rats, and no prenatal toxicity was observed by multiple administration in the same animal species. Ex vivo-expanded and cryopreserved hBMMSCs did not induce tumour formation in severe combined immunodeficient (SCID) mice.
Interpretation & conclusions:
Our results showed that the pooled hBMMSC population was non-toxic, non-teratogenic and non-tumorigenic in animals. Further studies need to be done to find out if it can be safely administered in human patients.
Keywords
Biodistribution
BMMSC
human
single and repeat dose toxicity
teratogenic
toxicity
tumorigenicity
Mesenchymal stromal cells (MSCs) are found in all adult tissues and are known to play a vital role in tissue repair and regeneration. Although these cells were originally isolated from the bone marrow1, MSCs have also been isolated and characterized from adipose tissue, umbilical cord blood, Wharton's jelly of the umbilical cord, amniotic fluid and membrane, placenta, dental pulp and foreskin2. These plastic adherent MSCs are self-renewing, multipotent and have an inherent potential to differentiate into cells of mesodermal origin such as osteocytes, adipocytes and chondrocytes in vitro with specific stimuli3 as well as into cells of other lineages including neuron-like cells, cardiomyocytes, endothelial cells and hepatocyte-like cells2. Although no MSC-specific markers have been identified yet, these cells commonly express surface markers such as CD73, CD90, CD105 and low levels of MHC class I, as well as adhesion molecules CD29, CD44, CD54, CD106 and CD166, and are negative for the expression of haematopoietic markers CD11b, CD14, CD19, CD34, CD45 and human leucocyte antigen -D related (HLA-DR)4.
The current therapeutic dogma suggests that MSCs have the ability to home to inflamed tissues in response to cytokines and chemokines and due to their potent anti-inflammatory properties can attenuate or suppress inflammation both in vitro and in vivo5. The likely therapeutic mechanism for MSCs is through the secretion of a whole gamut of paracrine factors with a wide range of physiological effects6. These properties of MSCs have attracted the attention of researchers to utilize these cells for diverse therapeutic applications such as critical limb ischaemia, acute myocardial ischaemia, inflammatory bowel disease, graft-versus-host disease (GvHD), osteoarthritis (OA), multiple sclerosis and organ transplantation7.
Ex vivo-expanded MSCs derived from various tissue sources can be used in both autologous and allogeneic settings for the evaluation of their therapeutic efficacy. The immune evasive and immunomodulatory properties are believed to allow the allogeneic MSCs to protect them from immune rejection in an unrelated recipient and render them suitable for allogeneic cell transplantation and therapy8. Multiple clinical studies have confirmed the safety of both allogeneic and autologous MSCs for the treatment of human diseases9.
The use of large-scale expanded cryopreserved allogeneic MSCs in cell therapy requires vigorous quality control to ensure that good manufacturing practices (GMPs) are followed, and the expansion and preservation process of the cells must not affect the safety and efficacy of the product. Clinical grade MSCs should also be free of aerobic and anaerobic bacteria, fungi, endotoxin and mycoplasma, thus ensuring the aseptic nature of the product10. In addition, the manufactured cells should undergo a battery of preclinical studies to ensure that the cells are non-toxic, safe and non-tumorigenic in nature.
A GMP-compliant cell therapy product (Stempeucel®) was developed which comprised adult human bone marrow-derived, cultured and pooled allogeneic and off-the-shelf cryopreserved MSCs that have been extensively characterized. To establish the in vivo safety and toxicity profiles of these cells, a series of preclinical studies in rodent and non-rodent animals using single donor- and pooled donors-derived bone marrow MSCs (BMMSC) were performed. The studies were designed on the basis of National Guidelines for Stem Cell Research (http://www.icmr.nic.in) and Schedule Y of the Drugs and Cosmetic Act, India (http://www.cdsco.nic.in). Cell toxicity was tested using various doses of human BMMSC (hBMMSC) following intravenous (i.v.) and intramuscular (i.m.) injections. The tumorigenic potential of the pooled cells was assessed in severe combined immunodeficient (SCID) mice, and prenatal developmental toxicity was conducted in pregnant rats to determine the safety profile of these cells. The tissue localization and kinetics of biodistribution of the labelled cells were also determined.
Material & Methods
Production of hBMMSC: The bone marrow aspiration study was conducted in Kasturba Medical College Hospital, Manipal, India after approval by the institutional ethics committee. Inclusion criteria included healthy consenting human volunteers within the age group of 18-40 yr of Indian origin. Exclusion criteria included volunteers with autoimmune disorders, diabetes mellitus, hypertension, significant cardiac diseases, malignancy, family history of inherited diseases, tested positive for tuberculosis, malaria, human immunodeficiency virus, hepatitis B virus, hepatitis C virus, cytomegalovirus and syphilis. Bone marrow aspiration was performed after obtaining informed written consent from healthy donors11. In brief, 60 ml of bone marrow aspirate was obtained from each donor, from which BMMSCs were isolated, expanded and characterized. The process of production of single donor-derived and pooled donor-derived hBMMSC in the manufacturing facility at Manipal has been described elsewhere1213. To obtain the pooled population, hBMMSC from three individual donors was pooled in equal proportion (1:1:1) at passage 2 (P2) and expanded to P3, which was considered as working cell bank (WCB). WCB was further expanded to P5 which was an investigational medicinal product (IMP) and all the experiments were conducted using cryopreserved hBMMSCs/IMP at P5.
Characterization of hBMMSC: Flow cytometric analysis was performed for characteristic surface antigen expression profile of hBMMSCs14. The antibody reagents and method of staining of these cells had been described previously in detail12. The trilineage differentiation (osteocytes, chondrocytes and adipocytes) of pooled hBMMSC was performed in vitro according to the published protocol3. Karyotyping of hBMMSC was performed by GTG-banding assay and DNA ploidy index was measured by flow cytometry (BD FACSCalibur, BD Biosciences, USA). In addition, these cells were also tested for sterility, bacterial endotoxin and mycoplasma to confirm that the IMP was sterile and endotoxin free. Cell viability was measured by flow cytometry using 7-amino actinomycin D12.
For immunosuppression assay, mitomycin C-treated hBMMSCs were used at different cell concentrations (2 × 105 - 2.5 × 104 cells/well) in one-way mixed lymphocyte reaction (MLR) with responder and stimulator peripheral blood mononuclear cells (PBMCs), and cell proliferation was measured using 5-bromo-2΄-deoxyuridine (BrdU) assay (QIA58, Merck Millipore, USA).
Preclinical animal studies: A series of safety/toxicological studies were performed in rodent and non-rodent species using single donor-derived BMMSCs as well as with the pooled cell product. These studies were carried out at various contract research organizations (CROs) and institutes in India as described below. These studies were approved by the respective Institutional Animal Ethics Committee of the CRO/institutions.
(i). Fourteen-day acute toxicity study was performed in rats (i.v. route) using single donor-derived hBMMSC at INTOX Pvt. Ltd., Pune, India, between January and June 2007.
(ii). Repeat dose toxicity study was performed in rats (i.v. and i.m. routes) using single donor-derived hBMMSC at INTOX, between March 2007 and September 2008.
(iii). Fourteen day acute toxicity study was performed in rats and rabbits (i.v. and i.m. routes) using pooled hBMMSC at ICMR- National Institute of Nutrition (NIN), Hyderabad, India, between August 2009 and September 2010.
(iv). Ninety day subchronic toxicity study was performed in rats and rabbits (i.v. and i.m. routes) using pooled hBMMSC at NIN, between September 2009 and October 2010.
(v). Tumorigenicity study was performed in SCID mice (s.c. and i.m. routes) using pooled hBMMSC at Syngene International Ltd., (Bengaluru, India), between December 2010 and September 2011.
(vi). Prenatal developmental toxicity study was performed in pregnant rats (i.v. route) using pooled hBMMSC at INTOX, between July 2010 and March 2011.
(vii). Biodistribution study was performed using pooled hBMMSC at Syngene International Ltd., between December 2012 and January 2013.
In all preclinical studies, animals were kept in polypropylene cages in an environmentally controlled room maintained at 18-24°C with 12 h light/dark cycle and 30-70 per cent relative humidity. All animals had free access to food and water. The animals were acclimatized to laboratory conditions for seven days before inclusion in the study.
Dose selection & cell preparation: The cell dose used in these studies was based on the proposed clinical dose of 2 × 106/kg body wt (b.w.) the most commonly used clinical dose for a similar type of cell therapy products using MSC1516. Cryopreserved hBMMSCs were thawed in 37°C water bath, centrifuged and resuspended in appropriate volume of PlasmaLyte A (Baxter, USA) and injected into the animals.
Fourteen day acute toxicity study using single donor-derived hBMMSC in rats: A dose range finding study was performed using ex vivo-expanded single donor-derived hBMMSC to determine the maximum tolerable dose. In this study, the cells were administered at doses of 5, 10, 15 and 20 × 106 cells/kg b.w. or vehicle to Sprague Dawley (SD) rats through i.v. route. All animals were observed for seven days. No abnormal clinical signs/mortality were observed (data not shown). Hence, the maximum dose of 20 × 106 cells/kg b.w. was selected for the study. The hBMMSCs were injected at a dose of 20 × 106 cells/kg b.w. to five male and five female SD rats through i.v. route. All animals were observed daily for mortality and clinical signs for 15 days. On day 15 all surviving animals were sacrificed using CO2 and subjected to complete necropsy.
Repeat dose administration of single donor-derived hBMMSC in rats: In this study, hBMMSC derived from a single donor was administered to rats at a dose of 15 × 106 cells/kg b.w. or vehicle as follows. SD rats were divided into four groups; each group consisted of 11 males and 11 females (Groups; G1- G4). Groups G1 and G2 were administered with hBMMSC at a dose of 15 × 106 cells/kg b.w. daily for 14 days through i.v. and i.m. routes, respectively. Groups G3 and G4 were injected with vehicle and served as a control for i.v. and i.m. routes, respectively. On day 15, six male and six female rats from each group were sacrificed and the remaining rats were kept for another 14 days to determine the persistence, reversibility or delayed occurrence of toxic effects. To assess the genotoxic potential, two additional groups (G5 & G6; 10 males and 10 females/group) were used and served as positive (cyclophosphamide treated) and negative control (saline injected), respectively. All the animals were observed daily for mortality and clinical signs. The body weight and food consumption were measured weekly once till the end of study. On days 15 and 29, animals were anaesthetized using mild isoflurane; blood was collected by retro-orbital sinus bleeding for haematology and biochemical parameters analysis (G1-G4). Complete necropsy and macroscopic examinations were performed on all animals. The organs were weighed and tissue histopathological examinations were performed. Bone marrow samples were collected from G2, G5 and G6 group rats on days 15 and 29 to assess the genotoxicity potential of i.m.-administered hBMMSC. Immunotoxicity potential of repeat i.m.-administered hBMMSC was evaluated by measuring basic type 1 tests17.
Fourteen-day acute toxicity study using pooled hBMMSC in rats and rabbits: In this study, pooled hBMMSCs (252 × 106 cells/kg b.w.) were injected into Fischer rats (180-200 g b.w., 5 males and 5 females/group) through i.v. and i.m. routes. Similarly, the cells (130.6 × 106 cells/kg b.w.) were administered into New Zealand White (NZW) rabbits (1-1.5 kg b.w., 2 or 3 males and 2 or 3 females/group). All these animals were observed for mortality and clinical signs for 14 days. On day 15, animals were euthanized and complete necropsy and gross pathological examinations were performed.
Ninety day subchronic toxicity study using pooled hBMMSC in rats and rabbits: A 90-day subchronic toxicity study was conducted in SD rats and NZW rabbits by administering hBMMSC through i.v. and i.m. routes. Rats were administered with low (12.6 × 106 cells/kg b.w.), medium (63 × 106 cells/kg b.w.) and high dose (126 × 106 cells/kg b.w.) of hBMMSC or vehicle. Each dose of cells was injected into six male and six female rats (180-200 g b.w.). Similarly, three male and three female rabbits (1-1.5 kg b.w.) received low (6.53 × 106 cells/kg b.w.), medium (32.65 × 106 cells/kg b.w.) and high dose (65.3 × 106 cells/kg b.w.) of hBMMSC or vehicle. The food consumption and body weights were measured as described above. Blood samples were collected from retro-orbital plexus at 48 h and days 15, 30, 60 and 90 after cell injection and used for determining haematologic and biochemical parameters. Animals were euthanized on days 15 and 90 after cell injection, complete necropsy was performed and tissue samples were processed for histopathological examination. Genotoxicity potential of pooled hBMMSC was determined18 using bone marrow samples at both time points.
The immune response against hBMMSC was measured by analyzing the immune cell subsets in the blood of i.v.-injected rats using antibodies for cell surface markers CD3-FITC, CD4-PE, CD8a-PerCP, CD45-PE (BD Pharmingen, USA) along with isotype control by flow cytometry. Inflammatory cytokine levels were also measured in serum samples using interleukin-1 beta (IL-1β), interferon-gamma (IFNγ) and tumour necrosis factor-alpha (TNFα) antibodies (Becton Dickinson, USA) on days 0 (baseline), 7 and 90.
Tumorigenicity study using pooled hBMMSC in SCID mice: The tumorigenicity study was performed in 5-6 wk old SCID mice (25 males and 25 females) using two different cell concentrations; 0.5 × 106 cells/mouse, subcutaneous (s.c.) route; 10 × 106 cells/mouse, s.c. and i.m. routes. For s.c. injection, cells were injected in a 200 μl volume of vehicle and for the i.m. injection volume was 50 μl/mouse. Vehicle-injected animals were used as negative control. The human colorectal cancer cell line, DLD-1 injected animals served as positive control. All animals were observed periodically for 26 wk and the tumour formation was observed twice a week. Animals were sacrificed when the tumour volume reached 2000 mm3. At the end of 26 wk period, all animals were sacrificed; necropsied and histopathological analysis was performed.
Prenatal developmental toxicity study in pregnant rats using pooled hBMMSC: The prenatal developmental toxicity of hBMMSC delivered through the i.v. route in Wistar rats was evaluated. The pregnant rats (n=10/group) received low (12 × 106 cells/kg b.w.), medium (60 × 106 cells/kg b.w.) and high dose (120 × 106 cells/kg b.w.) of hBMMSC or vehicle at days 5, 12 and 18. All the dams were monitored until day 20 for body weight change and abnormal clinical signs. Caesarean sections were performed on day 20 and foetuses were evaluated for structural abnormalities or altered growth.
Biodistribution of pooled hBMMSC in rat and mice: The biodistribution kinetics of pooled hBMMSC was performed in immunocompromised mice and rats through i.v. and i.m. routes of administration. The cells were labelled using CM-DiI fluorescent dye (Thermo Fisher Scientific, USA) as published before19. Imaging was performed using in vivo imaging system (Caliper Life Sciences, Germany) at various time intervals (minutes to days) to monitor the distribution of cells to various organs until the signal intensity disappeared.
Statistical analysis: Data analysis was performed for body weight changes, food consumption, haematological parameters, clinical chemistry, organ weights and organ weight to body weight ratio. The analysis was performed using one-way ANOVA followed by Dunnett's multiple comparison or Student's t test.
Results
Production and characterization of hBMMSC: Phenotypic analysis of the pooled hBMMSCs expressed high levels of MSC-associated antigens and very low or negligible expression of various haematopoietic cell markers (Table I). All the tested batches of cells complied with the sterility requirement, and karyotyping and DNA ploidy analysis did not reveal any genomic abnormality in the pooled preparation of hBMMSC. The MLR test performed with two HLA-mismatched PBMCs demonstrated that hBMMSCs mediated inhibition of lymphocyte proliferation (Table I). The in vitro trilineage differentiation results demonstrated the efficiency of pooled hBMMSCs to osteocytes, chondrocytes and adipocytes lineages, suggesting that these cells were multipotent in nature (Fig. 1). These results showed that the pooled hBMMSC population exhibited all the common properties of MSC. The single donor-derived hBMMSC also showed the same characteristics as observed with pooled population13.
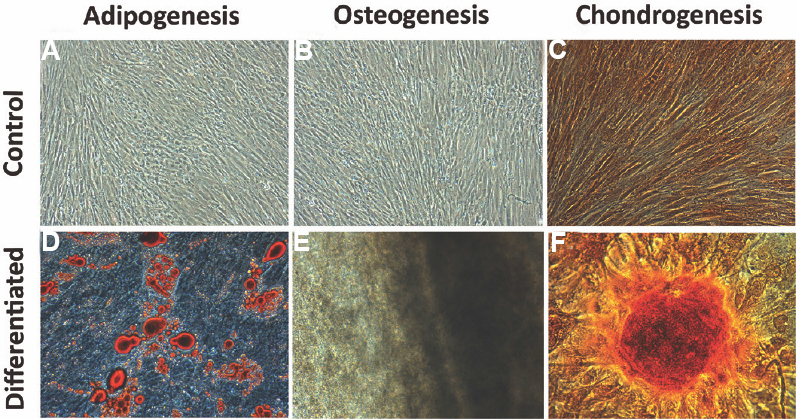
- Multilineage differentiation of pooled human bone marrow-derived mesenchymal stromal cells (hBMMSCs). Adipogenic differentiation was detected by oil droplet formation stained with Oil O red staining (D - differentiated), Osteogenic differentiation was confirmed by the stained mineralized matrix with alizarin red staining (E - differentiated), chondrogenic differentiation was confirmed through the presence of stained glycosaminoglycan by Alcian blue staining (F - differentiated). Undifferentiated control cultures of adipogenic, osteogenic and chondrogenic differentiation are shown in (A-C), respectively.
Fourteen day acute toxicity study in rats using single donor-derived hBMMSC: The dose range using single donor-derived hBMMSCs did not result in mortality or abnormal clinical signs in the animals for any of the cell dose (data not shown). The gross pathological examination did not reveal obvious signs of toxicity in any animal.
Repeat dose administration of single donor-derived hBMMSC in rats: Fourteen consecutive days of hBMMSC administration by i.v. and i.m. routes did not show mortality or abnormal clinical signs in rats (data not shown). There were no significant differences in body weights or food consumption throughout the study. A mild, but reversible swelling at the injection site was observed in some animals. No treatment-related change was observed in the cell-treated animals for haematological or biochemical parameters measured on days 15 and 29 (data not shown). There was no histological abnormality observed in either vehicle or hBMMSC-treated animals.
In addition, repeat i.m. administration of hBMMSC did not change the ratio of polychromatic erythrocytes (PCE) to total erythrocytes (TE) and the incidence of micronucleated PCEs (MnPCEs), suggesting that hBMMSC was not genotoxic. Repeated i.m. administration of hBMMSC did not alter any of the immune-related haematological and clinical chemistry parameters, and no treatment-related gross pathological changes and histopathological findings were observed in the lymphoid tissues (data not shown). Therefore, the no-observed adverse effect level (NOAEL) of hBMMSC in rats, following i.v. and i.m. administration for 14 days, was found to be ≥15 × 106 cells/kg b.w.
Fourteen day acute toxicity study using pooled hBMMSC in rats and rabbits: The pooled population of hBMMSC at a dose of 252 × 106 cells/kg b.w. did not result in mortality or abnormal clinical signs in rats following i.v. and i.m. administration. Similarly, i.m. administration of these cells (130 × 106 cells/kg b.w.) into rabbits was also found to be non-toxic and no mortality was observed (data not shown). However, in a parallel study conducted in rabbits, an instantaneous death of one male and one female animal occurred after i.v. administration of hBMMSC at the same dose. The necropsy examination suggested that the causality of death was incidental although death due to acute pulmonary embolism by cellular aggregates was not ruled out. All other animals remained healthy throughout the study. The minimum lethal dose, maximum tolerated dose and the median lethal dose of pooled hBMMSC were ≥252 × 106 cells/kg b.w. for rats and ≥130 × 106 cells/kg b.w. for rabbits for both i.v. and i.m. routes of administration.
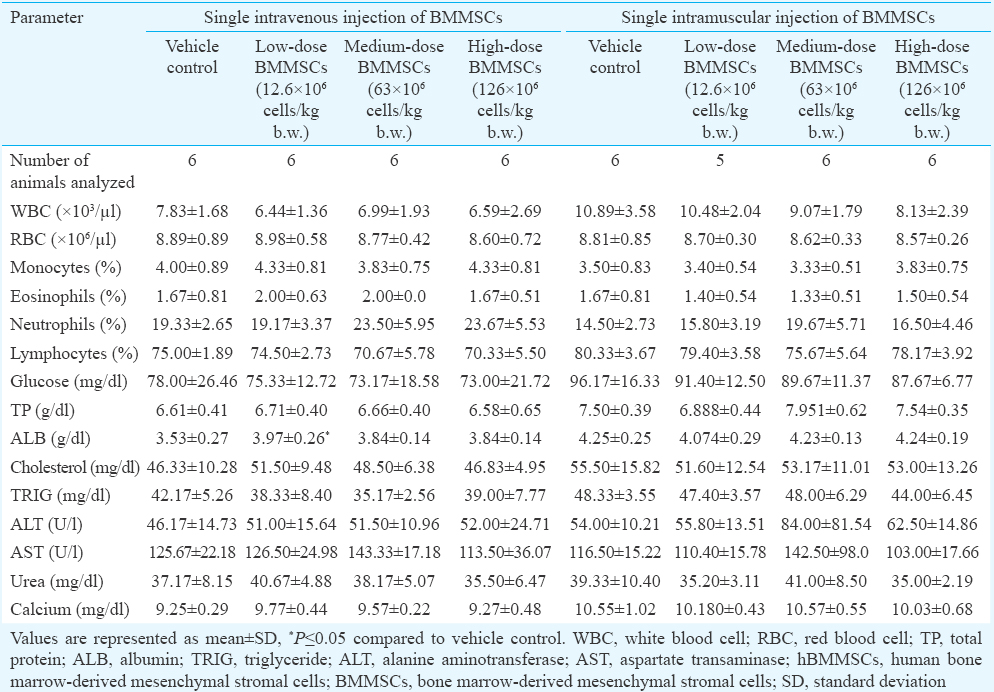
Ninety day subchronic toxicity study using pooled hBMMSC in rats and rabbits: There was no mortality observed in any of the treatment groups following i.v. injection of various doses of hBMMSC. Only one female rat died on day 4 after i.m. administration of hBMMSC, but the cause of death was not determined (Table II). No specific gross and histopathological changes were observed, suggesting that the cause of death was not due to cell injection.
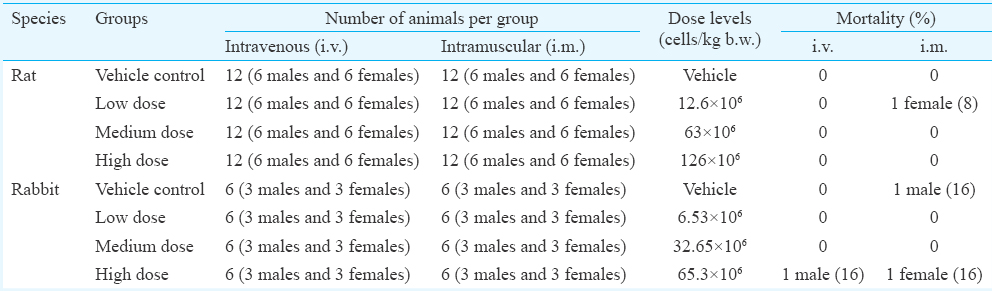
A similar study was conducted in rabbits using various doses of pooled hBMMSC through i.v. and i.m. routes. All, but three animals remained healthy throughout the study in terms of all physiological criteria. One male rabbit died in the high dose group on day 1 after i.v. administration. One female rabbit (high dose) and one male rabbit (vehicle control) also died on days 12 and 44, respectively, after i.m. administration (Table II). No treatment-related gross pathological and histopathological changes were observed in these animals, suggesting that the cause of death was due to other than cell administration. There was no treatment-related effect on the haematological or biochemical parameters measured on days 2, 15, 30 and 60 (data not shown) and day 90 (Tables III & IV) in the blood samples. Transient reduction in serum triglyceride and blood glucose levels was observed in some hBMMSC-treated animals on day 2 (data not shown). The genotoxicity potential assessed on days 15 and 90 did not reveal any hBMMSC treatment-related adverse effect. From the data, the NOAELs of hBMMSC following i.v. and i.m. administration in rats and rabbits were ≥126 × 106 cells/kg b.w. and 65.3 × 106 cells/kg b.w., respectively.
Assessment of immunological parameters following i.v. administration of hBMMSC in rats: We performed immune cell subset analysis by flow cytometry and also measured serum cytokine levels by ELISA at days 0 (baseline), 7 and 90 after cell injections. No significant differences were observed in the percentages of CD3+, CD4+ and CD8+ T lymphocytes as well as in the percentage of CD45+ (pan leucocytes) cells and the total leucocyte count in animals treated with hBMMSC compared to the vehicle control. Serum cytokines IL-1β, IFNγ and TNFα levels were also found to be similar between the vehicle and cell treatment groups (Fig. 2). These data suggested that administration of hBMMSC did not show any measurable short- or long-term effects on the lymphocyte profile or in the serum cytokine values in these animals.
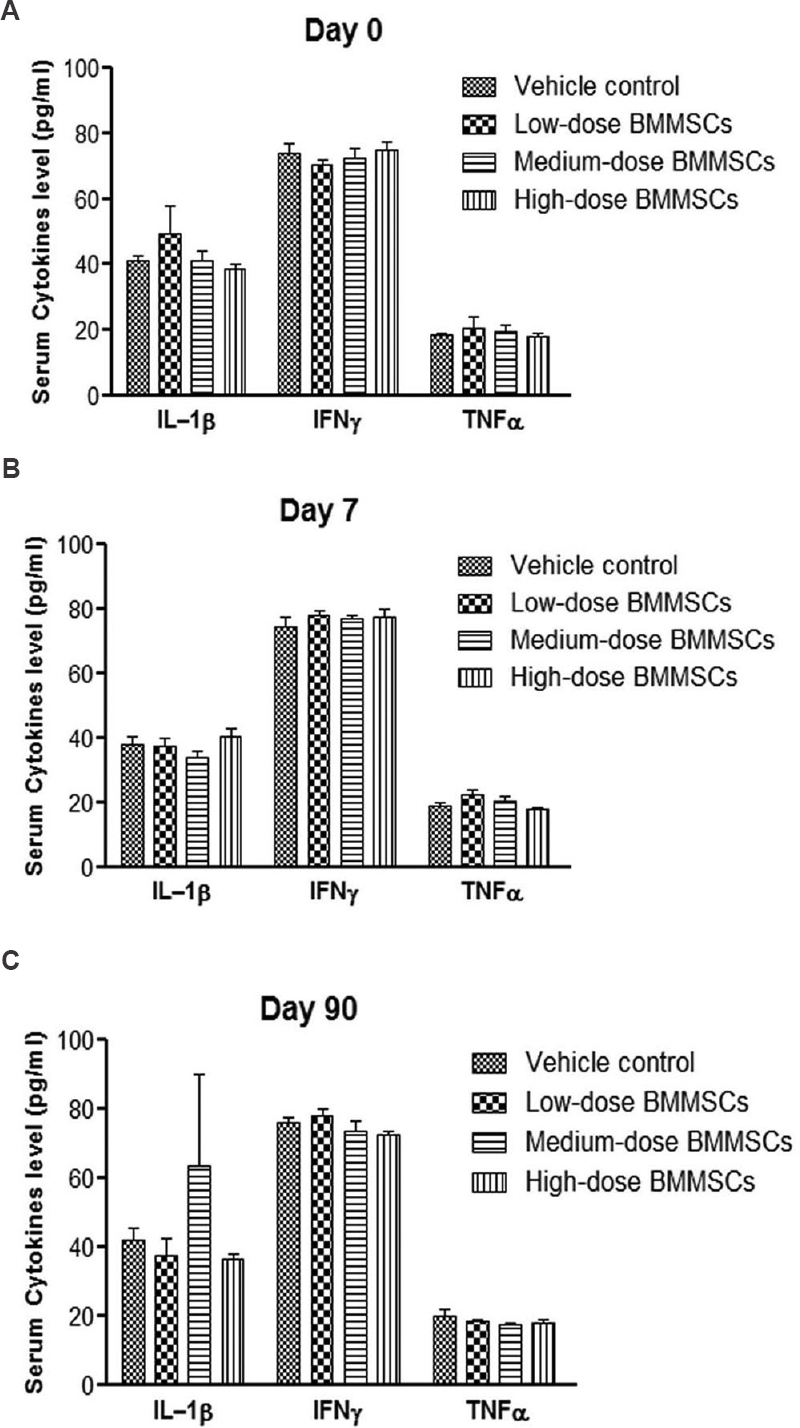
- Cytokine levels in the serum samples of rats (sex pooled) following single intravenous injection of pooled human bone marrow-derived mesenchymal stromal cells (BMMSCs) or vehicle. Serum cytokine levels were measured before cell injection (basal) at day 0 (A), day 7 (B) and day 90 (C) after cell injection. Human bone marrow-derived mesenchymal stromal cells were injected at low (12.6 × 106 cells/kg b.w.), medium (63 × 106 cells/kg b.w.) and high dose (126 × 106 cells/kg b.w.). No significant differences in the levels of cytokines were observed between cell- and vehicle-treated animals. Vertical bars represent standard error (n=6). IL-1β, interleukin-1 beta; IFNγ, interferon gamma; TNFα, tumour necrosis factor alpha.
Tumorigenicity study using pooled hBMMSC in SCID mice: All animals in the positive control group developed large tumours by 19-21 days (Fig. 3). None of the animals treated with pooled hBMMSC at a dose of 10 × 106 cells/mouse developed tumour during the six months period (Fig. 3A & B). No sign of tumour formation was observed at the site of cell injection (Fig. 3E & F), except for the positive control (Fig. 3D). No significant gross pathological or histopathological findings were seen in various doses of hBMMSC- or vehicle-treated animals (Fig. 3G, I & J). However, 10 per cent of animals from both vehicle- and hBMMSC-treated groups showed enlarged thymus and confirmed as spontaneous thymic lymphoma on histopathological examination (data not shown).
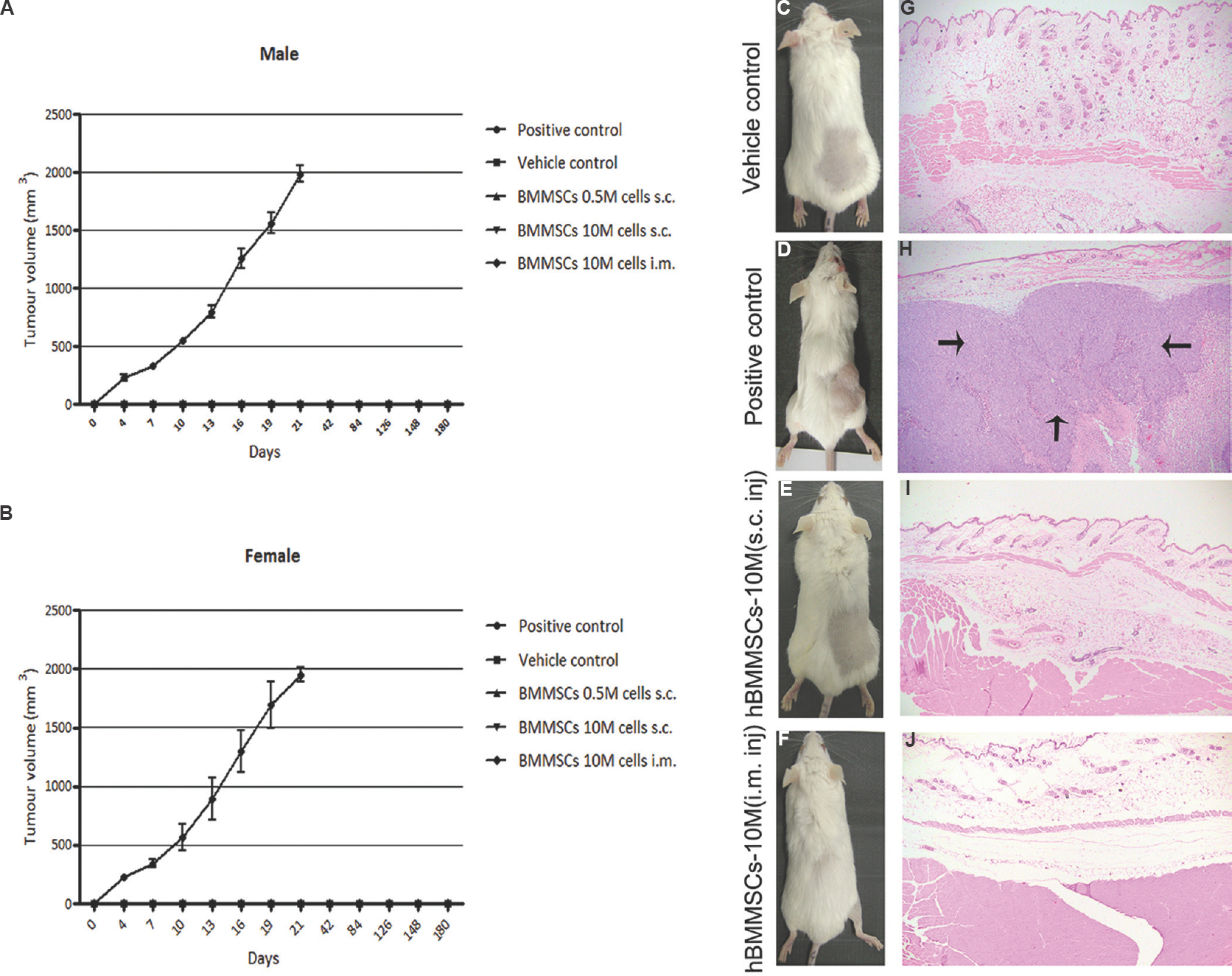
- Pooled human bone marrow-derived mesenchymal stromal cell (hBMMSC) population was non-tumorigenic in severe combined immunodeficient (SCID) mice. Tumour volumes were measured twice weekly in both male (A) and female (B) mice. Progressive tumour growth was detected visually at the site of cell injection in the positive control animals (D) and not detected in either vehicle- or human bone marrow-derived mesenchymal stromal cell-treated animals (C, E & F). Neoplastic cell growth with central area of necrosis (arrows) was observed in all (10/10) positive control animals (H) and absent in vehicle- or hBMMSCs-treated animals (G, I & J). Magnification, ×4 (G-J).
Prenatal developmental toxicity study in pregnant rats using pooled hBMMSC: Administration of pooled hBMMSC did not cause mortality in the pregnant rats. Caesarean section on gestation day 20 revealed no treatment related embryo-foetal toxicity and also the maternal necropsy did not show remarkable pathological findings in any of the three doses of hBMMSC (data not shown), suggesting that the ex vivo-expanded pooled hBMMSC population did not possess potential for inducing developmental toxicity in rats.
Biodistribution of pooled hBMMSC in rat and mice: The biodistribution pattern of i.v.-administered CM-DiI-labelled pooled hBMMSC in nude rats indicated that the cells were distributed into various locations of the animals within minutes after i.v. injection, and after a few hours, the cells were disseminated throughout the body. A large portion of the signal was detected in the thoracic cavity by day 1, while by day 4, the signal intensity in many organs reached peak fluorescence intensity. The majority of the cells disappeared by day 7, virtually no signal was detected on day 13. In contrast, the i.m.-administered cells in BALB/c nude mice for the most part remained localized in and around the thigh muscle area where the cells were injected. Since the cells remained confined in one area, no significant difference was observed in the signal intensity until day 4. The majority of cells were cleared out from the muscle by day 7, and little or no signal was detected at day 13 (Fig. 4).
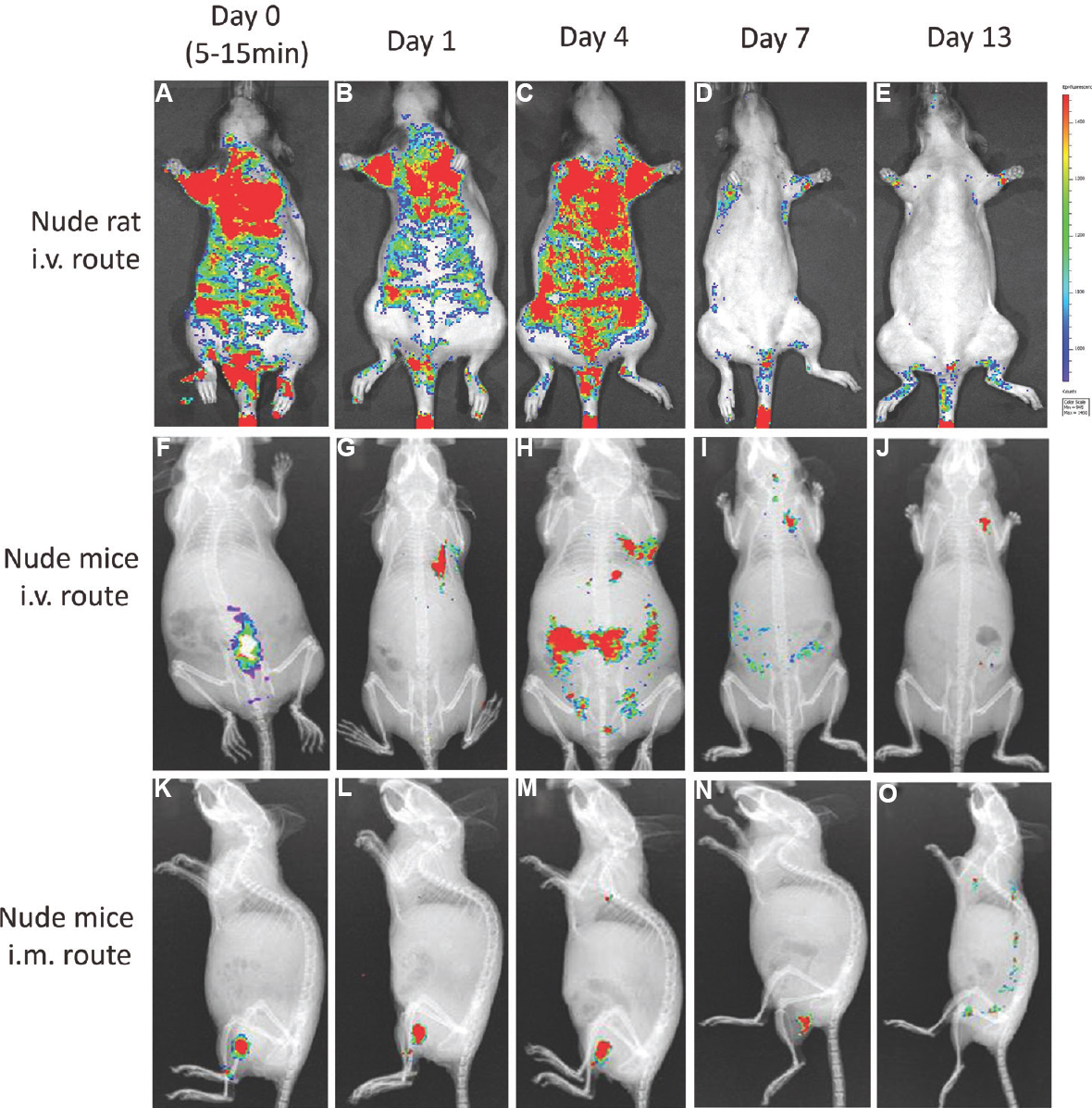
-
In vivo biodistribution profile of CM-DiI-labelled pooled human bone marrow-derived mesenchymal stromal cell (hBMMSC) at different time points (A-E & F-J) showing the distribution profile of hBMMSCs administered through intravenous route in nude rats and nude mice, respectively, and K-O showed biodistribution profile in nude mice through intramuscular route.
Discussion
Allogeneic transplantation of adult human MSCs from bone marrow and other adult tissues is a promising therapeutic option for tissue regeneration. Numerous clinical studies have demonstrated the safety and efficacy of MSCs for various clinical indications9. The approval of human allogeneic BMMSCs for the treatment of paediatric GvHD20 and an umbilical cord blood-derived allogeneic MSC21 for treating cartilage injury and OA has paved the way for using allogeneic MSC for other degenerative diseases. Clinical application of MSC requires large quantities of ex vivo-expanded cells under GMP manufacturing conditions. Extended ex vivo culture conditions may bring genetic alterations to these cells that might alter the safety profile of MSC in vivo. Therefore, comprehensive preclinical safety and toxicity studies are required before administering these cells into humans. Currently, most of the regulatory guidelines are tailored toward small molecule and biopharmaceuticals such as recombinant proteins and antibodies22. There is no standardized approach for evaluating in vivo host responses with regard to the safety of cell therapy products. In this study, comprehensive preclinical safety and toxicological studies were performed in various animal species were performed using different doses of hBMMSC.
A single-dose acute toxicity study followed by repeat-dose toxicity study demonstrated the safety of single donor-derived hBMMSC. In the repeat-dose experiment, genotoxicity and immunotoxicity potential of these cells were evaluated following repeat i.m. administration. The results demonstrated that repeat i.v. and i.m. administration of hBMMSC did not show measurable systemic toxicity. Intramuscular administration of hBMMSC did not reveal any abnormal variation in the PCE/TE ratio and in the incidence of micronucleated polychromatic erythrocytes (MnPCE). No abnormality in primary indicators of immune toxicity was observed. A similar study published by Gothelf et al23 using neurotropic factor secreting MSC (MSC-NTF) in C57BL/6 mice with three successive i.m. injections demonstrated that no cells were associated with systemic toxicity. In another study, repeat i.v. administration of human umbilical cord-derived MSC in cynomolgus monkeys showed no MSC-related toxicity24. Kol et al25 demonstrated that multiple i.v. administration of allogeneic adipose-derived (AT) MSC or BMMSC did not produce any organ toxicity or generalized inflammatory response in healthy equines. Although a direct comparison between these results with those generated from our study could not be drawn, it was evident that single or multiple administration of allogeneic or xenogeneic BMMSC did not induce proinflammatory responses in different animal species.
We also observed that the pooled population of three-donor-derived hBMMSC was non-toxic in both rats and rabbits. Although a few rabbits died immediately after i.v. administration of cells, no such death occurred in rats. It should be noted, however, that the risk of pulmonary sequestration and embolism following i.v. infusion of a high dose of ex vivo-expanded MSC (2.5 × 108 cells/kg b.w.) has been reported earlier26.
The 90 day long subchronic toxicity conducted in rodents and non-rodents did not result in any treatment-related long-term systemic toxicity either by i.v. or i.m. administration of hBMMSC. Similarly, no genotoxicity was observed in the bone marrow samples of these animals. Examination of immunological parameters in hBMMSC-injected animals did not elicit alterations in the levels of proinflammatory cytokines or in the number of circulating T-lymphocytes, at various time points after cell administration. Similar results were also reported by Ramot et al27, who demonstrated long-term safety of single and repeat i.m. administration of placental-derived MSCs (PLX - PAD) in NOD/SCID mice. In another study, it was reported that i.v. infusion of allogeneic BMMSC (5 × 106 MSCs/kg b.w.) followed by second i.m. injection of BMMSC from the same donor or a third allogeneic donor did not affect the overall health or immune status of the recipient baboons28.
Although hBMMSCs have been widely reported to be non-tumorigenic in vivo, large-scale expanded MSC from different laboratories requires confirmation that the expanded cells are non-tumorigenic. MSCs derived from bone marrow, AT-derived MSC (AdMSC) of humans and rhesus macaque showed altered cell cycle progression at early (P1) and higher passages (P20 and P30) in a long-term in vitro expansion29. However, none of these MSCs were reported to be tumorigenic in immune deficient mice after 120 days. To eliminate the possibility that pooled hBMMSCs could harbour tumorigenic potential, a long-term tumorigenicity study was undertaken. Our results showed that there was no evidence of tumour formation in any of the animals receiving hBMMSC through s.c. or i.m. route. Ra et al26 reported that subcutaneous administration of high dose of AdMSC (2 × 108 cells/kg b.w.) in SCID mice showed no evidence of tumour formation. Barkholt et al30 also concluded that no tumour formation was evident in mice injected with hBMMSC. We also tested the prenatal developmental toxicity in female pregnant rats by infusing different doses of pooled hBMMSC. The results demonstrated that three injections of hBMMSC did not induce any prenatal toxicity and incidence of external abnormality in the foetuses.
The biodistribution kinetics of pooled hBMMSC showed that i.v.-administered hBMMSC accumulated initially in the thoracic cavity and at latter time points disseminated to other organs and completely disappeared by day 13. Similar results were also observed by Gao et al31, where i.v.-administered MSC accumulated initially in the lungs and gradually redistributed to liver, spleen, kidney and bone marrow before disappearing from the circulation in rodents. Further, it has been demonstrated that MSCs could engraft and persist in multiple tissues in a xenogeneic environment by their unique immunologic characteristics32. In a recently published study the tissue distribution pattern of BMMSC delivered through i.v. and intra-arterial (i.a.) routes was compared in pigs33. While both routes were found to be safe, pulmonary entrapment of MSC was observed only in the i.v.-administered animals. Our results also corroborated with the findings of Prather et al34, where it was demonstrated that i.m.-administered MSC persisted only at the injection site and did not localize to other organs.
In conclusion a single or multiple administration of a pooled hBMMSC population did not elicit systemic or localized toxic effect in normal animals and the ex vivo-expanded cells were non-tumorigenic and non-teratogenic in nature. The preclinical efficacy and toxicity of the pooled hBMMSC population in various human disease models need to be determined in future studies.
Acknowledgment
The study was funded by Stempeutics Pvt Ltd, India. The authors thank Dr Siddikuzzaman, Shri Charan Thej and members of production and quality teams for technical support during the course of the study.
Conflicts of Interest: Authors declare no conflicts of interest, however, all authors are part of Stempeutics Research Pvt. Ltd., Bengaluru, India, which also funded the study.
References
- The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403.
- [Google Scholar]
- Human mesenchymal stem cells – Current trends and future prospective. Biosci Rep. 2015;35(2):e00191.
- [Google Scholar]
- Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7.
- [Google Scholar]
- Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-7.
- [Google Scholar]
- Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol Ther. 2012;20:14-20.
- [Google Scholar]
- Body management: Mesenchymal stem cells control the internal regenerator. Stem Cells Transl Med. 2015;4:695-701.
- [Google Scholar]
- Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667-79.
- [Google Scholar]
- Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559.
- [Google Scholar]
- Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19.
- [Google Scholar]
- A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143.
- [Google Scholar]
- Comparative cellular and molecular analyses of pooled bone marrow multipotent mesenchymal stromal cells during continuous passaging and after successive cryopreservation. J Cell Biochem. 2012;113:3153-64.
- [Google Scholar]
- Phenotypic and functional comparison of optimum culture conditions for upscaling of bone marrow-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2009;3:163-74.
- [Google Scholar]
- Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70:391-9.
- [Google Scholar]
- Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804-11.
- [Google Scholar]
- Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11-22.
- [Google Scholar]
- Food and Drug Administration, Redbook II, 1993, Chapter V.C. Available from: https://www.fda.gov/downloads/Food/GuidanceRegulation/UCM078748.pdf
- [Chemical mutagenesis in mammals. The Chinese hamster bone marrow as an in vivo test system. Hematological findings after treatment with trenimon] Humangenetik. 1970;11:35-54.
- [Google Scholar]
- Impact of passing mesenchymal stem cells through smaller bore size needles for subsequent use in patients for clinical or cosmetic indications. J Transl Med. 2012;10:229.
- [Google Scholar]
- Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20:229-35.
- [Google Scholar]
- Current concepts: The role of mesenchymal stem cells in the management of knee osteoarthritis. Sports Health. 2015;7:38-44.
- [Google Scholar]
- Preclinical biosafety evaluation of cell-based therapies: Emerging global paradigms. Toxicol Pathol. 2015;43:115-25.
- [Google Scholar]
- Safety of repeated transplantations of neurotrophic factors-secreting human mesenchymal stromal stem cells. Clin Transl Med. 2014;3:21.
- [Google Scholar]
- A toxicity study of multiple-administration human umbilical cord mesenchymal stem cells in cynomolgus monkeys. Stem Cells Dev. 2012;21:1401-8.
- [Google Scholar]
- Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res Ther. 2015;6:73.
- [Google Scholar]
- Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297-308.
- [Google Scholar]
- Safety and biodistribution profile of placental-derived mesenchymal stromal cells (PLX-PAD) following intramuscular delivery. Toxicol Pathol. 2009;37:606-16.
- [Google Scholar]
- Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711-21.
- [Google Scholar]
- Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229-38.
- [Google Scholar]
- Risk of tumorigenicity in mesenchymal stromal cell-based therapies - Bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15:753-9.
- [Google Scholar]
- The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12-20.
- [Google Scholar]
- Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation. 2012;19:273-85.
- [Google Scholar]
- Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17:392-402.
- [Google Scholar]
- The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemia. Cytotherapy. 2009;11:427-34.
- [Google Scholar]






