Translate this page into:
Preclinical evaluation of hydrogel sealed fluropassivated indigenous vascular prosthesis
Reprint requests: Dr Madathipat Unnikrishnan, Department of Cardiovascular & Thoracic Surgery, Division of Vascular Surgery, Sree Chitra Tirunal Institute for Medical Sciences & Technology, Thiruvananthapuram 695 011, Kerala, India e-mail: unnikrishnanmadathipat@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Polyethylene terephthalate (PET) graft, designed and developed at our institute for vascular reconstruction, is porous to promote optimal incorporation and neointima formation, requiring pre-clotting or biomodification by sealing the pores before implantation. The objective of this study was to characterize, test and perform preclinical evaluation of hydrogel (alginate dialdehyde cross-linked gelatin) sealed fluoropassivated PET vascular prosthesis in pig model, so as to avoid pre-clotting, for its safety and efficacy before employing the indigenous and less expensive graft for clinical use.
Methods:
Hydrogel sealed, fluoropassivated PET vascular prosthesis were tested for haemocompatibility and toxicity followed by small animal toxicology tests and in vivo experiments in pigs receiving implantation at thoracic aorta. All 33 animals received test as well as control grafts with a plan for phased explantation at 2, 12 and 26 weeks. All animals underwent completion angiogram at the end of procedure as well as before graft explantation.
Results:
Haemocompatibility tests for haemolysis and toxicity tests showed no adverse events in tested mice and rabbits. Completion angiogram showed intact anastamosis and patent graft in each animal in post-operative period and at explantation. Gross and histopathological examination showed well-encapsulated grafts, clean glistening neointima and no evidence of thrombus in both test and control grafts.
Interpretation & conclusions:
Hydrogel sealed, fluoropassivated PET vascular prosthesis was found non-toxic, haemocompatible and remained patent in in vivo studies at planned intervals.
Keywords
Aortic reconstruction
flouropassivated polyester graft
hydrogel sealing
in vivo large animal trial
polyethylene terephthalate vascular prosthesis
Patients with vascular diseases warrant reconstruction using prosthetic grafts to save life and limbs. Conventional grafts utilized for surgical reconstruction have been replaced by endoluminal prosthesis for aortic/arterial lesions in most centres in the world. However, in India, open vascular reconstruction is still in vogue in most hospitals. Sree Chitra vascular prosthesis, designed and developed indigenously, made of woven polyester requires pre-clotting to make it impervious to blood following implantation. Neovascularization of prosthetic graft plays an important role in the prevention of early graft failure and for patency of the vascular prosthesis1. Pores in polyester graft helpneovascularization and tissue ingrowth from perigraft bed, so as to stabilise neointima, a critical determinant of graft incorporation and its long-term patency2. However, the porosity of woven polyester fabric is 200±50 cc of normal saline/cm2/m at 120 mmHg, so leads to blood seepage at implantation that used to be prevented by a technique of blood pre-clotting. Over time, Dacron grafts were coated with gelatin, collagen or albumin in various commercially available grafts to seal the pores which got systematically degraded and replaced by native neointima3456. The incorporation of proteins and other biomolecules on the conduit surface results in positive interaction with blood and tissue environment thus improving its blood conducting and healing properties7.
Most surgeons prefer coated/sealed grafts so as to avoid the process of pre-clotting before implantation. Hence, we decided to develop technology for coating the graft to make it impervious at implantation using hydrogel, safety of which is proven and reported elsewhere891011. A technique of hydrogel coating at abluminal side and fluoropolymer on luminal side were adopted to negate thrombogenicity as well. We undertook in vivo experiments in pig as animal model to study the benefit of fluorocarbon coating on luminal side to increase thromboresistance and hydrogel sealing on the abluminal side to prevent blood seepage through graft pores.
Material & Methods
This study was conducted at Biomedical Technology Wing of Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), Thiruvananthapuram, Kerala, India, between January and December 2013.
Indigenous polyester vascular prosthesis: Large diameter vascular graft was made from polyethylene terephthalate (PET) as a seamless woven prosthesis designed by South India Textile Research Association, Coimbatore, akin to polyester graft in clinical use. Porosity was 200±50 cc/min/cm2 of normal saline at 120 mmHg. Extensively tested at our laboratory, implanted in pigs as animal models, graft completed implantation in controlled as well as multi-centric studies for 10 and 5 years, respectively with excellent results12.
Coating and sealing - principle: The principle of modification of PET prosthesis included coating the luminal side with polyvinylidine fluoride (PVDF), known to further lessen thrombogenicity of polyester, and sealing the abluminal side with hydrogel, developed at our institute for use as an embolization material. Hydrogel sealing makes the graft impervious at implantation which undergoes systematic degradation over two weeks to six months as graft gets incorporated during the healing process (unpublished observation).
Coating and sealing - method: Woven polyester graft was dip-coated in a solution of PVDF and dried in air oven to achieve a thin layer of fluoropolymer on the surface of graft. External surface was further coated with a hydrogel-forming solution prepared from sodium alginate and gelatin. Sodium alginate was oxidized to generate aldehydic reactive sites in the polymer. This was allowed to react with amino groups of gelatin to form three dimensional networks. Both alginates and gelatin are water soluble polymers, but when reacted into a three-dimensional network, these are not dissolved in water. Instead, these absorb huge quantities of water and form hydrogel. During the coating process, both oxidized alginate and gelatin were dissolved in water separately and just before coating these were mixed together and applied onto graft surface by spray coating technique. The coated grafts were placed in an oven to complete the reaction between gelatin and oxidized alginate and also to dry. Water permeability through the coated grafts was tested using an in-house fabricated apparatus, and it was found to be under 5 ml/min/cm2. Hydrogel-coated 10 and 12 mm diameter polyester grafts were tested in this study. The coated grafts were packed in containers and sterilized with ethylene oxide and made available for implantation. The thickness of the dried coating was found to be in the range 100-170 mm. This variation in coating thickness was due to the hills and valleys formed on the body of the graft due to crimping. When the graft was wet with saline, the coating thickness increased to 200-320 mm. The hydrogel absorbs water very quickly and attains 90 per cent of the final swollen weight within 10 min and attains equilibrium in about 30 min. The water absorbed by the hydrogel is about eight times its dry weight.
In vitro studies
Physical characteristics: Laboratory tests including burst strength, suture retention, fraying at edges, conformability and suturability, already tested in standard test grafts, were performed12.
Haemo-compatibility tests: Platelet consumption, leucocyte consumption and haemolysis (%) were determined after exposing the graft to human blood for 30 min. For this, blood from a healthy human volunteer was collected into anticoagulant citrate phosphate dextrose-adenine. Graft samples were placed in polystyrene culture plates for one hour with phosphate buffered saline before these were exposed to blood. To each plate, 10 ml blood was added and 5 ml sample was collected immediately for initial analysis. The remaining 5 ml blood was exposed to the materials for 30 min under agitation (75±5 rpm) using Environ shaker (Kuhner AG, Switzerland) thermostated at 35°C±2°C. Four empty polystyrene culture dishes were exposed with blood as a reference and four samples were used for each test. The blood counts at initial and after 30 min exposure were analyzed using a Haematology Analyser (Sysmex-K 4500, GMI, Minnesota, USA). Percentage haemolysis was determined using the following procedure. The total haemoglobin (Hb) in the whole blood samples were measured using the automated analyser. Free Hb liberated into the plasma after exposure to the sample was measured using a Diode array spectrophotometer (Hewlett Packard, Germany). The percentage haemolysis was calculated using the formula: Haemolysis (%)=(Free Hb/Total Hb)×100
Small animal toxicity studies: Test graft coated with PVDF and alginate-gelatin extracted in physiological saline (PS) and cottonseed oil (CSO) at 37°C for 72h were collected and used for acute systemic toxicity in mice, intracutaneous irritation test in rabbits and intramuscular implantation in rabbits. A test material of 10×1 mm size was implanted in to the paravertebral muscle of rabbits. The sample sizes of in-house acclimatized small animals used in this study were as follows:
-
Acute systemic toxicity - Intravenous application test - 10 animals (mice), Control - 5 animals. Intraperitoneal application test - 10 animals (mice), Control - 5 animals.
-
Intracutaneous irritation test - 3 rabbits (5 test sites/5 control sites).
-
In vitro haemolysis - n=3 (rabbit blood).
-
Implantation in muscle - n=9 rabbits [3 animals each, three time periods (1, 4 and 12 wk)].
The animal studies were conducted in accordance with good laboratory practice (GLP) and in an accredited laboratory (COFRAC, France) that deals with the toxicological evaluation of biomaterials and medical device. The tests were performed as regulatory tests as per ISO (International Organization for Standardization) standards and not as research mode. Hence, the power of the study was not estimated. All animal studies were approved by the Institutional Animal Ethics Committee (IAEC).
Methods of animal toxicity studies conducted were as follows:
Acute systemic toxicity: The study was conducted as per ISO 10993-1113. Test for acute systemic toxicity test were conducted in accordance with Organisation for Economic Co-operation and Development (OECD) principles of GLP14. The physiological saline (PS) extract of the test material (hydrogel coated graft material), and control (PS alone) were injected intravenously to the mice and the CSO extract of the test material and control (CSO alone) were injected intraperitoneally to the mice and the animals were observed immediately after injection and at 4, 24, 48 and 72 h for the evidence of abnormalities like any clinical signs, loss in body weight or death. The result of the systemic toxicity was evaluated on the basis of toxic signs, symptoms, body weight reduction or death of animals.
Intracutaneous irritation test: The PS and CSO extracts of the test material were aseptically injected into five sites (0.2 ml/site) on the upper left hand side and right hand side of three rabbits. The PS (control) alone and CSO (control) alone were injected into five sites on the lower left hand side and lower right hand side of the same rabbits. The grading of erythema and oedema of test and control sites of all animals at 24, 48 and 72 h were recorded as per ISO 10993-1015.
In vitro haemolysis test: The study was conducted as per ASTM F756-0016 and in accordance with OECD principles of GLP14. The test consisted of a protocol for a haemolysis test under static conditions with either an extract of the material or direct contact of the material with blood. Test and control material extracts were exposed to contact with rabbit blood under defined static conditions, and the increase in released Hb was measured. Comparisons were made with the control and test specimens tested under identical conditions.
Implantation in muscle: The study was conducted as per ISO 10993-617 and in accordance with OECD principles of GLP14. Rabbits were anaesthetized using ketamine (80 mg/kg body weight) + xylaxin (5 mg/kg body weight). The skin of the anaesthetized rabbits was lightly swabbed with 70 per cent alcohol and air dried. Five incisions were made on the skin and inserted five test implant materials intramuscularly (paravertebral muscle) along one side of the spine (right side), and about 25 mm apart from each other. Similarly, five control implant material (SCTIMST, Thiruvananthapuram) was intramuscularly implanted in the contralateral muscle (left side) of each rabbits. The incision was then closed using sterilized sutures. The study was conducted in nine rabbits (whose paravertebral muscle was sufficiently large), three animals each for one, four and 12 weeks. The body weight of the animals was not <2000 g.
In vivo implantation studies (preclinical)
The objective of the study was to evaluate safety and efficacy of the coated indigenous test graft in pig as animal model in the form of adequacy and integrity of the sealing in terms of bleeding at implantation, suturability, suture retention and handling qualities as noted by the surgeon. Patency assessment, thrombus formation if any, late leakage, seroma or aneurysm formation, dilatation, infection or any other adverse events/toxicity, in comparison to control grafts already in clinical use was performed.
Experimental design: A total of 30 disease free and healthy pigs aged 3-6 months weighing 25-35 kg procured from Ankamali, Kerala, after three weeks of quarantine were selected and divided into three groups. Three groups were assigned explantation to study healing characteristics at 2, 12 and 26 wk with six animals each as test and three each as control. Weslowski's experiments showed that healing pattern in pigs over three months was equivalent to three years in man18. In addition, large animal like pig posed significant supportive and financial impact, limiting the number. Each group underwent implantation using test and control prosthesis with hydrogel-coated fluoropassivated Chitra woven PET grafts and albumin coated commercially available polyester grafts, respectively. Pigs belonging to either sex were randomly selected and allotted to each group receiving implantations of six test and three control grafts for a test duration of 2, 12 and 26 wk each.
Surgical procedure of implantation: Under general anaesthesia and standard aseptic precautions, through midline ventral neck cut down the carotid artery was cannulated following modified Seldinger technique19 for invasive blood pressure monitoring, carotid-femoral shunt and for aortogram. Femoral artery was similarly cannulated for carotid-femoral shunt which sustained the retrografe perfusion to spinal cord and distal branches during aortic cross-clamping period.
Thoracotomy was performed through 5th intercostal space in case of short graft and 4th and 8th intercostal spaces for long graft implantations. The segment of approximately 8 cm of thoracic aorta distal to the left subclavian artery was dissected by incising the mediastinal pleura. Small vascular branches were ligated either with titanium ligating clips or 3/0 silk ligatures. After heparinization at 150 IU/kg body weight, aorta was cross-clamped proximally and distally once the activating clotting time was beyond 400 sec. Either test or control graft of <10 cm was sutured as an interposing graft with end-to-end anastomosis using 4/0 monofilament polypropylene suture (Fig. 1). In four animals i.e. one in three months test group, two in six months test group and one in six months control group, long grafts of >10 cm length were implanted following end-to-end anastomosis proximally and end-to-side anastomosis distally as a bypass graft. The native aorta was ligated in continuity proximally so as to provide retrograde flow into intercostal arteries and so prevent spinal cord ischaemia. After ensuring complete haemostasis, the anastomosis was wrapped with oxidized cellulose strips, and the parietal pleura was opposed with interrupted sutures. Chest wall was closed in layers and surgical wound was managed as per standard technique. An aortogram was acquired after implantation through 7F introducer sheath in the carotid artery with tip of a 7F Judkins right coronary catheter placed at the aortic arch proximal to the proximal anastomosis using ‘Omnipaque 350’ as the contrast agent (Fig. 2). Antibiotic coverage was provided with ceftriaxone 25 mg/kg once daily and gentamicin 1 mg/kg twice daily. Analgesic, antipyretic and anti-inflammatory agents were given (5 mg meloxicam, 300 mg paracetamol and 100 mg tramadol, respectively). Anti-platelet medication with aspirin 75 mg was started on the 5th post-operative day and continued till the end of the study.
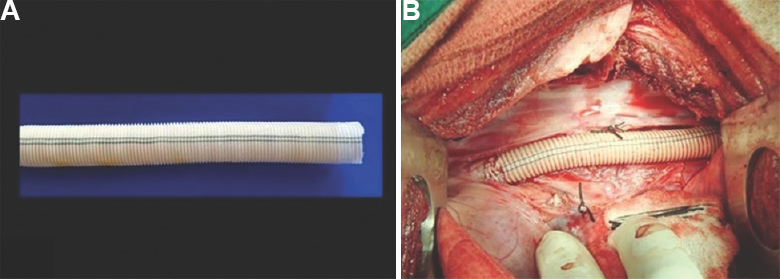
- (A) Hydrogel sealed fluoropassivated woven polyester vascular prosthesis (test graft) - 10 mm internal diameter. (B) Intraoperative photograph showing interposition test graft in thoracic aortic position in pig using left posterolateral thoracotomy under general anaesthesia.
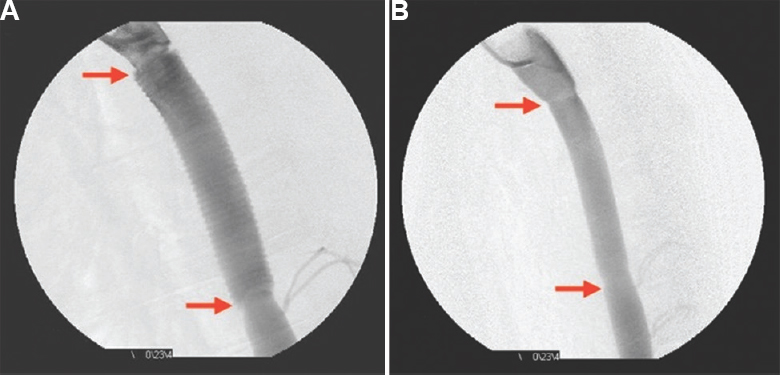
- (A) Check digital subtraction angiogram showing intact proximal and distal anastomoses (red arrows), normal graft flow including the well-visualized crimping on the graft. (B) Digital subtraction angiogram at 26 wk after implantation of hydrogel sealed test graft showing excellent functional status before explantation after euthanizing the animal under general anaesthesia.
Preclinical experiments were conducted in pig model with the approval of Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Animal Welfare Division, New Delhi.
Explantation - necropsy and histopathology: At the end of the study period, animals were anaesthetized and after systemic heparinization, aortogram was acquired after a left lateral carotid cut-down as per the procedure mentioned above. The animals were sacrificed with excess dose of thiopentone sodium, pancuronium bromide and potassium chloride administered intravenously. A complete autopsy was performed by the pathologist thereafter. Grafts were cut open longitudinally and observed for thrombi, pseudoaneurysms and endothelialization. Formalin-fixed samples were dehydrated in a series of graded isopropyl alcohol solutions, xylene and embedded in paraffin as described elsewhere20. For histological evaluation, 5 μm paraffin-embedded tissue sections were stained with haematoxylin and eosin (H and E) and Masson's trichrome stain for basic light microscopic analysis and Von Kossa's stain to identify calcification. Histology was assessed qualitatively by H and E staining for cell nuclei and general matrix morphology, Masson's trichrome for collagen and smooth muscle cells.
Results
In vitro studies
Physical characteristics: Hydrogel sealing on the abluminal side gave a gentle yellow tinge to the test graft. Test graft appeared clean, with excellent aesthetic appearance. Burst strength, suture retention, conformability were already reported for the indigenous vascular prosthesis12.
Haemocompatibility tests: The results of the haemocompatibility studies showed that due to coating leucocyte consumption and haemolysis were decreased by 23 and 13 per cent, respectively. About seven per cent increase in platelet consumption was observed for the coated graft (Table).

Small animal toxicity studies: The results of the acute systemic toxicity test indicated that PS and CSO extract of the test material and control injected animals (intravenously and intraperitoneally) did not show any abnormalities or loss of body weight during the observation period. Hence, PS and CSO extract of the test vascular graft coated with hydrogel met the requirements of the test as per ISO 10993-1113.
The results of the intracutaneous irritation test indicated that PS and CSO extract induced an average irritation score of ‘0’ in each extract following intradermal injection. None of the animals in the test and control groups (both PS and CSO) of the guinea pig maximization (sensitization) test showed any adverse skin reaction during the induction or challenge period. The extract of test material (both PS and CSO) induced a numerical grading of ‘0’ for erythema and oedema. Hence, the test material met the requirement of the test as per ISO 10993-1015.
The histopathological comprehensive result of the intramuscular implantation test indicated that the test material was non-irritant at one, four and 12 wk post-implantation. Hence, the test material met the requirements of the test as per ISO 10993-617.
In vivo experiments - implantation data: Although originally 30 experiments were planned in all, three additional implantation were conducted following the death of two animals due to bleeding into chest and another one was sacrificed when paraplegia resulted due to prolonged aortic cross-clamping (>60 min). Three animals in addition underwent implantation of uncoated Chitra graft. However, in all 33 animals, excellent handling and suturing qualities of the test graft were noted with no bleeding at all through graft interstices comparable to the control grafts. Anastomotic bleeding, if encountered, was addressed with extra pledgeted sutures. Aortograms performed showed patent graft, intact anastomosis and excellent blood flow through graft into distal aorta.
In vivo experiments - explantation data: On gross examination, lumen was patent throughout the length of the graft region in both groups. Anastomosis at both ends was intact, and there were no pseudoaneurysms. No thrombi were observed at the anastomoses or in the lumen in test and control grafts. Neointima was observed in the graft lumen as a distinct transparent layer in test and control grafts (Fig. 3). No signs of infection were observed in the graft region and at the anastomoses in both groups. Other body organs appeared normal. Even at two weeks, a thin neointima was formed at the luminal surface of the graft but could be distinguished only under microscope. Thicker neointima was observed at three and six months which were identical in appearance and was clearly distinguishable with naked eye.
On histopathological examination, proximal and distal anastomoses were intact, and healing was observed at the anastomoses in test and control grafts. In the graft region, fibrous tissue and mild infiltration of chronic inflammatory cells and foreign body giant cells were noted. No microthrombi were observed at the anastomoses and in the graft lumen of both groups. Neointima was a distinct transparent layer with regular and smooth flow surface at the anastomoses and body and was lined with endothelial-like cells. In the mid-graft region, neointima was moderately thick at focal areas, moderately thin at focal areas in a few cases and lined with endothelial-like cells. Calcification was absent in graft region in both grafts. Neointima was observed in the graft lumen as distinct transparent layer in test and control grafts. No signs of infection were observed in the graft region, and at the anastomoses in both groups. No inflammatory response was visible at 26 wk. No trace of hydrogel was observed at this time period indicating that the entire hydrogel was resorbed (Fig. 4).
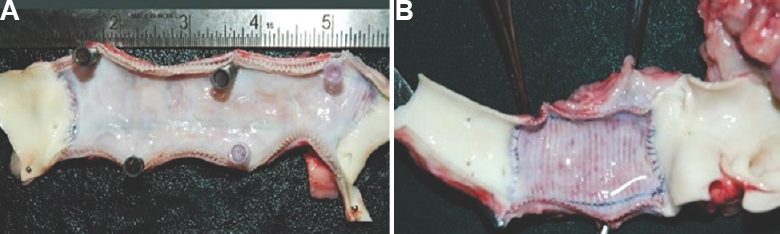
- Gross photograph of the slit explanted graft at 26 wk showing thin, clean, glistening and lustrous neointima with tidy proximal and distal anastomosis and absence of thrombus in test graft (A) and control graft (B).

- Low power microphotographs (H & E, ×1.2) uniform endothelial-lined neointima (black arrow) on the inner aspect of the graft (arrowhead) six months after implantation in porcine model. The neointimal tissue thickness was 997.8 μm (standard deviation 484.6) for test graft (A) and 779.1μm (standard deviation 413.1) for control graft (B).
Discussion
Vascular prosthesis in surgical practice for reconstruction of aorta and arteries needs to possess specific characteristics to provide flawless function in the human body on implantation. The ideal prosthetic vascular graft should be non-thrombogenic, compatible at high and low shear rates and have compliance similar to the native arteries16. Additional desirable qualities include suturability, tensile strength host incorporation and resistance to infection21. Laboratory testing followed by small and later large animal trials is mandatory to study safety and efficacy.
Healing of prosthesis depends on several factors and presence of micropores in woven/knitted prosthesis enhances incorporation inside the body by ingrowth of cells and fibroblasts from perigraft capsule to stabilize and promote neointima formation which is key for long-term patency and resistance to infection. This property is enhanced by pores that require to be sealed to have no bleeding at implantation, and the material used should be safe, non-toxic and should undergo graded degradation over several months for the pores to function in the intended fashion22. The hydrogel which sealed pores were fully resorbable and allowed tissue infiltration and integration with the graft. Histopathologic data revealed complete integration of the graft with tissues.
We chose to coat fluorocarbon to make the thrombogenicity to least/negligible level inside, and seal the pores using hydrogel so as to make the graft impervious to blood at implantation. Having extensively tested and completed human implantation studies lasting over 10 yr including multi-centric trial23, our PET graft required further studies to prove safety of the coated and sealed prosthesis before human implantation could be commenced. Sodium alginate-gelatin cross-linkage is bound to the graft and gets activated upon immersing it in normal saline for five minutes before implantation at which time graft pores are completely plugged. Hydrogel thus used for sealing showed haemocompatibility, non-toxicity and systemic safety in in vitro experiments as reported by Manju et al10. Haemolytic potential of PET was also reduced when it was coated with hydrogel. Improved adhesion and proliferation of endothelial cells were observed. Joseph et al11 found that PVDF coating improved in vitro haemocompatibility of PET fabric and reduced platelet consumption by 50 per cent and leucocyte consumption by 24 per cent. About 60 per cent reduction in partial thromboplastin time (PTT) was observed. Ao et al24 noted that fluoropassivated Dacron resulted in less intimal hyperplasia in sheep carotid model than regular Dacron graft. The hydrogel coating is expected to be systematically degraded from two weeks, getting completed in 3-6 months time when neointima would be formed from the body that has received the device.
There are distinctive advantages to the use of pigs in that they share similar anatomic and physiologic characteristics with humans particularly pertaining to the cardiac and vascular systems25. Classic animal experimental data reported by Wesolowski18 on growing pigs weighing 20-39 kg formed the benchmark for graft research as implantation data of three months in growing pigs in terms of healing patterns and characteristics were noted to be equivalent to three years in man. Hence, we chose pigs as the animal model in addition to the fact that preclinical trial of the standard uncoated graft was also successfully completed in pigs.
The handling characteristics of the test graft were comparable to the control grafts and were confirmed to be impervious to blood following implantation. Aortograms performed after implantation and before explantation showed good lumen with uninterrupted blood flow through the conduit. The test graft was found to be well incorporated in perigraft capsule, both anastomosis intact with no thrombus and clean and lustrous neointima at explantation comparing well with widely used control graft. Since thrombogenicity is inversely proportional to quality of neointima12, long-term graft patency will also be higher when the neointima is well formed.
The smaller sample size and shorter duration of explantation time were the limitations of the present study.
In conclusion, the present study showed that hydrogel sealed fluoropassivated PET vascular prosthesis was comparable to control graft in clinical use and was found non-toxic, haemocompatible and remained patent in in vivo studies.
Acknowledgment
Authors acknowledge the support of our former Director, Dr K.R. Radhakrishnan and present Director Dr Asha Kishore in accomplishing this project, and thank Shrimati Vasanthy S. and Shri Liji Kumar for their artwork. This study received financial assistance from Technology Development Fund, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala.
Conflicts of Interest: None.
References
- Neo-endothelialisation of PTFE microvascular grafts: A five-year experience. Microsurgery. 1995;16:404-11.
- [Google Scholar]
- Current status of prosthetic bypass grafts: A review. J Biomed Mater Res B Appl Biomater. 2005;74:570-81.
- [Google Scholar]
- Can collagen impregnated polyester arterial prostheses be recommended as small diameter blood conduits? ASAIOJ. 1996;42:974-83.
- [Google Scholar]
- Impregnated polyester prostheses: A theoretical advantage. J Mal Vasc. 1996;21(Suppl A):41-7.
- [Google Scholar]
- Comparative in vivo study on the healing qualities of four different presealed vascular prostheses. J Vasc Surg. 1993;17:538-45.
- [Google Scholar]
- Albumin coating of a knitted polyester arterial prosthesis: An alternative to preclotting. Ann Thorac Surg. 1984;37:457-65.
- [Google Scholar]
- Biomechanics and biocompatibility of the perfect conduit-can we build one? Ann Cardiothorac Surg. 2013;2:435-43.
- [Google Scholar]
- Phenotypic modulation of cell types around implanted polyethylene terephthalate fabric in rabbit muscle. Toxicol Pathol. 2013;41:497-507.
- [Google Scholar]
- Implant pathology of polyvinylidene fluoride coated polyethylene terephthalate fabric in rabbits. Indian J Vet Pathol. 2007;31:11-6.
- [Google Scholar]
- Evaluation of alginate dialdehyde cross-linked gelatin hydrogel as a biodegradable sealant for polyester vascular graft. J Biomed Mater Res B Appl Biomater. 2011;98:139-49.
- [Google Scholar]
- Characterization of surface modified polyester fabric. J Mater Sci Mater Med. 2009;20(Suppl 1):S153-9.
- [Google Scholar]
- 1099. Biological evaluation of medical devices - Part 11: Test for Systemic Toxicity. ISO 10993-11: 2017 (en). Available from: https://www.iso.org/obp/ui/#iso:std:iso:10993:-11:dis:ed-3:v1:en:sec:H
- OECD Principles of Good Laboratory Practice (as revised in 1997). Paris: OECD; 1998.
- Biological evaluation of medical devices - Part 10: Tests for irritation and skin sensitization. ISO 10993-10:2010 (en). Available from: https://www.iso.org/obp/ui/#iso:std:iso:10993:-10:ed-3:v1:en
- Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM F756-00. Philadelphia: ASTM; 2008.
- Biological evaluation of medical devices - Part 6: Tests for local effects after implantation. ISO 10993-6: 2016 (en). Available from: https://www.iso.org/obp/ui/#iso:std:iso:10993:-6:ed-3:v1:en
- Vascular prostheses: The need for standards - Historical and surgical perspectives. In: Kambic HE, Kantrowitz A, Sung P, eds. Vascular graft update: Safety and performance. Philadelphia: American Society for Testing and Materials (ASTM); 1986. p. :253-77.
- [Google Scholar]
- Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368-76.
- [Google Scholar]
- Bancroft JD, Gamble M, eds. Theory and Practice of Histological Techniques (6th ed). Philadelphia: Elsevier Health Sciences; 2008.
- An albumin-coated polyester arterial graft: In vivo assessment of biocompatibility and healing characteristics. Biomaterials. 1996;17:3-14.
- [Google Scholar]
- Adult coarctation of the aorta repaired with a prosthetic bypass and its long-term follow-up. Eur J Cardiothorac Surg. 2014;45:1114.
- [Google Scholar]
- Development of intimal hyperplasia in six different vascular prostheses. Eur J Vasc Endovasc Surg. 2000;20:241-9.
- [Google Scholar]
- , Frazier KS.Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49:344-56.
- [Google Scholar]






