Translate this page into:
Plasma & urinary catecholamines & urinary vanillylmandelic acid levels in patients with generalized vitiligo
For correspondence: Dr. Somesh Gupta, Department of Dermatology & Venereology, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: someshgupta@hotmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Vitiligo is an acquired skin disease characterized by depigmented areas of the skin. Increased release of catecholamines from autonomic nerve endings in microenvironment of melanocytes in affected skin might be involved in the aetiopathogenesis of vitiligo. Levels of catecholamines are considered as being related to onset or worsening of the disease. Therefore, in this study, the role of catecholamines was evaluated in mapping disease stability and outcome of vitiligo patients undergoing melanocyte transfer.
Methods:
In this study, circulatory and urinary levels of catecholamine (CA) and vanillylmandelic acid (VMA) were determined in 45 individuals (30 vitiligo patients and 15 healthy controls) using ELISA.
Results:
A significant increase for plasma and urinary catecholamines along with VMA was observed as compared to healthy controls. When the pre- and post-intervention levels were analyzed in responders and non-responders, respectively, only dopamine showed significant decline in urine, rest of the molecules in plasma as well as urine showed non-significant decline except VMA which showed insignificant increase.
Interpretation & conclusions:
Levels of plasma/urinary epinephrine, and plasma dopamine, could not be established as biomarkers for disease stability or successful outcome of autologous melanocyte transfer in generalized vitiligo patients. However, dopamine (urine) might be of help in determining the stability in patients with generalized vitiligo undergoing melanocyte transfer. Further studies need to be done on a large sample of patients to confirm our findings.
Keywords
Dopamine
epinephrine
melanocyte transfer
norepinephrine
vanillylmandelic acid
vitiligo
Vitiligo is an acquired skin disease characterized by depigmented areas of the skin that occurs in 0.1-8.8 per cent of the population. Incidence of vitiligo is found to be 0.5-2.5 per cent in India with a high prevalence in the States of Gujarat and Rajasthan1. The disease may affect the individuals of both sexes and is mostly characterized by loss of functional melanocytes2. Besides loss of colour, there is no other structural change. Despite much research, the aetiology of vitiligo and the causes of melanocyte death are not clear. Conventionally, there have been three hypotheses to explain the pathogenesis of vitiligo: neural, immune and self-destructive, but none can completely explain the disease and are probably interrelated3.
The increased release of catecholamines (CA) from the autonomic nerve endings in the microenvironment of melanocytes in the affected skin areas might be involved in the aetiopathogenesis of vitiligo through two main mechanisms: a direct cytotoxic action of CA and/or their o-diphenol catabolites4 and an indirect action, skin and mucosa arterioles possess receptors, activation of which by CA discharge may cause a severe vasoconstriction, leading to epidermal and dermal hypoxia with excessive production of toxic oxyradicals generated by different pathways5. In both cases, a genetic predisposition due to insufficient radical scavengers in the affected areas should be taken into account4.
There are two main biochemical abnormalities associated with vitiligo. These include disturbed antioxidant defence6 as evidenced by high levels of hydrogen peroxide (H2O2) in the epidermis and elevated serum and urine levels of catecholamines. Elevated CA levels worsen the condition in the presence of altered redox state leading to tyrosinase haptenation by quinones, triggering or aggravating melanocytes damage5. Catecholamines (o-diphenols) are better substrates of tyrosinase than tyrosine (a monophenol) and may, therefore, compete for the enzyme in the absence of cofactors acting as substitute substrates7. The H2O2-dependent increase in the levels of CA competing with tyrosine for oxidative transformation by tyrosinase may thus be of pivotal importance in the pathogenic mechanism of idiopathic vitiligo. The levels of CA which are consistently released as a consequence of emotional and/or stressful events are considered as being related to the onset or worsening of the disease4.
There is lack of information on the role of CA in mapping disease stability and outcome of vitiligo patients undergoing melanocyte transfer. Therefore, in this study the levels of CA and urinary vanillylmandelic acid (VMA) were studied and associated with stability of disease and outcome of transplantation in vitiligo patients as potential markers to predict the disease outcome.
Material & Methods
The study was a prospective interventional study, in which plasma/urinary catecholamine levels and urinary VMA were measured in vitiligo patients before and after six months of autologous melanocyte transfer. This study was approved by the Institutional Ethics Committee and the patients who signed informed consent and were willing to come for follow up six months after transplantation were included in this study. A total of 15 apparently healthy controls and 30 consecutive patients with generalized vitiligo (vitiligo vulgaris and acrofacial vitiligo) who were not on any systemic drugs and registered at Dermatology outpatient department of All India Institute of Medical Sciences (AIIMS), New Delhi, India, and who fulfilled the inclusion-exclusion criteria, were selected. Blood and urine samples of healthy controls from the healthy adults accompanying patients attending skin outpatient department, as well as students and staff of the departments of Biochemistry and Dermatology and Venereology, AIIMS, New Delhi, were collected. The study was conducted from 2010 to 2013. Clinical characteristics of the vitiligo patients and controls are shown in Table I. None of the patients had a family history of vitiligo. There was also no history of other autoimmune disease in any of the patients. Patients with lesional surface area involvement of <10 per cent with no history of appearance of new lesions or progression of existing lesions in the past three months or more were included. Patients with focal or segmental vitiligo, history of koebner phenomenon in the past three months, keloidal tendencies, history of bleeding diathesis and with predominantly facial lesions and with other autoimmune disorders were excluded. Pregnant and lactating women were also excluded. Controls having any active infection or inflammation were excluded from the study.
The patients were divided into three groups as follows: (i) Group 1 (n=11): No appearance of new lesion or progression of existing lesion for more than three months but less than one year; (ii) Group 2 (n=7): No appearance of new lesion or progression of existing lesion for one year or more but less than two years; and (iii) Group 3 (n=12): No appearance of new lesion or progression of existing lesion for two years or more.
Sample collection: After overnight, fasting blood was collected between 0800 and 0900 h to avoid circadian variation. Blood was collected using an anticoagulant (ethylenediaminetetraacetic acid) vial and plasma was separated. Sample was stored at −80°C until use. Intake of coffee, tea, sodium, bananas, chocolate, cocoa, citrus fruits, vanilla and smoking was avoided for at least 24 h before sample collection. Urine samples (24 h) were collected in a container containing 3 ml of 6M HCl as preservative. Samples were refrigerated immediately after collection. Aliquots of urine (20 ml) in three tubes were frozen at −80°C until assay.
Pretreatment evaluation: History regarding occupation, marital status, age at onset, duration of disease, progression, sites of involvement, family history and associated diseases was taken. For assessment of area involved, rule of nine and hand palm method were used. A single patch was chosen for transplantation and evaluation of the extent was assessed using photographic image analysis and drawings made on a transparent sheet.
Procedure of autologous melanocyte transfer using suction blisters: Epidermal grafting was chosen as melanocyte transfer procedure. It was done using disposable syringes of 10 ml attached to three-way cannulas, a 50 ml syringe for aspiration of air to produce the vacuum and a manometer to measure negative pressure. The ideal pressure gradient required to produce suction blisters varies according to age of the patient ranging from 300 to 500 mm of Hg. Site of blister induction was lateral aspect of thigh. The recipient site was prepared by dermabrasion using diamond impregnated cylindrical fraises891011.
Harvesting the graft: After a unilocular blister was ready, the periphery of the blister was cut with curved iris scissors. One edge of a sterile glass slide smeared with an antibiotic ointment was kept near the blister. The graft was lifted gently and everted on the glass slide with the dermal side facing upward. Graft was spread to its full size and transfixed on the dermabraded recipient patch. Area was covered with non-adherent antibiotic gauze dressing which was removed on the seventh day. The patient was put on either narrowband ultraviolet B (NbUVB) therapy or, if the patient was unable to visit hospital for this, psoralen photochemotherapy using natural sunlight (PUVAsol) was recommended121314151617.
Assessment of outcome of the transplant: Final evaluation of repigmentation was done after six months. The outline of the patch on the transparent sheet was replaced on the patch and the areas of repigmentation marked. The percentage of repigmentation was calculated using the formula12:
% repigmentation = area of repigmentation/total area of patch×100
In addition, photographs of the patch taken before and six-month after transplantation were compared. Those with >75 per cent repigmentation were labelled as responders and those with <75 per cent repigmentation were labelled as non-responders.
Catecholamine ELISA: Levels of CA were estimated by commercially available ELISA kit (CAT ELISA, DLD Diagnostika GmBH, Germany). The quantitative measurement of epinephrine (E), norepinephrine (NE) and dopamine (DA) was done in plasma and urine. E, NE and DA were extracted using a cis-diol-specific affinity gel and acylated to N-acyl-E, N-acyl-NE and N-acyl-DA and converted enzymatically into N-acylnormetanephrine, N-acylmetanephrine and N-acyl-3-methoxytyramine, respectively. E, NE and DA were bound to the solid phase of the microtiter plate. The antibody bound to solid phase CA was detected by anti-rabbit IgG/peroxidase. The substrate 3,3’,5,5’-tetramethylbenzidine (TMB) / peroxidase reaction was monitored at 450 nm using ELISA reader (Biotek, US). The amount of antibody bound to solid phase CA is inversely proportional to the CA concentration of the sample.
Plasma & urinary catecholamine and vanillylmandelic acid (VMA) levels: Levels of VMA were estimated by commercially available VMA ELISA kit (DRG International Inc., USA). Plasma as well as urinary E, NE, DA and VMA present in the samples or standard and a monoclonal anti- E, NE, DA and VMA conjugated to biotin were simultaneously added in respective microtiter plates already coated with monoclonal antibody specific for different cytokines. Following incubation, unbound E, NE, DA and VMA were removed by a washing step. Biotinylated anticytokine antibody was added and incubated. Streptavidin-horseradish peroxidase (HRP) was added and bound to the biotinylated anticytokine antibody. After incubation and washing, substrate solution was added to the wells. A coloured product was formed, proportional to the amount of E, NE, DA and VMA present in the sample. The reaction was terminated by the addition of sulphuric acid, and the absorbance was measured at 450 nm using ELISA reader (Biotek, US).
Statistical analysis: Sample size needed for this study, keeping the power as 80 per cent was calculated to be 30 for cases, while 15 was the minimum number for control group. Data were analyzed by SPSS version 15.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were compared between the groups by Wilcoxon rank sum test and independent t test. Change within the group was seen by paired t test/Wilcoxon Signed-rank test.
Results
The patients in the three clinical groups did not differ significantly from each other in any of the baseline characteristics. There was a preponderance of females over males overall (66.66%) and in individual groups: 63.63 per cent in group 1, 57 per cent in group 2 and 75 per cent in group 3. There was no family history of vitiligo in any of the patients. A total of 30 patients (10 males, 20 females), with a mean age of 22.9±4.7 (range 17-40 yr) were enrolled. The control group (n=15) included 11 males and four females, with a mean age of 28.3±6.8 yr (range 18-38 yr). There was no significant difference in mean age of patients and controls. Fig. 1 shows the percentage of patients who responded to the transplantation in all the study groups.
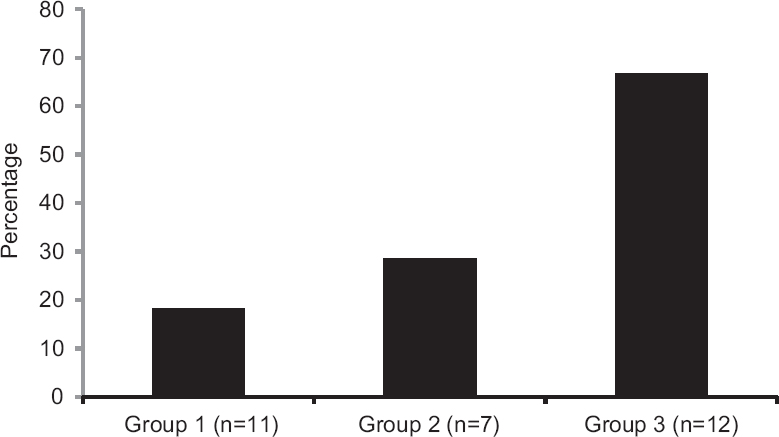
- Number of responders and non-responders in three different clinically determined stability groups. Group 1, < three months < one year; Group 2, ≥ one year < two years; Group 3, ≥ three years.
Circulatory levels of catecholamines: Mean values of catecholamines studied in pre- and post-intervention (transplantation followed by phototherapy/photochemotherapy) vitiligo patients and controls are shown in Table II. There was a significant increase in the mean levels of CA in pre-intervention vitiligo patients as compared to healthy controls, while only DA showed a significant increase in post-intervention patients. On comparing the pre- and post-intervention mean values, catecholamines showed significant decrease post-intervention. Table III demonstrates the statistical significance of the results in plasma of responders and non-responders.
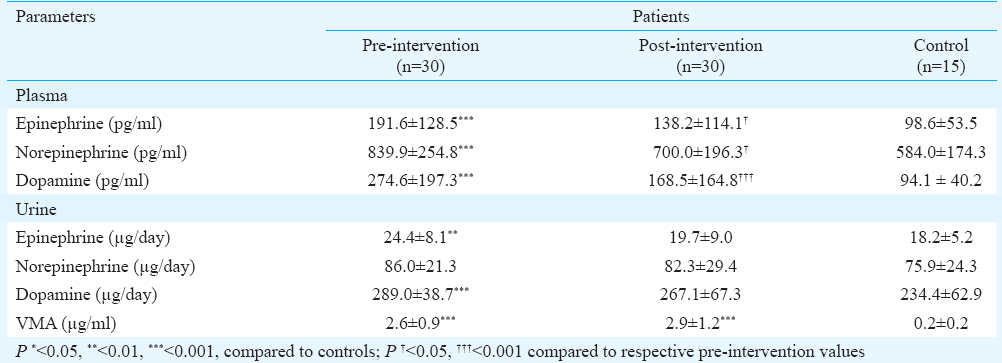

Urinary levels of catecholamines and vanillylmandelic acid: Mean values of CA and VMA studied in pre- and post-intervention vitiligo patients and the controls are shown in Table II. There was a significant increase in mean levels of E, DA in pre-intervention vitiligo patients as compared to healthy controls except NE, whereas significant increase was observed for VMA. There was no significant increase in the mean CA levels in post-intervention vitiligo patients as compared to healthy controls, whereas a significant increase was observed for VMA. When the pre- and post-intervention mean values were compared, E showed significant decrease (Table II). Table III demonstrates significance of the results in urine of responders and non-responders. Fig. 2 shows the level of VMA between pre- and post-intervention of responders and non-responders as compared to healthy controls.
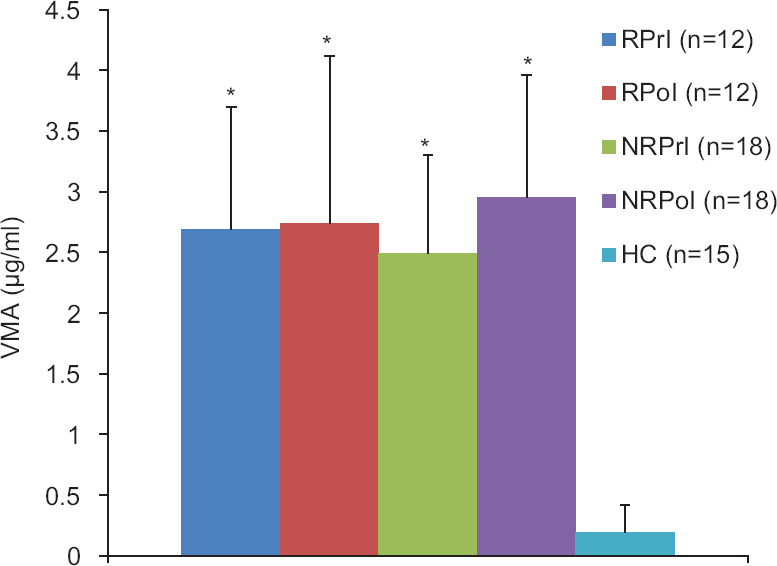
- Pre- and post-intervention levels of urinary VMA in responders, non-responders and healthy controls. VMA, vanillylmandelic acid; RPrI, responder pre-intervention; RPoI, responder post-intervention; NRPrI, non-responder pre-intervention; NRPoI, non-responder post-intervention; HC, healthy controls. *P<0.05 compared to healthy controls.
Discussion
Vitiligo is an acquired idiopathic pigmentary disorder characterized by hypopigmented to depigmented macules. Surgical modalities of treatment become necessary when the lesions become stable and refractory to medical therapy. The outcome of surgery is good in stable lesions whereas it is poor for unstable lesions, as shown in the present study and in our earlier reports16. Vitiligo has a complex pathogenesis where biochemical and immunological factors17 have been proposed to play a role and clinical or historical stability alone may not be reliable. Gupta and Kumar18 reported that in patients with clinically stable disease, only 50-60 per cent patients with bilateral vitiligo showed <75 per cent repigmentation. Therefore, it is important to consider biochemical and cellular stability along with clinical stability before undertaking any surgery for vitiligo19. The results of the present study suggested only dopamine (urine) might be of some help in determining the stability in patients with generalized vitiligo undergoing melanocyte transfer. This might facilitate better selection in patients undergoing surgery.
In this study, association between vitiligo associated stress and the increase in plasma and urinary CA levels and urinary VMA levels was studied due to active phase of the disease. A significant decline was found in the CA levels after transplantation. When pre- and post-intervention values were compared, responders showed significant decline in plasma and urinary E and DA whereas insignificant increase and decrease in plasma and urinary NE, respectively. This could probably be due to immune suppression, alteration in cytokine expression and cell cycle arrest induced by phototherapy20. The conjunction of psoralens with epidermal DNA inhibits DNA replication and causes cell cycle arrest. Psoralen photosensitization also causes an alteration in the expression of cytokines and cytokine receptors. Infiltrating lymphocytes are strongly suppressed by PUVA and NbUVB, with variable effects on different T-cell subsets. Psoralens and UV radiation also stimulate melanogenesis20. Reduced emotional stress of the patients may also be one of the factors. While undergoing phototherapy, patients interact with other patients with similar problems and also undergo counselling.
Preliminary studies showed significantly higher levels of plasma and urinary monoamines and their metabolites in patients at recent onset of the disease than chronic sufferers21. In 12 per cent of the cases, the development of vitiligo was attributed to severe emotional stress; however, the mechanism is not yet properly investigated22. Different strategies have been adopted to study these neural markers and their metabolites. Schallreuter and Wood23 noticed an increase in NE production in both plasma and urine. Bilgiç and Bilgiç24 reported a case of vitiligo lesion development after use of atomoxetine drug (inhibitor of NE reuptake sites) in a patient with attention-deficit/hyperactivity disorder. Chakraborty et al25 also evidenced higher urinary excretion values of indole metabolites in vitiligo patients. Other studies on these markers have focused on cellular values with increased production of CA being observed in lesional keratinocytes. The increased plasma levels of NE and increased urinary concentration of CA catabolites homovanillic acid (HVA) and VMA were significantly associated with disease activity. Shahin et al21 reported elevated levels of plasma and urinary catecholamines and 5-hydroxyindoleacetic acid in non-segmental vitiligo patients. Cucchi et al5 showed a gradual decrease in the levels of catecholamine and their metabolites with the duration of the disease suggesting that increase might occur in response to initial stress. Moreover, the moderate increases in plasma monoamine and metabolite concentrations, noted in patients with more recent onset of vitiligo, tend to support the hypothesis that these increases represent an epiphenomenon rather than the principal cause of depigmentation. Bolognia and Pawelek26 showed that a neurotransmitter such as NE or similar agents released at nerve endings would be involved in the destruction of the melanocytes in a segmental cutaneous region.
Morrone et al4 conducted a study on urinary CA of patients affected with different types of vitiligo and showed that a significant increase of urinary levels of HVA and VMA, characterized the onset and the progressive active phases of vitiligo, irrespective of the type or distribution using gas chromatography-mass spectrometry. A study by Cucchi et al27 showed higher levels of plasma NE, E, normetnephrine (NMN) and metanephrine (MN) in patients with vitiligo. Patients with active disease showed significantly higher levels of NE and NMN than those at stable phase. The patients with progressive vitiligo and those with more recent onset (one year) showed significantly increased levels of E, NE and MN in comparison with longer-term sufferers5. In addition, urinary levels of NE, DA, NMN and MN were found to be significantly higher in patients than in controls. The patients with progressive vitiligo presented increased urinary excretion values for all parameters (in particular, NE levels) than other patients527. Reimann et al28 reported the altered expression of genes associated with the dopamine pathway in vitiligo patients. Lin et al29 reported that apigenin attenuates dopamine-induced apoptosis in melanocytes. Another study demonstrated Akt/PKB (protein kinase B) activation by dopamine which ultimately promotes cell death in melanocytes30.
The limitation of this study was its small sample size. Therefore, the study needs to be done in larger patient cohort and controls so that a link between stability and the levels of these molecules could be established. We have observed statistically significant decline in the levels of plasma and urinary E and DA after the transplantation in responders.
In conclusion, the levels of plasma/urinary E, and dopamine could not be established as biomarkers for disease stability or successful outcome (responders) of autologous melanocyte transfer in the generalized vitiligo patients from the findings of this study.
Financial support & sponsorship: Authors acknowledge the Indian Council of Medical Research, New Delhi, India, for providing financial support to carry out this study
Conflicts of Interest: None.
References
- Vitiligo: Pathomechanisms and genetic polymorphism of susceptible genes. Indian J Exp Biol. 2006;44:526-39.
- [Google Scholar]
- Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191:407-16.
- [Google Scholar]
- Higher plasma catecholamine and metabolite levels in the early phase of nonsegmental vitiligo. Pigment Cell Res. 2000;13:28-32.
- [Google Scholar]
- Circulatory levels of antioxidants and lipid peroxidation in Indian patients with generalized and localized vitiligo. Arch Dermatol Res. 2009;301:731-7.
- [Google Scholar]
- Catecholamine level and its relation to anxiety and depression in patients with vitiligo. J Egypt Women Dermatol Soc. 2009;6:74-9.
- [Google Scholar]
- Suction blister induction time: 15 minutes or 150 minutes? Dermatol Surg. 2000;26:754-6.
- [Google Scholar]
- Surgical pearl: Standardized suction syringe for epidermal grafting. J Am Acad Dermatol. 2005;52:348-50.
- [Google Scholar]
- Suction blister device for separation of viable epidermis from dermis. J Invest Dermatol. 1968;50:129-37.
- [Google Scholar]
- A novel scoring system for evaluation of results of autologous transplantation methods in vitiligo. Indian J Dermatol Venereol Leprol. 2002;68:33-7.
- [Google Scholar]
- Autologous epidermal grafting with PUVA-irradiated donor skin for the treatment of vitiligo. Int J Dermatol. 1998;37:551-4.
- [Google Scholar]
- Treatment of vitiligo with suction epidermal grafting by the use of an ultrapulse CO2 laser with a computerized pattern generator. Dermatol Surg. 2001;27:565-8.
- [Google Scholar]
- Suction blister grafting for stable vitiligo using pulsed erbium: YAG laser ablation for recipient site. Int J Dermatol. 2000;39:471-3.
- [Google Scholar]
- Study of clinical, biochemical and immunological factors determining stability of disease in patients with generalized vitiligo undergoing melanocyte transplantation. Br J Dermatol. 2012;166:1230-6.
- [Google Scholar]
- Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-β) in patients with vitiligo. J Am Acad Dermatol. 2012;66:510-1.
- [Google Scholar]
- Epidermal grafting in vitiligo: Influence of age, site of lesion, and type of disease on outcome. J Am Acad Dermatol. 2003;49:99-104.
- [Google Scholar]
- The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Coll Antropol. 2011;35(Suppl 2):147-51.
- [Google Scholar]
- Detection of plasma and urinary monoamines and their metabolites in nonsegmental vitiligo. Acta Dermatovenerol Croat. 2012;20:14-20.
- [Google Scholar]
- Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. J Invest Dermatol. 1997;109:310-3.
- [Google Scholar]
- Possible atomoxetine-induced vitiligo: A case report. Atten Defic Hyperact Disord. 2015;7:179-81.
- [Google Scholar]
- Vitiligo, psoralen, and melanogenesis: Some observations and understanding. Pigment Cell Res. 1996;9:107-16.
- [Google Scholar]
- Catecholamines increase in the urine of non-segmental vitiligo especially during its active phase. Pigment Cell Res. 2003;16:111-6.
- [Google Scholar]
- Expression profile of genes associated with the dopamine pathway in vitiligo skin biopsies and blood sera. Dermatology. 2012;224:168-76.
- [Google Scholar]
- Apigenin attenuates dopamine-induced apoptosis in melanocytes via oxidative stress-related p38, c-Jun NH2-terminal kinase and Akt signaling. J Dermatol Sci. 2011;63:10-6.
- [Google Scholar]
- Potential redox-sensitive Akt activation by dopamine activates Bad and promotes cell death in melanocytes. Oxid Med Cell Longev. 2010;3:219-24.
- [Google Scholar]






