Translate this page into:
Pioglitazone utilization, efficacy & safety in Indian type 2 diabetic patients: A systematic review & comparison with European Medicines Agency Assessment Report
Reprint requests: Dr Nilima Kshirsagar, National Chair Clinical Pharmacology (ICMR), National Institute for Research in Reproductive Health, J. M. Street, Parel, Mumbai 400 012, Maharashtra, India e-mail: kshirsagarna@yahoo.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
With pioglitazone ban and subsequent revoking in India along with varying regulatory decisions in other countries, it was decided to carry out a systematic review on its safety, efficacy and drug utilization in patients with type 2 diabetes mellitus (T2DM) in India and compare with the data from the European Medicines Agency Assessment Report (EMA-AR).
Methods:
Systematic review was performed as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, searching Medline/PubMed, Google Scholar and Science Direct databases using ‘pioglitazone AND India AND human’ and ‘pioglitazone AND India AND human AND patient’ and compared with EMA-AR. Spontaneous reports in World Health Organization VigiBase from India were compared with VigiBase data from other countries.
Results:
Sixty six publications, 26 (efficacy), 32 (drug utilization) and eight (safety), were retrieved. In India, pioglitazone was used at 15-30 mg/day mostly with metformin and sulphonylurea, being prescribed to 26.7 and 8.4 per cent patients in north and south, respectively. The efficacy in clinical trials (CTs) was similar to those in EMA-AR. Incidence of bladder cancer in pioglitazone exposed and non-exposed patients was not significantly different in an Indian retrospective cohort study. There were two cases and a series of eight cases of bladder cancer published but none reported in VigiBase.
Interpretation & conclusions:
In India, probably due to lower dose, lower background incidence of bladder cancer and smaller sample size in epidemiological studies, association of bladder cancer with pioglitazone was not found to be significant. Reporting of CTs and adverse drug reactions to Clinical Trials Registry of India and Pharmacovigilance Programme of India, respectively, along with compliance studies with warning given in package insert and epidemiological studies with larger sample size are needed.
Keywords
Benefit
risk ratio
bladder cancer
efficacy
India
pioglitazone
safety
Pioglitazone, an agonist of peroxisome proliferator-activated receptor gamma, acts as an insulin sensitizer, thereby improving glycaemic control in patients with type 2 diabetes mellitus (T2DM). Reports about an increased risk of bladder cancer in patients exposed to pioglitazone led to its withdrawal from the French market1. The United States Food and Drug Administration (FDA) did not suspend the market authorization but added a black box warning for bladder cancer risk2. Indian drug regulatory authorities withdrew pioglitazone in June 2013 but then revoked the ban due to lack of sufficient evidence and recommendation by the Drug Technical Advisory Board (DTAB)3. The European Medicines Agency (EMA) put forth an assessment report on safety, efficacy and risk management plan for pioglitazone in December 20114.
The EMA assessed the association of pioglitazone with bladder cancer. The Scientific Advisory Group in Diabetes/Endocrinology of EMA concluded that pioglitazone was useful in the treatment of T2DM as second-line drug when metformin was not effective or contraindicated and that its use should be restricted in duration (less than two years), cumulative dose (>28,000 mg) and patients with bladder cancer risks4.
In the light of the varying regulatory decisions taken by different countries and the EMA Assessment Report (EMA-AR), we carried out a systematic review (SR) of the published literature from India to determine the efficacy, safety and drug utilization pattern of pioglitazone in patients with T2DM and compared it with the data from EMA-AR. The data from VigiBase, maintained by the World Health Organization Uppsala Monitoring Centre (WHO-UMC) on pioglitazone were also analysed.
Material & Methods
The systematic review was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines5. Three databases, namely, Medline/PubMed, Google Scholar and Science Direct were searched and all studies on pioglitazone carried out in India published in English language between 1999 and 2014 were included.
Study characteristics: The search terms used were ‘pioglitazone AND India AND human’ and ‘pioglitazone AND India AND human AND patient’ (Figure). The search was further expanded using other search terms ‘safety’, ‘efficacy’, ‘bladder cancer’, ‘drug utilization’ and ‘thiazolidinedione’. The ‘Create Alert’ facility in PubMed was subscribed to keep a track of any latest article. The last date of PubMed alert was April 20, 2015. Letters / emails were written to authors and journal indices of articles included in SR were hand searched to identify any publication missed in the search. The full text of all articles were obtained and read independently by both the authors, and the data were extracted in a pilot form and the final spreadsheet was prepared.
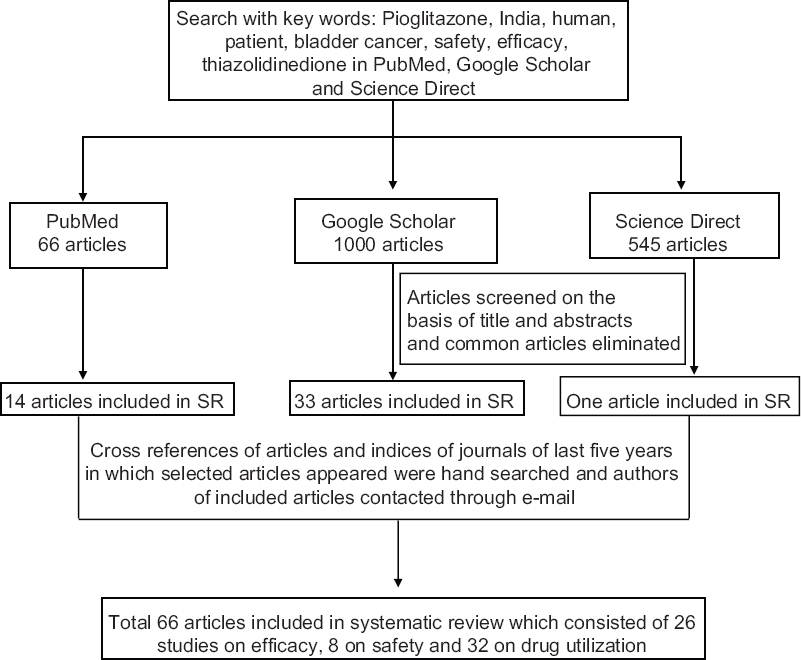
- Flowchart showing selection of articles for inclusion in systematic review (SR).
Inclusion & exclusion criteria: As per the PICO (population, intervention, control and outcome) criteria5, studies included in the SR were those: (i) that were carried out in Indian T2DM patients (and carried out in India) (population); (ii) with pioglitazone either alone or in combination with other antidiabetic drugs (intervention); (iii) comparing it with baseline or other antidiabetic drugs (comparison); and (iv) efficacy parameters such as percentage decrease in glycosylated haemoglobin (HbA1c), changes in lipid profile; safety parameters such as cases of bladder cancer and utilization pattern such as prescribed dose and combination of pioglitazone (outcomes).
Studies were excluded if related to pioglitazone use in other conditions such as (i) polycystic ovary syndrome; (ii) psoriasis; (iii) type 1 diabetes mellitus; (iv) in vitro studies; and (v) reviews, commentaries and editorials.
Drug utilization studies: The data extracted included (i) region in which the study was carried out (north, south, east, west and central India); (ii) the setting in which the study was carried out (in- or out-patient); (iii) type of health care setting (community/government hospital/private hospital); (iv) number of prescriptions studied; (v) patient's age, body mass index, gender, duration of diabetes, co-morbidities; and (vi) the use of pioglitazone (dose, whether as monotherapy or in combination with other drugs). The overall use of pioglitazone (percentage) in each region was calculated from the data provided in individual studies (usage and number of prescriptions).
Efficacy studies: The data extracted in the spreadsheet included changes in glucose and lipid profile with respect to baseline namely, fasting plasma glucose, postprandial plasma glucose, HbA1c, triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). The dose and duration of pioglitazone and also its effects on requirement of other antidiabetic drugs such as insulin and sulphonylureas, were noted.
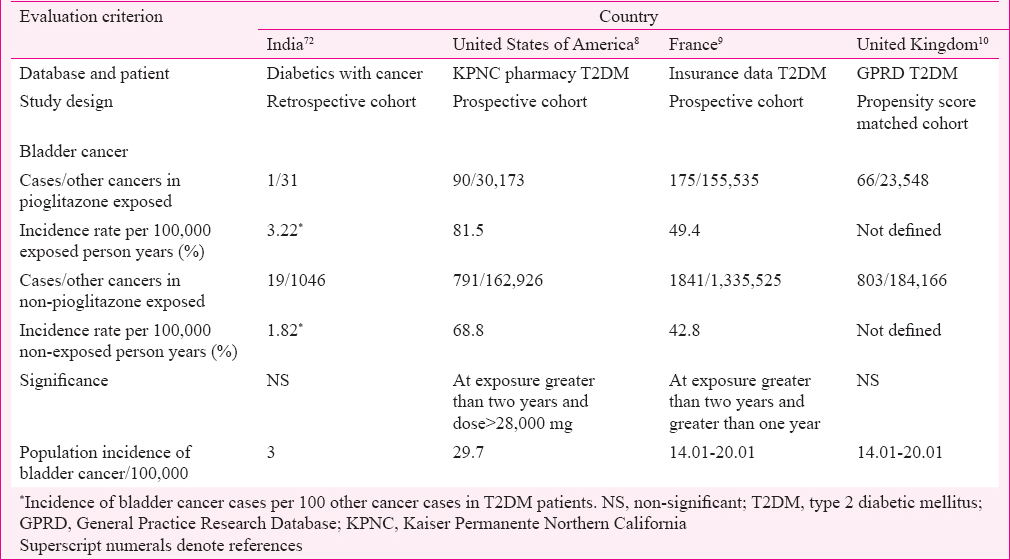
Safety studies: The data extracted included the following details about patients (case history): (i) age; (ii) gender; (iii) duration of T2DM; (iv) dose of pioglitazone; (v) duration of pioglitazone use; (vi) investigations; and (vii) outcomes. Data from India were compared with the data and publications with similar methodology/data sets analyzed in references from the EMA-AR. EMA Assessment Report was chosen for comparison as it is freely available in the public domain and gives comprehensive information on benefit-risk evaluation of pioglitazone4. The drug utilization data from India were compared with that of the General Practice Research Database (GPRD), United Kingdom4. Results from clinical trials (CTs) conducted in India were compared with that of PROactive study carried out in Europe67. Epidemiological studies on bladder cancer associated with pioglitazone from India were compared with Kaiser Permanente Northern California (KPNC) (USA)8, French cohort study9 and GPRD study (UK)10.
Spontaneous reports from WHO-UMC: EMA-AR did not have information on data from VigiBase. However, to get an estimate of spontaneous reports for pioglitazone, a summary of adverse drug reactions (ADRs) associated with pioglitazone was obtained from the WHO-UMC VigiBase, global individual case safety report (ICSR) database through personal communication. The reporting period in this database was from 2000 to November 18, 2014 for countries such as USA, UK, Japan and France while for India it was from 2011 onwards. The VigiBase data were provided as a spreadsheet which included summary of all ADRs, country-wise and year-wise ADRs. We extracted ADRs related to urinary bladder for India, USA, France, UK and Japan from this spreadsheet as the latter three represented regulated markets. The bladder-related abnormalities in the spreadsheet included bladder calculus, bladder carcinoma, bladder discomfort, bladder fibrosis and bladder neoplasm benign.
Results
Details of search of the databases are given in the Figure. Sixty six articles fulfilled the inclusion and exclusion criteria and were taken up for the SR. Of these, 13 each were randomized controlled trials and prospective cohort studies, 34 were cross-sectional studies, three case reports, two case series and one was a retrospective cohort study. Thirty two articles were on drug utilization, 26 on efficacy and eight were on safety.
Usage pattern: There were 32 publications of observational ‘real life’, prescription monitoring, drug utilization studies, of which 15 were prospective cohort studies, five were retrospective cohort studies and 12 were cross-sectional studies. These studies were carried out in tertiary care (5 public and 27 private) hospitals. The study duration varied from two months to two years. The number of prescriptions studied ranged from 64 to 1185, and duration of T2DM was from 0 to 1 year (newly diagnosed) to about 10 yr, but duration of pioglitazone use was not specified.
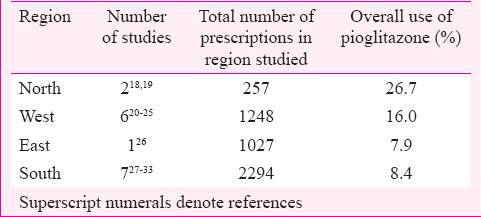
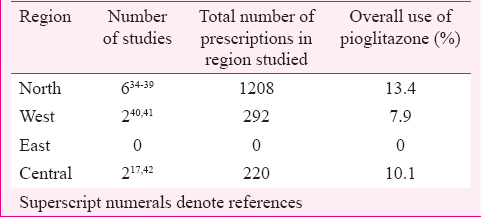
Pioglitazone was used mostly in combination with metformin and sulphonylurea, and the prescribed dose was 15-30 mg. In one study, it was used in combination with metformin, sulphonylurea and dipeptidyl peptidase-IV inhibitor at 15 mg/day dose11. In five studies patients from only inpatient departments were included1213141516, one study included patients from both inpatient and outpatient departments17 while 25 studies included patients from outpatient departments only18192021222324252627282930313233343536373839404142. Pioglitazone was prescribed to 6.5-11 per cent of T2DM patients in inpatient departments1213141516. The extent of the use of pioglitazone in outpatient departments (26 studies) was described as percentage of T2DM patients in 16 studies (Table I) and as percentage of antidiabetic drug prescriptions in 10 studies (Table II). There was regional variation in pioglitazone use across India, being more in the north compared to the south (Table II).
Efficacy studies
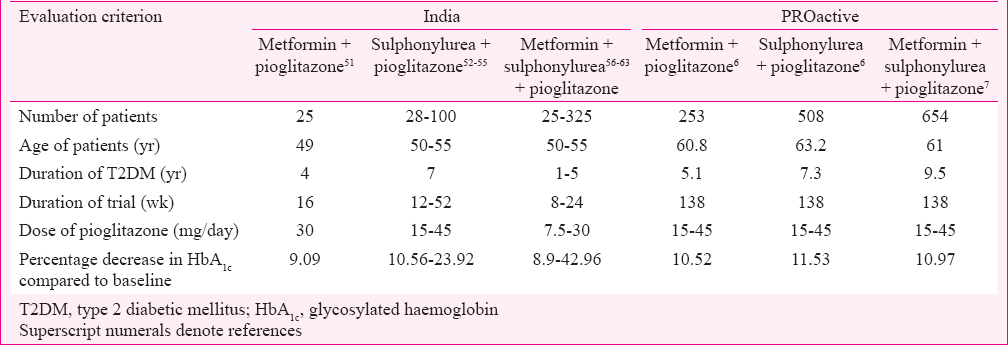
Glycaemic control: There were 26 studies (13 randomized and 13 non-randomized, controlled, comparative CTs) with primary objective to determine efficacy of pioglitazone in T2DM patients. Eight were monotherapy4344454647484950, one add-on to metformin51, four add-on to sulfonylurea52535455, and eight were add-on to sulphonylurea and metformin combination5657585960616263. Two studies evaluated add-on to metformin, sulphonylurea and insulin6465; three evaluated and noted decrease in α-amylase, glucokinase and gamma-glutamyl transferase activities666768. The results from 13 studies from India and from PROactive studies carried out in Europe are summarized in (Table III). Studies in India have been done in younger patients, with shorter duration of T2DM, shorter trial duration, and lower dose of pioglitazone; however, the decrease in HbA1c (efficacy) was found to be comparable to PROactive studies. In one study, 7.5 mg of pioglitazone given with insulin, sulphonylurea, metformin and α-glucosidase inhibitor was found to be effective in achieving good glycaemic control59.
Other effects: Pioglitazone did not have any detrimental effect on lipid profile in Indian patients, which is comparable to that in PROactive study. It was shown to decrease serum TG (10-35%), TC (5-30%) and LDL-C (1.4-31.5%)454647485052 and increase HDL-C (2.5-14.4%)4547. It also demonstrated an increase in apolipoprotein-A1 and a decrease in apolipoprotein-B which corresponded with increase in HDL-C and decrease in LDL-C, respectively4446. It decreased significantly homeostasis model of assessment of insulin resistance, plasma fasting insulin and increased adiponectin (2.7 fold) compared to baseline43454656. It was observed to produce a significant decrease in C-reactive protein, tumour necrosis factor-alpha and serum malondialdehyde and increase in serum magnesium, angiotensin, interleukin-8 and serum glutathione5368. In one study, a significant decrease in systolic (11.8%) and diastolic (11.6%) blood pressure was noted when compared to sulphonylureas or other insulin secretagogues47.
Safety
Bladder cancer: There were two case reports and one case series of eight patients697071. Of these 10 patients, nine were males, age being 65 yr and 64 yr in the case reports and 43-76 yr in the case series, dose used was 15-30 mg/day, duration of use was 1-9 years, eight had developed transitional cell carcinoma, one had bladder dysplasia while the nature of malignancy of one patient was not reported. One patient died of metastasis to lung and liver, another had recurrence of haematuria on rechallenge with pioglitazone while one was in remission for 11 months after receiving six cycles of mitomycin chemotherapy. The outcome of the remaining patients was not reported.
In a retrospective cohort study carried out in India on 1077 diabetics with cancer, 20 were bladder cancer cases, of whom one was in the pioglitazone exposed group which consisted of 31 patients with exposure for more than two years and dose 7.5-15 mg/day. No significant association was found between pioglitazone use and bladder cancer72. In comparison, KPNC study (USA) which included 193,099 diabetic patients found significant risk of bladder cancer in pioglitazone exposed patients with cumulative dose more than 28,000 mg and cumulative duration more than two years8. The French cohort study with 1,491,060 diabetic patients found significant association with exposure more than one year and two years9 while GPRD study of the UK in 207,714 diabetics found no significant association10 (Table IV).
In the WHO-UMC, VigiBase, there were 101 spontaneous reports on pioglitazone from India, most common ones being hypoglycaemia (19.8%) and oedema (19.8%) but none on bladder abnormalities while globally there were 1862 reported cases of bladder carcinoma associated with pioglitazone.
Other safety issues: In CTs, compared to baseline, significant increase in body weight (1.6±1.3 kg), increase in body mass index (0.7±0.3 kg/m2), reduction in haemoglobin and compared to placebo reduction in total, free and bioavailable testosterone with increase in androstenedione were observed4449556573. No significant change in liver function tests, urine composition (pH, proteinuria, pyuria or haematuria) compared to baseline was observed59. Hypoglycaemia, haematemesis, myocardial infarction, renal stone and fracture were reported in CTs4448. Incidence of fracture in pioglitazone group (1.9%) was not significantly different compared to placebo group (1.9%) during a three-year study48. In a case report a patient with Grave's disease and T2DM developed chemosis, proptosis and lid retraction while on pioglitazone with reversal on its discontinuation and treatment with steroids and surgical decompression74. Increased micronuclei formation75 and reversible atrial and mitral valve regurgitation with pioglitazone use76 have also been reported.
Discussion
With pioglitazone being initially banned by the Indian drug regulatory authority and later the ban being revoked, along with varying regulatory decisions in European countries, the need was felt to analyze its utilization, efficacy, safety in Indian T2DM patients and compare it with EMA-AR. The data available in EMA-AR were comprehensive, however, data available from India were limited with respect to prescription monitoring studies, controlled CTs for efficacy, case reports on ADRs, and epidemiological study on ADRs. Compared to the data from EMA-AR, in India, information on drug utilization in terms of patient years of use is not available. However, analysis of studies from India has brought out important differences in drug utilization. In the GPRD (UK) database, pioglitazone is used most commonly with metformin at 30 mg4. The defined daily dose of pioglitazone is 30 mg77. In India, it is mostly used in combination with metformin and sulphonylurea at a dose of 15-30 mg/day2628. There is also regional variation in pioglitazone prescription with greater use in northern and western India compared to central and southern India with no studies from east India. Whether the lower body weight of Indians78 makes up for the lower dose used and gives similar exposure as in European population with higher dose and body weight has not been studied.
Efficacy of pioglitazone has been studied in randomized and non-randomized comparative studies in India. The observed effects on glycaemic control and lipids have been similar to PROactive study.
There is a lack of adequate epidemiological data and spontaneous reporting of ADRs from India. Sample size, dose and duration of use are important variables, in the epidemiological studies. In the French cohort study with large sample size, significant association between pioglitazone use and bladder cancer was noted even with more than one year of use9. In the KPNC study with smaller sample size compared to French cohort, risk for bladder cancer was noted with cumulative exposure greater than two years and cumulative dose greater than 28,000 mg, while in GPRD study, which was a propensity score matched study, no association between pioglitazone and bladder cancer was noted810.
Pioglitazone is metabolized mainly by CYP2C879, and any variation of this enzyme affecting exposure predisposing to development of bladder cancer through formation of carcinogenic metabolites has not been reported. Factors predisposing to the development of bladder cancer are male sex, increasing age, cigarette smoking, exposure to chemicals such as aromatic amines, acrolein, arsenic, aniline dye, Schistosoma haematobium infection, pelvic irradiation, chronic irritation and indwelling catheter80. The incidence of bladder cancer per 100,000 population in India has been reported to be three, while in the USA and UK, it is 29.7 and 14.01-20.01, respectively81. Whether and how this background rate will affect the pioglitazone potential for carcenogenicity needs to be studied.
Subsequent to the EMA assessment in 2011, 10 yr follow up of PROactive study patients and KPNC cohort study final analysis with 10 yr follow up did not find significant association between pioglitazone and bladder cancer8283. Though details are not yet published, there may be variables such as discontinuation of pioglitazone to be considered.
In CTs, case reports and spontaneous reports in the Pharmacovigilance Programme of India (PvPI), most common ADRs reported were weight gain, oedema and hypoglycaemia4873, all of which are listed in summary of product characteristics (SmPC) and EMA-AR for pioglitazone. Average weight gain in Indian trials (1.68 kg) had been lesser than in PROactive study (3.8 kg) which may be because in Indian CTs636473 dose of pioglitazone was 7.5-30 mg/day while in PROactive it was 45 mg/day and duration of treatment was also longer84. Bone fractures were reported but incidence was similar to that with placebo which could be because of smaller number of patients/women (n=204/26) in the study48 which is contradictory to a meta-analysis (n=24,544) in which a significant increase in incidence of bone fracture in women was noted, with 896 cases of fracture being reported in 22 randomised CTs85. Decrease in serum testosterone observed in one study from India was not reported in SmPC or EMA-AR, and its clinical significance has also not been established49. A case report of worsening of thyroid ophthalmopathy with pioglitazone use has been reported from India and this warrants caution in using pioglitazone in patients with thyroid abnormalities74. There are no published case reports and ICSRs in VigiBase of macular oedema, a listed ADR for pioglitazone from India.
As per the recommendation by DTAB in July-August 2013, Indian package inserts of pioglitazone containing products carry boxed warning contraindicating its use as a first-line therapy in diabetes mellitus, use in patients with active bladder cancer and uninvestigated haematuria as well as discontinuation if adequate effect is not observed in 3-6 months3. Elderly patients should be started on lowest possible dose and regularly monitored because of risk of bladder cancer and heart failure. However, the boxed warning does not provide any guidance on cumulative dose or duration of use.
Our SR had certain limitations. We might have inadvertently missed out on some publications (retrieval bias). We were not able to identify any reporting or publication bias in the publications included in this SR. The quality of randomized controlled trials used for efficacy studies was not assessed. Further as studies of different levels of evidence (case reports to randomized controlled trials) were included, the strength of evidence for the various outcomes could not be sufficiently estimated.
In conclusion, regional variation in pioglitazone use was observed in India, with higher use in north and west compared to south and central regions. In India, pioglitazone is used more often as triple drug combination instead of dual drug therapy with metformin and at lower doses compared to UK. Compared to KPNC or French studies which found significant association between incidence of bladder cancer and pioglitazone cumulative dose >28,000 mg and duration of use more than one year, in retrospective cohort study from India with 15-30 mg pioglitazone taken for more than two years, association between incidence of bladder cancer and pioglitazone use was not seen. Smaller sample size, lower dose of pioglitazone and lower background incidence of bladder cancer might be some of the possible reasons for not finding an association. In controlled CTs, though on fewer patients, at shorter duration of treatment and with lower dose, efficacy of pioglitazone on HbA1c and lipids was similar to that in PROactive studies. ADRs have not been reported to PvPI and it is required that spontaneous reporting must be encouraged. Studies on compliance to warnings given in the package insert for pioglitazone and epidemiological studies with larger sample size and regional representation are needed.
Conflicts of Interest: None.
References
- French regulators suspend pioglitazone citing cancer risk. Available from: http://www.medscape.com/viewarticle/744345
- FDA Drug Safety Communication: Update to ongoing safety review of Actos (pioglitazone) and increased risk of bladder cancer. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm259150.htm
- Assessment Report for Actos, Glustin, Competact, Glubrava, Tandemact. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report _-_Variation/human/000285/WC500126656.pdf
- Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-9, W64.
- [Google Scholar]
- PROactive investigators. Long-term glycaemic effects of pioglitazone compared with placebo as add-on treatment to metformin or sulphonylurea monotherapy in PROactive (PROactive 18) Diabet Med. 2009;26:1242-9.
- [Google Scholar]
- PROactive investigators. Long-term glycaemic control with metformin-sulphonylurea-pioglitazone triple therapy in PROactive (PROactive 17) Diabet Med. 2009;26:1033-9.
- [Google Scholar]
- Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-22.
- [Google Scholar]
- Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953-62.
- [Google Scholar]
- Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-9.
- [Google Scholar]
- Usage of pioglitazone at Medanta, the Medicity. Indian J Endocrinol Metab. 2014;18:111-2.
- [Google Scholar]
- Study of drug utilization pattern of antihyperglycemic agents in a South Indian tertiary care teaching hospital. Indian J Pharmacol. 2012;44:210-4.
- [Google Scholar]
- Investigation of in-patient prescribing patterns of oral antidiabetic drugs in a tertiary care teaching hospital. Afr J Pharmacol Ther. 2013;2:54-8.
- [Google Scholar]
- Retrospective prescription-based survey in type 2 diabetes in an Indian tertiary care hospital. Int J Res Pharm Sci. 2011;2:417-20.
- [Google Scholar]
- Drug use evaluation of diabetes mellitus in hospitalized patients of a tertiary care referral hospital. J Basic Clin Physiol Pharmacol. 2012;23:173-7.
- [Google Scholar]
- Study on prescribing pattern and potentials drug-drug interactions in type-2 diabetes mellitus (inpatients) in a tertiary care teaching hospitals. Pharm Lett. 2011;3:13-9.
- [Google Scholar]
- Pattern of antidiabetic drugs used in outpatient and hospitalized patients in a tertiary health institute of central India. J Contemp Med Dent. 2014;2:48-54.
- [Google Scholar]
- Prescribing pattern of antidiabetic drugs and achievement of glycaemic control in T2DM patients tertiary care hospital in North India. Int J Diabetes Dev Ctries. 2013;33:140-6.
- [Google Scholar]
- Evaluation of antidiabetic prescriptions from medical reimbursement applications at Banaras Hindu University health care facility. J Pharm Care. 2014;2:49-54.
- [Google Scholar]
- Prescribing trends of antidiabetics in diabetic patients and hypertensive diabetic patients in an urban secondary care hospital. Int J Pharm Biol Arch. 2010;1:249-55.
- [Google Scholar]
- Utilization of some newer oral antidiabetic agents in a tertiary care hospital. Natl J Physiol Pharm Pharmacol. 2012;2:146-51.
- [Google Scholar]
- Evaluation of antidiabetic prescriptions, cost and adherence to treatment guidelines: a prospective, cross-sectional study at a tertiary care teaching hospital. J Basic Clin Pharm. 2013;4:82-7.
- [Google Scholar]
- Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. J Basic Clin Pharm. 2014;5:79-83.
- [Google Scholar]
- Pattern of antidiabetic drugs use in type-2 diabetic patients in a medicine outpatient clinic of a tertiary care teaching hospital. Int J Basic Clin Pharmacol. 2013;2:485-91.
- [Google Scholar]
- Screening of prescriptions in patients of type-2 diabetes mellitus in a tertiary care teaching hospital. Int J Pharm Res Biosci. 2014;3:401-9.
- [Google Scholar]
- Prescription pattern study in type 2 diabetes mellitus in an Indian referral hospital. Internet J Pharmacol. 2009;7(1)
- [Google Scholar]
- Prescribing practices of oral anti-diabetic drugs in the treatment of type-2 diabetes mellitus at St. Martha's hospital, Bangalore. Int J Pharmacother. 2013;3:86-90.
- [Google Scholar]
- Comparison of usage of conventional and newer oral hypoglycaemic drugs in type 2 diabetes mellitus. Int J Pharm Biol Arch. 2013;4:1172-4.
- [Google Scholar]
- A study on drug utilization of oral hypoglycemic agents in type-2 diabetic patients. Asian J Pharm Clin Res. 2011;4:60-4.
- [Google Scholar]
- Drug utilisation study in geriatric type 2 diabetic patients. J Clin Diagn Res. 2007;1:440-3.
- [Google Scholar]
- Comparison of use of antidiabetic agents among geriatric and non-geriatric population. Pharmacol Online. 2011;3:299-304.
- [Google Scholar]
- A study on insulin usage among diabetic patients in a tertiary care teaching hospital. Indo Am J Pharm Res. 2013;3:8474-9.
- [Google Scholar]
- Prescribing pattern of antidiabetic drugs in urban population of Hyderabad. Natl J Physiol Pharm Pharmacol. 2015;5:5-9.
- [Google Scholar]
- Utilization pattern of oral hypoglycaemic agents for diabetes mellitus type 2 patients attending out-patient depatment at a university hospital in New Delhi. Pharmacol Pharm. 2014;5:636-45.
- [Google Scholar]
- Drug utilization of oral hypoglycemic agents in a university teaching hospital in India. J Clin Pharm Ther. 2010;35:267-77.
- [Google Scholar]
- Study of drug prescribing pattern in diabetes mellitus patients in a tertiary care teaching hospital at Dehradun, Uttarakhand. Int J Med Sci Public Health. 2014;3:1351-4.
- [Google Scholar]
- Prescription pattern and patient adherence study of the antidiabetic drugs in research institute cum hospital. Int J Res Pharm Sci. 2013;4:60-75.
- [Google Scholar]
- Prescribing patterns of antidiabetic medications in a tertiary care teaching hospital, Bareilly, UP, India. J Pharm Sci Innov. 2013;2:41-6.
- [Google Scholar]
- A study of drug prescribing pattern and cost analysis among diabetic patients in a tertiary care teaching institute in North India. J Drug Deliv Ther. 2013;3:56-61.
- [Google Scholar]
- Changing pattern of prescribing antidiabetic agents in patients suffering from diabetes mellitus. Int J Basic Clin Pharmacol. 2013;2:47-50.
- [Google Scholar]
- Comparison of lipid profile pattern in obese and non obese type 2 diabetic patients and to study the prescription pattern of antidiabetic drugs. Int J Pharm Sci Rev Res. 2010;4:53-8.
- [Google Scholar]
- Prescribing pattern of antidiabetic drugs in Indore city hospital. Indian J Pharm Sci. 2008;70:637-40.
- [Google Scholar]
- Use of glimepiride and insulin sensitizers in the treatment of type 2 diabetes – A study in Indians. J Assoc Physicians India. 2004;52:459-63.
- [Google Scholar]
- A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of saroglitazar 2 and 4 mg compared to pioglitazone 45 mg in diabetic dyslipidemia (PRESS V) J Diabetes Sci Technol. 2014;8:132-41.
- [Google Scholar]
- Effects of pioglitazone and metformin on plasma adiponectin in newly detected type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2006;65:722-8.
- [Google Scholar]
- Effect of pioglitazone and its combination with statins in coronary artery disease patients with hyperinsulinemia. Can J Physiol Pharmacol. 2007;85:628-33.
- [Google Scholar]
- Effect of pioglitazone and rosiglitazone on mediators of endothelial dysfunction, markers of angiogenesis and inflammatory cytokines in type-2 diabetes. Acta Diabetol. 2009;46:27-33.
- [Google Scholar]
- Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2) Diabetologia. 2009;52:1019-26.
- [Google Scholar]
- Effect of pioglitazone on testosterone in eugonadal men with type 2 diabetes mellitus: a randomized double-blind placebo-controlled study. Clin Endocrinol (Oxf). 2013;78:454-9.
- [Google Scholar]
- Evaluation and comparison of the effect of two insulin sensitizers on glycemic and lipid control in patients with type II diabetes mellitus. Int J Res Phytochem Pharmacol. 2012;2:64-9.
- [Google Scholar]
- Effect of addition of either sitagliptin or pioglitazone in patients with uncontrolled type 2 diabetes mellitus on metformin: a randomized controlled trial. In: J Pharmacol Pharmacother. Vol 4. 2013. p. :27-32.
- [Google Scholar]
- Impact of glitazones on metabolic and haemodynamic parameters in patients with type 2 diabetes mellitus. Singapore Med J. 2009;50:395-9.
- [Google Scholar]
- Serum lipid peroxides and magnesium levels following three months of treatment with pioglitazone in patients with type-2 diabetes mellitus. Diabetes Metab Syndr. 2013;7:35-7.
- [Google Scholar]
- Efficacy, safety and cost-effectiveness of insulin sensitizers as add-on therapy in metabolic syndrome in patients with secondary sulfonylurea failure: a comparative study. J Pharmacol Pharmacother. 2010;1:82-6.
- [Google Scholar]
- Comparative evaluation of glycaemic control and changes in lipid profile with combined oral hypoglycaemics (a sulfonylurea plus pioglitazone versus sulfonylurea plus metformin) in patients with type 2 diabetes mellitus. Indian Med Gaz. 2011;11:442-7.
- [Google Scholar]
- Comparison of the effect of glitazones and DPP-IV inhibitors in type 2 diabetics as add on therapy on insulin sensitivity and serum hs-CRP levels. JK Sci. 2013;15:69-72.
- [Google Scholar]
- Effects of metformin and pioglitazone on impaired glucose tolerance patients – An open level prospective study. J Diabetes Mellitus. 2012;2:316-20.
- [Google Scholar]
- An open level study to assess the glycemic control effect of metformin and Pioglitazone as add on therapy along with sulfonylurea in uncomplicated type 2 diabetes mellitus. J Diabetes Mellitus. 2012;2:191-5.
- [Google Scholar]
- Efficacy and safety of pioglitazone in type 2 diabetes in the Indian patients: results of an observational study. Indian J Endocrinol Metab. 2013;17:709-15.
- [Google Scholar]
- Evaluation of efficacy and safety of fixed dose combination of glimepiride 2 mg pluspioglitazone 15 mg plus metformin SR 500 mg in the management of patients with type-2 diabetes mellitus. J Indian Med Assoc. 2005;103:447-50.
- [Google Scholar]
- Comparison of efficacy of add-on therapy of vildagliptin versus pioglitazone among type 2 diabetes mellitus patients inadequately controlled on dual therapy of metformin plus sulfonylurea. Asian J Med Sci. 2014;5:77-81.
- [Google Scholar]
- Comparative evaluation of voglibose versus pioglitazone on glycaemic control and lipid profile in patients of type 2 diabetes mellitus on glimepiride and metformin in Punjabi population. Int J Basic Clin Pharmacol. 2012;1:160-7.
- [Google Scholar]
- Induction of long-term glycemic control in type 2 diabetic patients using pioglitazone and metformin combination. J Assoc Physicians India. 2007;55:333-7.
- [Google Scholar]
- Beneficial effects of triple drug combination of pioglitazone with glibenclamide and metformin in type 2 diabetes mellitus patients on insulin therapy. J Assoc Physicians India. 2003;51:1061-4.
- [Google Scholar]
- Efficacy of pioglitazone as an add on drug with insulin, glibenclamide and metformin in patients with uncontrolled type 2 diabetes mellitus. Diabetol Croat. 2002;31:51-7.
- [Google Scholar]
- Differential expression of glucokinase activity in Indian type-2 diabetes patients. Int J Pharmacol. 2010;6:500-4.
- [Google Scholar]
- γ-glutamyltransferase expressions in Indian type 2 diabetic patients. Int J Biol Pharm Allied Sci. 2014;3:280-8.
- [Google Scholar]
- Eight cases of bladder cancer in pioglitazone users from India. J Assoc Physicians India. 2012;60:66.
- [Google Scholar]
- Pioglitazone induced carcinoma of urinary bladder: a case report. Br Biomed Bull. 2013;1:131-5.
- [Google Scholar]
- A retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425-7.
- [Google Scholar]
- Pioglitazone induced weight changes in type 2 diabetic patients. Int J Collaborative Res Intern Med Public Health. 2011;3:534-40.
- [Google Scholar]
- Thiazolidinedione precipitated thyroid associated ophthalmopathy. J Assoc Physicians India. 2010;58:255-7.
- [Google Scholar]
- Increased frequency of micronuclei in diabetes mellitus patients using pioglitazone and glimepiride in combination. Food Chem Toxicol. 2010;48:3432-5.
- [Google Scholar]
- Reversible mitral and aortic regurgitation due to pioglitazone. Endocr Pract. 2012;18:e32-6.
- [Google Scholar]
- ATC/ DDD Methodology. WHO Collaborating Centre for Drug Statistics Methodology. Available from: http://www.whocc.no/atc_ddd_index/
- Clinical and biochemical profile of lean type 2 diabetes mellitus. Indian J Endocrinol Metab. 2011;15(Suppl 1):S40-3.
- [Google Scholar]
- The role of human CYP2C8 and CYP2C9 variants in pioglitazone metabolism in vitro. Basic Clin Pharmacol Toxicol. 2009;105:374-9.
- [Google Scholar]
- International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66:59-73.
- [Google Scholar]
- Takeda Announces Completion of the Post-marketing Commitment to Submit Data to the FDA, the EMA and the PMDA for Pioglitazone Containing Medicines Including ACTOS. Available from: https://www.takeda.com/news/2014/20140829_6714.html
- Observational follow-up of the PROactive study: a 6-year update. Diabetes Obes Metab. 2014;16:63-74.
- [Google Scholar]
- PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009;32:187-202.
- [Google Scholar]
- Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014;68:115-23.
- [Google Scholar]






