Translate this page into:
Pefloxacin as a surrogate marker for quinolone susceptibility in Salmonella enterica serovars Typhi & Paratyphi A in India
Reprint requests: Dr. Arti Kapil, Department of Microbiology, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: akapilmicro@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The emergence of resistance to fluoroquinolones in enteric fever despite the pathogen being susceptible by in vitro laboratory results, led to repeated changes in Clinical and Laboratory Standard Institute (CLSI) guidelines for this class of antibiotics to have specific and sensitive interpretative criteria. In 2015, CLSI added pefloxacin disk diffusion criteria as a surrogate marker for fluoroquinolone susceptibility. This study was carried out to evaluate the use of pefloxacin as a surrogate marker for ciprofloxacin, ofloxacin and levofloxacin susceptibility in clinical isolates of Salmonella Typhi and S. Paratyphi A.
Methods:
A total of 412 strains of S. Typhi and S. Paratyphi A were studied for pefloxacin disk diffusion test as a surrogate marker for susceptibility to ciprofloxacin, ofloxacin and levofloxacin as per CLSI and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. Molecular mechanisms of resistance to fluoroquinolones were also determined and correlated with pefloxacin susceptibility breakpoints.
Results:
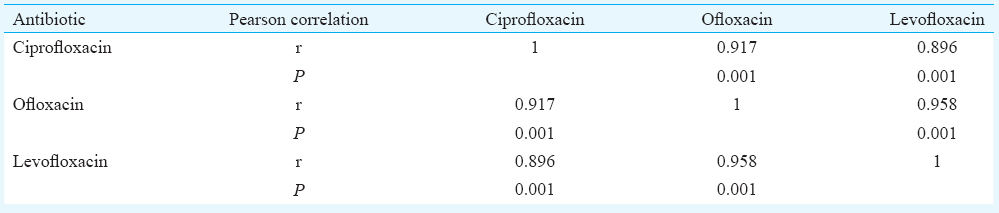
Of the total 412 strains, 34 were susceptible to ciprofloxacin and 33 each to levofloxacin and ofloxacin using CLSI minimum inhibitory concentration (MIC) breakpoints. There was a positive correlation between MICs with correlation coefficients 0.917, 0.896 and 0.958 for the association between ciprofloxacin and ofloxacin, ciprofloxacin and levofloxacin and ofloxacin and levofloxacin, respectively (P <0.001). The sensitivity, specificity and positive predictive value of pefloxacin as a surrogate marker using ciprofloxacin MIC as a gold standard were 100, 99.5 and 94.4 per cent, while 100, 99.2 and 91.7 per cent taking ofloxacin and levofloxacin MIC as gold standard. Mutations in target genes correlated with the pefloxacin susceptibility results.
Interpretation & conclusions:
Our results showed that pefloxacin served as a good surrogate marker for the detection of susceptibility to ciprofloxacin, ofloxacin and levofloxacin in S. Typhi and S. Paratyphi A. Further studies are required to confirm these findings.
Keywords
Antimicrobial resistance
ciprofloxacin
fluoroquinolones
pefloxacin
Salmonella
surrogate marker
Despite the availability of vaccines and antimicrobial agents, enteric fever causes high morbidity and mortality and accounts for 11.9 million typhoid fever illnesses and 129,000 deaths in developing countries12. The treatment continues to pose major therapeutic challenge due to the emergence of antimicrobial resistance in Salmonella enterica serovars Typhi and Paratyphi A, two major pathogens causing enteric fever3. For multiple drug resistant strains, which were resistant to first-line drugs i.e. chloramphenicol, amoxicillin and co-trimoxazole, ciprofloxacin became the first line of treatment in enteric fever4. However, soon after, the reports of clinical failure to ciprofloxacin therapy started to appear even when the laboratory reports interpreted the strain as susceptible using the Clinical and Laboratory Standard Institute (CLSI) guidelines at that time56. The guidelines being used at that time had same interpretative criteria for all members of Enterobacteriaceae. It was reported in a few cases that despite adequate ciprofloxacin treatment the infection relapsed and the subsequent culture during relapse had a higher minimum inhibitory concentration (MIC) to ciprofloxacin, though still sensitive as per the CLSI criteria. Nalidixic acid, the first-generation quinolone correlated with clinical failure to ciprofloxacin7 and was recommended as a surrogate marker for extraintestinal salmonellae by CLSI in 20098. Later as more data were available, CLSI revised ciprofloxacin breakpoints in 2012 for typhoidal salmonellae9 and subsequently added other quinolones – ofloxacin and levofloxacin in 201310. In 2015, pefloxacin was added as a surrogate marker for fluoroquinolones susceptibility11. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) has recommended the use of pefloxacin disk diffusion as a surrogate marker to determine the susceptibility for ciprofloxacin but does not have a recommendation for ofloxacin and levofloxacin12.
Quinolones act on the DNA gyrase and topoisomerase enzymes, inhibit replication and transcription activities and cause fragmentation of DNA13. The mechanism of resistance to this class of antibiotics is most commonly associated with the mutations in quinolone resistance determining region (QRDR) of target genes gyrA, gyrB, parC and parE14. The isolates with a single mutation in any of the target enzyme show reduced susceptibility to fluoroquinolones, and further accumulation of mutations results in double or triple mutant isolates which have higher MIC to quinolones due to the cumulative effect of mutations15. Other mechanisms of resistance are the presence of plasmid-mediated qnr genes and overexpression of efflux pumps but are rarely present in typhoidal salmonellae16.
These complex and multiple mechanisms impart resistance and their interplay may be the reason responsible for discordance between the clinical and laboratory results. A single mechanism cannot be specifically attributed to increase in MIC and clinical failure. The advantage of fluoroquinolones is their bactericidal activity, better tolerance and ease of administration. As guidelines for ciprofloxacin for susceptibility in S. Typhi and S. Paratyphi A are being revised frequently and levofloxacin and ofloxacin recommendations are available as MIC breakpoints only, it is ideal to have a surrogate marker which can be used to determine the resistance on the basis of simple disk diffusion method that can be done in a routine clinical microbiology laboratory in low resource setting also.
The present study was carried out to evaluate the present CLSI guidelines11 in clinical isolates of S. Typhi and S. Paratyphi A from India to determine the use of pefloxacin as a surrogate marker in these isolates.
Material & Methods
A total number of 412 S. Typhi and S. Paratyphi A strains isolated from the enteric fever patients were included in the study. Strains were collected from two centres; 352 strains (303 S. Typhi and 49 S. Paratyphi A) were obtained from All India Institute of Medical Sciences (AIIMS), New Delhi. This included all the strains isolated during 1993-2015 available from archived collection of cryopreserved strains in the department of Microbiology, AIIMS. Sixty strains isolated from Christian Medical College, Vellore, isolated during 2010-2013 were also included. The study was approved by the Institutional Ethical Committees of the participating institutes.
All strains were obtained from blood cultures from the patients suspected to have enteric fever and were preserved as glycerol stocks at -70°C. The stocks were revived and reconfirmed by standard biochemical methods comprising motility, citrate utilization, glucose fermentation, H2S production, dulcitol fermentation and decarboxylase reaction17. The identification was further confirmed by slide agglutination test with specific antisera (Statens Serum Institute, Copenhagen, Denmark).
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was done using Kirby-Bauer disk diffusion method18 for nalidixic acid (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), ofloxacin (5 μg) and pefloxacin (5 μg) (Oxoid Ltd., United Kingdom)11. MIC was determined for ciprofloxacin, ofloxacin and levofloxacin with E-test as per manufacturer's guidelines (BioMerieux, France). Susceptibility interpretation was done using CLSI guidelines11. For result interpretation, intermediate and resistant strains were considered together as resistant. Escherichia coli ATCC 25922 was used as a quality control strain for disk diffusion and MIC determination.
Sequencing of topoisomerase genes
QRDR of gyrA, gyrB, parC and parE genes were sequenced using previous primers as standardized in our laboratory and determined the relation between mutations to these genes and pefloxacin zone diameters1415.
Statistical analysis
Antimicrobial susceptibility data were analyzed in STATA (Version 11.1, Stata Corp., College Station, Texas, USA). Non-parametric test for trend was performed to determine the temporal patterns of MIC to ciprofloxacin, ofloxacin and levofloxacin. Correlation analysis was done to find out the relationship between MIC trends of ciprofloxacin, levofloxacin and ofloxacin. Correlation coefficients of zone diameter for pefloxacin, ciprofloxacin, ofloxacin, levofloxacin and nalidixic acid were also determined.
Sensitivity, specificity and positive predictive value were determined for the use of pefloxacin disk diffusion as a surrogate marker for susceptibility to ciprofloxacin, ofloxacin and levofloxacin using their respective MICs as gold standard and pefloxacin disk diffusion result was used as test.
Results
Of the 412 isolates, only 34 (8.25%) were susceptible to ciprofloxacin. Ciprofloxacin susceptibility percentage was also compared between CLSI 2015 and CLSI 2011 i.e. before the revision of breakpoints. As per the earlier interpretation criteria 329 (80.03%) isolates were susceptible to ciprofloxacin, but with revised criteria, only 34 (8.25%) remained susceptible while 309 (75%) showed decreased susceptibility and 69 (16.75%) were resistant. For ofloxacin, 33 (8%) isolates were susceptible, 309 (75%) were intermediate and 70 (16.99%) were resistant. Similarly for levofloxacin, susceptible, intermediate and resistant isolates were 33 (8%), 307 (74.5%) and 72 (17.47%), respectively. MIC50 and MIC90 values were found to be 0.38 and 8 μg/ml for ciprofloxacin, 1 and 24 μg/ml for ofloxacin and 1.5 and 32 μg/ml for levofloxacin, respectively.
MIC values of ciprofloxacin, ofloxacin and levofloxacin showed a definite increase over time which was significant (P <0.001, P <0.003 and P <0.021, respectively). There was a positive correlation between MIC to ciprofloxacin and ofloxacin having correlation coefficient 0.917 (P <0.001), and also between ciprofloxacin and levofloxacin and between levofloxacin and ofloxacin (Table I). Correlation coefficients of zone diameter for ciprofloxacin, ofloxacin, levofloxacin, pefloxacin and nalidixic acid are given in Table II. Scatter plot analysis was done to find out corresponding disk diffusion zones of ciprofloxacin and pefloxacin. The breakpoints for susceptible zones of inhibition for pefloxacin and ciprofloxacin corresponded to each other (Fig. 1).


- Scatter plot analysis to find out corresponding disk diffusion zones of pefloxacin and ciprofloxacin. ZOI, zone of inhibition.
Ciprofloxacin susceptible isolates showed a zone of inhibition from 25 to 32 mm using 5 μg pefloxacin disks. However, for pefloxacin susceptible isolates, MIC of ciprofloxacin ranged from 0.002 to 0.38 μg/ml and for pefloxacin resistant isolates MIC range was between 0.125 to 32 μg/ml (Fig. 2).

- Frequency histogram showing segregated minimum inhibitory concentration (MIC) distribution of ciprofloxacin for pefloxacin susceptible and resistant Salmonella strains. Blue bar represents pefloxacin susceptible strains which have ciprofloxacin MIC range from 0.002 to 0.38 μg/ml while black bars represent pefloxacin resistant strains having ciprofloxacin MIC range between 0.125 to 32 μg/ml.
Pefloxacin zone diameters and fluoroquinolones MIC results were compared to evaluate the predictive efficacy of pefloxacin as a surrogate marker for fluoroquinolones susceptibility. Of the total 412 isolates tested for pefloxacin susceptibility, 36 were sensitive to pefloxacin, and 376 were resistant. All pefloxacin resistant isolates were resistant to ciprofloxacin, ofloxacin and levofloxacin. Among 36 pefloxacin susceptible isolates, 34 were susceptible to ciprofloxacin, and two were intermediate, while 33 were susceptible to ofloxacin and levofloxacin and three were intermediate to these drugs.
The sensitivity, specificity and positive predictive values of pefloxacin as a surrogate marker using ciprofloxacin MIC as a gold standard were 100, 99.5 and 94.4 per cent, respectively. Similarly, the sensitivity, specificity and positive predictive values of pefloxacin as a surrogate marker using ofloxacin and levofloxacin MIC were 100, 99.2 and 91.7 per cent, respectively.
Mechanisms of resistance
Sequence analysis of gyrA, gyrB, parC and parE revealed the presence of mutations in gyrA and parC genes, but no mutation was detected in gyrB or parE genes. MIC and zone diameter range of mutants and wild type isolates is given in Table III. All isolates with pefloxacin zone of inhibitions ≥24 mm were wild type i.e. did not possess any mutation in QRDR of DNA gyrase or topoisomerase genes.

When compared the susceptibility percentage of two serovars it was observed that fluoroquinolones resistance was significantly higher in S. Paratyphi A. All S. Paratyphi A isolates were in resistance or intermediate category i.e. fluoroquinolone susceptibility in S. Paratyphi A serovar was 0 per cent as per CLSI 201511.
Discussion
Emerging resistance to antityphoidal drugs has been regularly reported from India19. Though there has been a decrease in the prevalence of chloramphenicol resistant strains, probably due to the decreased use of chloramphenicol, but an increase in resistance to ciprofloxacin has also been observed from India2021. Following the ciprofloxacin resistance, ceftriaxone became the drug of choice to treat enteric fever2223 but the reports on clinical failure to ceftriaxone started emerging from Asian region24.
Fluoroquinolones have good oral absorption, are bactericidal and are well tolerated. CLSI published evidence-based revision of ciprofloxacin MIC and disk diffusion interpretative criteria in 2012 where susceptibility breakpoints for MIC value has been lowered from ≤1 to ≤0.06 μg/ml, and zone diameter has been increased from ≥21 to ≥31 mm9. Studies have shown that the change in CLSI recommendation for ciprofloxacin breakpoints has a significant impact on whether an isolate is classified as susceptible, intermediate or resistant2526. Subsequently, in 2013, ofloxacin and levofloxacin MIC interpretative criteria were included as ≤0.12 μg/ml for susceptible isolates10, followed by the recommendation of the use of pefloxacin as a surrogate marker for fluoroquinolones susceptibility. Pefloxacin showed a sensitivity and specificity of 100 and 99.5 per cent respectively, with a positive predictive value of 94.4 per cent for ciprofloxacin susceptibility. All pefloxacin susceptible isolates were also susceptible to ciprofloxacin except two isolates having intermediate MIC to ciprofloxacin which were susceptible according to pefloxacin disk diffusion test. These variations need to be studied in a larger number of strains across the world.
EUCAST has also recommended the use of pefloxacin as a marker for ciprofloxacin susceptibility12, but has no recommendation for ofloxacin and levofloxacin susceptibility. For ciprofloxacin, there are only susceptible and resistant categories which are a better method of reporting according to EUCAST as it avoids the confusion in the clinical decision-making with the intermediate category. In the present study, all intermediate strains fell into resistant category according to surrogate marker interpretation.
It has been shown that pefloxacin disk diffusion provides a better separation for ciprofloxacin susceptibility than any other disk diffusion, even better than ciprofloxacin itself2728. In addition, levofloxacin and ofloxacin have only MIC breakpoints and do not have disk diffusion criteria, which makes pefloxacin testing by disk diffusion a convenient method in resource poor settings where MIC determination is not possible2930. A linear increase was observed in MIC to fluoroquinolones with time. The resistance percentage was higher in S. Paratyphi A than S. Typhi. Based on the molecular mechanism of resistance comparing pefloxacin susceptibility with mutations in the QRDR region of DNA gyrase, it was possible to segregate sensitive wild type from resistant mutant.
A word of caution is that while reporting the susceptibility results for fluoroquinolones in a culture positive enteric fever, pefloxacin should not be reported as it is a surrogate marker only and is not used in clinical practice.
The main limitation of the study was that the strains studied were from hospitals representing two regions of India only. There is a need to generate a larger community-based data to determine the concordance between the interpretation of surrogate marker and clinical outcomes.
Acknowledgment
The study was financially supported by the Indian Council of Medical Research (ICMR), New Delhi, India.
Conflicts of Interest: None.
References
- Burden of typhoid fever in low-income and middle-income countries: A systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2:e570-80.
- [Google Scholar]
- Antibiogram pattern and seasonality of Salmonella serotypes in a North Indian tertiary care hospital. Epidemiol Infect. 2006;134:961-6.
- [Google Scholar]
- Clinical outcomes in typhoid fever: Adverse impact of infection with nalidixic acid-resistant Salmonella typhi. BMC Infect Dis. 2005;5:37.
- [Google Scholar]
- Nalidixic acid susceptibility test to screen ciprofloxacin resistance in Salmonella typhi. Indian J Med Res. 2002;115:49-54.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial susceptibility testing; 19th informational supplement, CLSI Document M100-19. Wayne, PA: CLSI; 2009.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial susceptibility testing; 22nd informational supplement, CLSI Document M100-22. Wayne, PA: CLSI; 2012.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial susceptibility testing; 23rd informational supplement, CLSI Document M100-23. Wayne, PA: CLSI; 2013.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial susceptibility testing; 25th informational supplement, CLSI Document M100-25. Wayne, PA: CLSI; 2015.
- [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. 2014. Breakpoint tables for interpretation of MICs and Zone Diameters. Version 4.0. Available from: http://www.eucast.org/clinical_breakpoints/
- [Google Scholar]
- Structure based in silico analysis of quinolone resistance in clinical isolates of Salmonella typhi from India. PLoS One. 2015;10:e0126560.
- [Google Scholar]
- Reduced susceptibility to ciprofloxacin and gyrA gene mutation in North Indian strains of Salmonella enterica serotype typhi and serotype Paratyphi A. Microb Drug Resist. 2004;10:146-53.
- [Google Scholar]
- Induction of resistant mutants of Salmonella enterica serotype Typhi under ciprofloxacin selective pressure. Indian J Med Res. 2014;139:746-53.
- [Google Scholar]
- Study of the role of efflux pump in ciprofloxacin resistance in Salmonella enterica serotype Typhi. Indian J Med Microbiol. 2013;31:374-8.
- [Google Scholar]
- Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, eds. Mackie & McCartney practical medical microbiology. London: Churchill Livingstone; 1996. p. :131-49.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial disk susceptibility tests; Approved standards, CLSI Document M02-A12 (12th ed). Wayne, PA: CLSI; 2015.
- [Google Scholar]
- Occurrence & antibiogram of Salmonella Typhi & S. Paratyphi A isolated from Rourkela, Orissa. Indian J Med Res. 2011;133:431-3.
- [Google Scholar]
- Re-emergence of chloramphenicol-sensitive Salmonella Typhi. Lancet. 1999;353:1241-2.
- [Google Scholar]
- Emergence of fluoroquinolone resistance in Salmonella enterica serovar Typhi in Andaman and Nicobar Islands, India. Indian J Med Res. 2012;136:98-101.
- [Google Scholar]
- Change in antimicrobial resistance pattern of Salmonella Typhi in central India. Indian J Med Res. 2002;115:248-50.
- [Google Scholar]
- Antimicrobial susceptibility of Salmonella enterica serovars in a tertiary care hospital in Southern India. Indian J Med Res. 2013;137:800-2.
- [Google Scholar]
- ACC-1 beta-Lactamase-producing Salmonella enterica serovar Typhi, India. Emerg Infect Dis. 2010;16:1170-1.
- [Google Scholar]
- Revised ciprofloxacin breakpoints for Salmonella Typhi : Its implications in India. Indian J Med Microbiol. 2014;32:161-3.
- [Google Scholar]
- Pitfalls of interpreting ciprofloxacin minimum inhibitory concentrations in Salmonella enterica serovar Typhi. Indian J Med Res. 2012;136:884.
- [Google Scholar]
- Development of a pefloxacin disk diffusion method for detection of fluoroquinolone-resistant Salmonella enterica. J Clin Microbiol. 2015;53:3411-7.
- [Google Scholar]
- Evaluation of surrogate disk tests for detection of ciprofloxacin and levofloxacin resistance in clinical isolates of Salmonella enterica. J Clin Microbiol. 2015;53:3405-10.
- [Google Scholar]
- Performance of E-test and disk diffusion for detection of ciprofloxacin and levofloxacin resistance in Salmonella enterica. J Clin Microbiol. 2015;53:298-301.
- [Google Scholar]
- Fluoroquinolone susceptibility testing of Salmonella enterica: Detection of acquired resistance and selection of zone diameter breakpoints for levofloxacin and ofloxacin. J Clin Microbiol. 2014;52:877-84.
- [Google Scholar]






