Translate this page into:
Pasteurization of bone for tumour eradication prior to reimplantation – An in vitro & pre-clinical efficacy study
Reprint requests: Prof. Ajay Puri, Orthopaedic Oncologist, Tata Memorial Centre, Parel, Mumbai 400 012, India e-mail: docpuri@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
In current era of limb-salvage therapy, pasteurization of bone sarcomas is receiving growing attention as a potential extracorporeal treatment and cost-effective alternative to allografts and radiation before surgical reimplantation. Detailed in vitro and in vivo pre-clinical study to evaluate efficacy of pasteurization to eradicate malignant cells has not been reported yet. The present study was carried out to assess the efficacy of pasteurization to kill tumour cells both in vitro and in vivo.
Methods:
Surgically resected specimens of osteosarcomas (n=4) were cut into equal halves and one section was pasteurized by heating at 60°C to 65°C for 40 min. Paired samples before and after pasteurization were studied in vitro for DNA ploidy, evaluation of histological change and elimination of mitotic activity. These tissues were transplanted in immune-deficient NOD-SCID mice to evaluate effect on tumour-generating ability, presence of human nuclei, osteopontin and cytokine/chemokines released in tumour-transplanted mice.
Results:
Non-pasteurized tumour samples had viable tumour cells which exhibited significant growth in culture, increased proliferative ability and clonogenic potential while respective pasteurized tumour tissues did not grow in culture and did not exhibit clonogenicity. Flow cytometry revealed that propidium iodide positive dead cells increased significantly (P< 0.01) post pasteurization. Seven of 12 non-pasteurized tumour transplanted mice demonstrated tumour-forming ability as against 0 of 12 in pasteurized tumour transplanted mice. Solid tumour xenografts exhibited strong expression of anti-human nuclei and osteopontin by immunohistochemistry as well as secretary human interluekin-6 (IL-6) while pasteurized mice failed to express these markers.
Interpretation & conclusions:
This study has provided a basis to establish pasteurization as being efficacious in ensuring tumour eradication from resected bone tumour specimens. Pasteurized tumour bearing bone can thus safely be used to reconstruct large defects after tumour resection.
Keywords
Bone
extracorporeal treatment
pasteurization
reimplantation
tumour eradication
Limb-sparing modalities are the standard of care today for patients with resectable primary tumours123. Various methods using autografts, allografts, vascularized fibula, etc. have been described to reconstruct these defects4. Allografting using processed bone from the tissue bank has problems of immunologic response, high risk of disease transmission and high incidence of graft fracture. It takes a long time to incorporate due to denaturing of the osteoinductive proteins in the processing. Using a vascularized fibula involves skilled microsurgical setup and long duration surgery lasting generally 10-12 h. An option which can be used universally without much expense is, therefore, needed.
Immediate reimplantation of resected bone in surgical treatment for cancer has been done after extracorporeal treatment such as heating, freezing, or irradiation to devitalize the tumour-infiltrated segment. These procedures besides weakening the bone also destroy the osteoinductive bone-morphogenic proteins resulting in delayed incorporation as well as risk of fracture1567. Irradiation sterilization requires the specimen to be taken out of the operating theatre to a facility where radiation is available which prolongs the surgical time. Irradiated bone which is subjected to 50 Gy, is biomechanically inferior to normal bone. Radiation facilities are available only in a few centres in India. This increases the significance of pasteurisation which can be carried out without the need for extensive infrastructure facilities.
Studies and clinical applications of hyperthermia-treated bone proved the superiority of pasteurization of bone8910. The superiority depends on the lethal effect of pasteurization on malignant cells while preserving the bone-inducing property. Urist and Iwata5 showed induction of bone by the response of the mesenchymal cells in the recipient bed to bone morphogenetic protein (BMP) transferred from the bone implant. They also showed that in thermal exposures greater than 70°C, or irradiation sterilization, the biologic activity of BMP was completely destroyed. The pasteurized autograft is a simple and easily accessible method in limb salvage, provides best fit to the host and is an economic alternative to allograft, with no risk of disease transmission and possibly lower rates of ultimate failure and graft-related complications1112. The pasteurized bone has a wide application in oncology practice as well as in trauma management.
Unlike earlier clinical studies which14691113 have demonstrated the surgical outcomes of pasteurized bone, the present study focused on demonstrating the efficacy of pasteurization in tumour eradication both in vitro and in vivo. Our pre-clinical in vitro/in vivo study highlights the various pathways in which pasteurization affects tumour cells. This study will also serve as a pilot to validate the efficacy of pasteurisation as an effective method of tumour-kill before undertaking its actual clinical use.
Material & Methods
Patients and tissue samples: Samples from four high grade osteosarcoma patients who had been treated by neo adjuvant chemotherapy prior to amputation were collected from the Department of Orthopedic Oncology, Tata Memorial Hospital, Mumbai, India, during December 2010 to June 2011. The affected regions included one right proximal tibia, two distal ends of left femur and one distal end of right femur. The study protocol was approved by the Institutional Human Ethics Committee. Written informed consent was obtained from all patients. Surgically resected samples were cut into equal halves to have matched pairs of specimens for pathology and laboratory (in vitro/in vivo) study, before and after pasteurization treatment. One section was treated in water bath containing sterile water at 60-65°C for 40 min. This sample was further tested as pasteurized sample. The other sample was used for further testing as non-pasteurized sample.
Histological and immunohistochemical analysis: Human tissues before and after pasteurization were fixed in 10 per cent buffered formalin and paraffin blocks were prepared. Histology was performed on 5 µm thick sections of paraffin blocks using routine haematoxylin and eosin staining to confirm the presence of viable osteosarcoma cells and evaluate the per cent necrosis.
Cell culture studies: Surgically resected tumour specimens collected before and after treatment were processed and cultured for outgrowth of proliferative cells. The cells were cultured at 37°C in a humidified atmosphere of a 5 per cent CO2 in air, in 25 cm2 flasks containing 5 ml of Iscoves’ Modified Dulbecco's Medium (IMDM; Invitrogen, USA) supplemented with 10 per cent foetal calf serum (FCS; Invitrogen, USA), 2 mM L-glutamine (Sigma, USA), 100 IU/ml penicillin and 50 μg/ml streptomycin. The medium was changed every third day and, for sub-culture, the cell monolayer was washed twice with phosphate-buffered saline (PBS, 0.01M, pH 7.5) and incubated with trypsin-EDTA solution (0.25% trypsin, 1 mM EDTA; Stem Cell Technologies, Vancouver, Canada) for 10 min at 37°C to detach the cells.
Cell proliferation assay: The mitotic potential of cells obtained from non-pasteurized and pasteurized tissues was measured by a 3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma). Cells (5000 or 10000/well) were seeded into 96-well flat-bottom culture plates. Cells were allowed to adhere for 24 h and then 20 μ1 MTT (5 mg/ml) was added to each well, and the cells were incubated at 37°C for additional 4 h. The reaction was stopped by lysing the cells with 150 μl DMSO for 5 min. Optical densities of supernatants were determined on a microplate reader (Biotek FL-600, Bio-Tek, Winooski, VT) at 540 nm.
Cell cycle analysis: Osteosarcoma tumour tissues collected before and after pasteurization were minced to obtain single cell suspension. Cells were washed, and fixed in 70 per cent chilled ethanol. Cells were washed with PBS and were suspended in 500 µl of 1×PBS and incubated for 30 min at 37°C in dark in presence of 40 µg of propidium iodide (PI, Sigma, USA) and 100 µg of RNAase (Sigma). Subsequently, a single cell suspension was obtained by passing it through a 1ml syringe. Lymphocytes obtained from 5 ml peripheral blood of healthy individual were treated similarly and served as normal diploid control. For acquisition gates were set using normal lymphocytes and then tumour samples were acquired on a FACSCalibur (Becton Dickinson, USA). Samples were analysed using ModFit software (Becton Dickinson, USA).
Cell viability by propidium iodide staining: Cells obtained from tissues before and after pasteurization were stained with 5 µl of 400 µg/ml of propidium iodide for 30 min and cells were acquired on FACSCalibur. Samples were analysed using CellQuest software (Becton Dickinson, USA) for per cent population exhibiting PI staining positivity.
Clonogenic assay: Colony forming or clonogenic potential of fresh tumours and pasteurized tumour specimens was analyzed in soft agar colony assay. Single cell suspensions were prepared by mincing tissue from the solid tumour and the cells were plated out in 0-3 per cent agar over a layer of 0-5 per cent agar in 30 mm Petri dishes. Cells were grown for 7 to 10 days in incubator at 37° C with humidified atmosphere and 5 per cent CO2 in air. Colonies were observed under inverted light microscope Carl Zeiss, Germany.
Evaluation of tumorigenic potential: All procedures involving animals were done as per guidelines approved by the Institutional Animal Ethics Committee. NOD/LtSZ-SCID/SCID mice (obtained from Jackson Laboratory, Bar Harbor, ME, USA) at 6 to 8 wk of age were transplanted with 6mm x 6mm x 6mm piece fresh osteosarcoma tissue subcutaneously (s.c.). The procedure was performed under general anaesthesia obtained with intraperitoneal injection of ketamin (35 mg/kg body wt) and xylazin (5 mg/kg body wt). The surgical incision was closed using tissue adhesive. To prevent the loss of data due to animal mortality, three mice were assigned to each group (non-pasteurized tumour tissue, n=3; pasteurized tumour tissue, n=3 per human sample). The animals were randomized and used for transplantation of tumour fragments from surgical samples prior and post pasteurization.
The animals were housed for 3 months during which they were monitored at regular intervals for health and body weight. Size of tumours formed was measured using Vernier caliper. These animals were, sacrificed and lung and xenograft tumour were collected for analysis of presence of human cells/DNA. Non-transplanted healthy mice served as normal control. Their lung wet weight and body weight were measured.
Histological and immunohistochemical analysis: Presence of human nuclei and human bone-specific protein osteopontin was analysed in tumour and lung tissues of mice to check for development of local or metastatic human tumour. Mouse lung / xenograft tumour specimens collected after sacrificing animals, were formalin fixed and paraffin blocks were prepared. Histology was performed on 5 µm thick sections of paraffin blocks using routine haematoxylin and eosin staining to confirm the presence of osteosarcoma cells. Immunohistochemistry was performed on deparaffinzed sections by using monoclonal anti-human nuclei antibody (clone 3E1.3; Millipore Corporation, USA), anti-osteopontin antibody (Clone 190312; R & D Systems Inc.; USA) and anti-vimentin antibody (Sigma Inc, MO, USA) to assess functional activity of bone cells metastasized to lung. This was followed by standard streptavidin biotin complex protocol for the localization of antigen-antibody complex14.
Cytokine bead assay for intracellular cytokines using flow cytometry: The use of serum inflammatory markers in the evaluation of the inflammatory response has been shown to be clinically useful and sensitive in prediction of potential for fracture or bone injury. Serum samples from mice were tested for human pro-inflammatory cytokine interleukin-6 (IL-6) and mouse inflammatory chemokine KC/ IL-8 using Cytokine Flex Kit by Cytometric Bead Assay as per the manufacturer's instructions (BD BioSciences, USA). Stained samples were acquired on FACSAria (BD BioSciences). Data were analysed as indicated by the manufacturer (FlowCytomixPro version 2.2, USA). Data were represented in pg/ml of cytokines.
Assessment of DNA concentration, purity, and quality: Total DNA was isolated from cells or tissue samples using the DNA isolation Kit (Qiagen, Germany) according to the manufacturer's instructions. The concentration of DNA was determined by measurement of absorbance at 260 nm (A260) using Nanodrop spectrophotometer (Thermoscientific, USA). Only samples with A260:A280 ratio > 1.8 were regarded as satisfactorily pure. The quality of DNA was also evaluated by gel electrophoresis.
Quantitative real-time-PCR: Quantitative real-time PCR assay (RQ-PCR) was performed to detect RNAse P expression that was used to detect presence of human DNA in mouse xenograft and lung tissues. The TaqMan master mix and RNAse P primers were obtained from Applied BioSystems (assay id - Hs02696070_cn). RQ-PCR was done on an ABI 7900HT Sequence Detection System (Applied Biosystems, Japan) under the following conditions: for amplification 50°C for 2 min, for initial denaturation 95°C for 10 min then 40 cycles of 95°C for 30 sec, and 60°C for 1 min for annealing. The gene expression CT value from each sample was plotted.
Statistical analysis: Results were expressed as mean ± S.E. Statistical analysis was performed using SPSS software for Windows system (SPSS, Inc. Chicago, USA).
Results
In vitro evaluation of efficacy of pasteurization
Histopathology data of human tissues: Paired samples from before and after pasteurization of widely resected specimens of bone sarcomas (n=4) were studied for the histological change caused by pasteurization (Table I). These cases were previously treated with chemotherapy hence showed some baseline necrosis. Histopathology demonstrated that in sample No. 1 and 5 necrosis due to chemotherapy was low which increased significantly post-pasteurization. While necrosis observed in sample Nos. 3 and 4 was high post-chemotherapy prior to surgery and exhibited minor change after pasteurization (Table I).
Cell viability assay: Efficacy of pasteurization on cell viability of tissue sample before and after pasteurization was evaluated by staining cells with PI and acquiring cells on flow cytometer. Fig. 1A and Table I exhibit that per cent PI+ dead cell population increased significantly post-pasteurization (non-pasteurized tumour: 15.04 ± 5.9%; pasteurized tumour: 75.3 ± 5.9 % ; P< 0.01).

- Efficiency of pasteurization in killing tumour cells in osteosarcoma tumour tissue by in vitro assays. Panel A depicts per cent PI+ dead cells in osteosarcoma human tumour. The thin lines exhibit increase in PI+ dead cells (before and after pasteurization) in four samples studied while, thick line demonstrates mean per cent PI+ cells. Mean value ± standard error (S.E.) is given for both non-pasteurized and pasteurized tumour. Statistical significance by Student's t test is given as p=0.001. Panel B exhibits effect of pasteurization on cell culture and growth of tumours from sample no. 1 (a, b) and sample no. 3 (c,d) as observed by inverted phase contrast light microscopy. Non-pasteurized samples (a, c) gave good cell growth in culture (d 17) while pasteurization completely abrogated cell growth upon culture (b, d). All images were taken at magnification 10X.
Cell culture assay: Tissue samples were processed to make single cell suspension and cultured in complete media. Cell growth was monitored for 1-2 months. Non-pasteurized tissues of all tumour samples gave primary cell cultures whereas pasteurized cells failed to show any viable cells upon long-term culture (Table I; Fig. 1B).
Cell proliferation assay: Effect of pasteurization on reduction in metabolic activity which is a measure of reduction in viability was analyzed by MTT assay. It was observed that the metabolic activity of cells was greatly affected post-pasteurization in individual samples (Table I, P< 0.001). The ‘Paired t Test’ applied to all four samples demonstrated that pasteurization efficiently inhibited tumour cell proliferation (P< 0.05).
DNA ploidy analysis: Single cell suspension from tissues was analysed for ploidy status by PI and flow cytometry. Ploidy study exhibited that 2 of 4 samples (Sample Nos. 1 and 3) had diploid as well as aneuploid population. In these cases diploid population increased while aneuploid population reduced significantly after pasteurization treatment (Table II, Fig. 2). In the remaining two samples (sample nos. 4 and 5) which exhibited 100 per cent near-diploid cells and no aneuploid population, pasteurization maintained diploid status. It was observed that apoptotic cell per cent increased after pasteurization (Table II) in three of four samples studied. In sample no. 4 level of apoptosis was unaffetcted post-pasteurization.

- Effect of pasteurization on DNA ploidy of cell populations. This figure demonstrates data of sample no.1. In histograms of cell cycle phases before (A) and after (C) pasteurization, red peaks (A,C) show G1 phase of diploid population, while yellow peak (A) shows G1 phase of anueploid population. The peacock blue peak (C) shows subG0 i.e. apoptotic population. Panels B (before pasteurization) and D (after pasteurization) indicate dot plot of cell populations in their various cell cycle phases. Rightmost values demonstrate the per cent apoptotic cells, diploid population and anueploid population.
Clonogenic potential by soft-agar colony assay: Clonogenic assay was standardized using MG63 cells in soft agar colony assay. MG63 cells demonstrated colony forming ability (Data not shown). In two of four cases, non-pasteurized tumour cells demonstrated viable cells and colonies post 10 days while respective treated cells exhibited clumps of dead cells (Fig. 3). Other two non-pasteurized samples failed to give colonies in soft agar assay.

- Clonogenic assay. The panels exhibit colony forming ability of non-pasteurized (A) and pasteurized (B) sample of patient no. 5 on soft agar plate. The images were clicked using inverted phase contrast microscope. The magnification was 10x for each panel.
In vivo pre-clinical evaluation of efficacy of pasteurization
Effect on tumorigenicity of osteosarcoma tissues in mice models: The tumorigenic potential of non-pasteurized and pasteurized tumours (n=4) to form xenografts was analysed by transplantation in NOD-SCID mice as part of pre-clinical study. Mice transplanted with 5-6 mm pieces of osteosarcoma tumour before and after pasteurization treatment, were observed for body weight and xenograft tumour formation upto 90 days. Ratio of lung wet weight to body weight was found to be reduced in mice transplanted with non-pasteurized tumour compared to that observed in mice transplanted with pasteurized tumour or normal control mice. However, the difference was not significant (data not shown).
Non-transplanted control mice did not show subcutaneous tumour growth. In three of four sample sets, non-pasteurized tumours demonstrated solid tumour xenograft (7/12 mice) forming ability while pasteurization treatment successfully hampered the tumour forming ability (0/12 mice) of these tissues. Fig. 4A shows tumorigenic potential of non-pasteurized tumour in representative sample no. 1 while pasteurized counterpart of the same tissue failed to give tumours in mice at day 87 (Fig. 4B). Xenograft tumours from two mice (sample no. 1) were maintained as xenograft passage in mice upto P3 passage.

- Effect of pasteurization on tumorigenic potential of bone tumors by in vivo pre-clinical assays. The data are of representative sample no. 1. (A) Top panel shows mice at 7-day post transplantation of non-pasteurized tumour pieces of 6-8 mm in size. Lower panel shows mice at day 87 post-transplantation. Two of the mice developed xenograft solid tumour which were characterized and also passaged subsequently for next 4 passages (B) The panels show loss of tumorigenicity of osteosarcoma tumour after pasteurization. Upper subpanels show mice at day 7 while lower panels show mice at day of sacrifice i.e. day 90.
Immunohistochemistry for human markers on mouse tissues: Immunofluorescence experiments exhibited that primary culture developed from one of the osteosarcoma samples demonstrated positive staining for human nuclei and bone-specific protein osteopontin and vimentin markers. Anti-human nuclei demonstrated nuclear staining while osteopontin demonstrated nuclear as well as cytoplasmic staining. Immunohistochemistry experiments demonstrated that mouse solid tumour xenografts were positive for human nuclei (Fig. 5B, Table III) and osteopontin (Figs. 5C, 5G; Table III). Since anti-human nuclei antibody and osteopontin antibodies were specific for human species, they did not show reactivity with mouse skin present adjacent to xenograft sections (Fig. 5F and 5G; Yellow arrows human xenograft, black arrow mouse skin). Lung sections of xenograft bearing mice failed to show presence of these markers (Table III). The data indicated that these mice did not show lung metastasis. It was observed that lung sections of pasteurized mice and non-transplanted control mice did not show reactivity to anti-human nuclei and osteopontin antibody indicating absence of human cells in mice lung tissue.

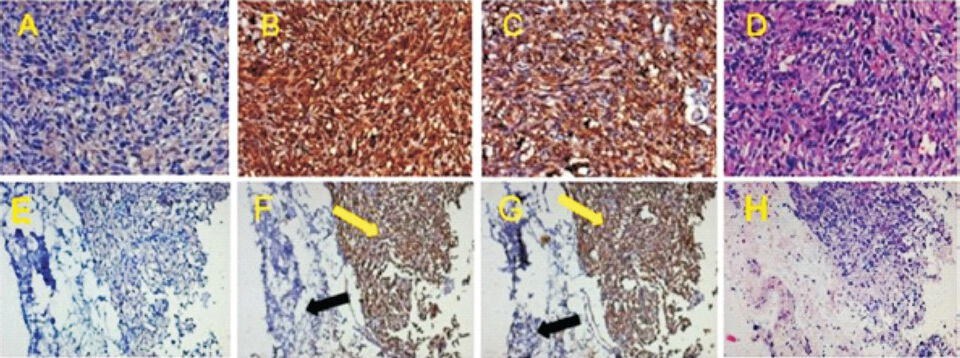
- Characterization of human xenografts in mice by immunohistochemistry for anti-human nuclei, human osteopontin and hematoxylin-eosin staining. This figure gives representative immunohistochemistry data of xenograft solid tumours from mouse 209 (panels A to D) and mouse 950 (panels E to H) transplanted with non-pasteurized tumour of sample no. 1 (tumours were obtained from n=3 human samples transplanted). PBS treated mouse paraffin-sections served as negative control (A, E). Panels (D, H) exhibit section tissue morphology by hematoxylin and eosin staining. In xenograft section with anti-human nuclei, all nuclei are strongly stained (panel B). Anti-osteopontin antibody stained nuclear as well as cytoplasmic staining in these sections (panel C). Images in A to D were at magnification 20X. Sections E to H involve some part of mouse skin as tumours were subcutaneous. Yellow arrows show strongly stained human specific nuclei (B) and osteopontin (C) and black arrows show non-stained mouse skin section. All images in E to H were at magnification 10X.
Detection of human cytokine IL-6 and mouse KC/ IL-8 in mice serum: Serum samples of tumour transplanted (pasteurized and non-pasteurized) mice and control mice were evaluated for the presence of secreted human cytokine IL-6 and mouse inflammatory chemokine KC. It was observed that serum of xenograft bearing mice (non-pasteurized tumour transplanted) of sample no. 1 exhibited presence of human IL-6 while serum of mice transplanted with pasteurized tumours and control mice failed to show human IL-6. Serum samples of mice bearing tumour xenograft from other samples (samples nos. 3, 4 and 5) did not show detectable levels of human IL-6 (Table III). Xenograft tumours from sample no. 1 could be passaged and maintained as mouse xenografts. Serum samples of these mice (P0, P1, P2 and P3) demonstrated presence of significant levels of human IL-6.
Inflammatory chemokine KC (homologue of human IL-8) was found to be increased in mice transplanted with non-pasteurized tumour (n=10; mean ± SE 9.1 ± 2.07 pg/ml) and pasteurized tumour (n=10; mean ± SE 12.46 ± 3.5 pg/ml) compared to that in healthy mice control (n=6, mean ± SE 5.6 ± 0.91 pg/ml), however, differences between all three groups were non-significant (Table III). The levels of inflammatory chemokine KC were significantly increased in serum of mice which were passages P0, P1, P2 and P3 of sample no. 1.
Detection of human house-keeping gene in mouse tissues: Real-time PCR for human DNA performed on transplanted mice organs demonstrated that solid tumour xenografts (sample nos 1 and 3) were positive for human DNA while lung tissue from respective mice did not show presence of human DNA (Table III). Sample no. 4 which had small tumours and IHC showed very small patches identified by anti-human antibody, failed to show presence of human DNA by real-time PCR. DNA from lung tissues of mice transplanted with pasteurized tumour and lungs of healthy non-transplanted control mice did not show presence of human DNA. These data corroborated with immunohistochemistry data regarding absence of lung metastasis. DNA from various passages of xenografts of sample no. 1 tumours exhibited significant amount of human DNA while their lung tissues did not show presence of human DNA except one lung tissue (P2 passage) which demonstrated very low levels of human DNA present.
Discussion
The use of autologous purged tumour bone in limb sparing surgery in lieu of allografts has exciting potential. Extracorporeal devitalization and reimplantation requires definite destruction of all tumour cells prior to reimplantation. Friedgood15 was the first to show that Walker rat sarcoma cells were killed after heat treatment at 44°C for 30 min. Later Dickson16 demonstrated that diathermy heating with radiofrequency of 13.56 MHz led to tumour destruction with minimal or no damage to surrounding normal tissues suggesting its potential use in treatment of limb tumours in humans.
Earlier published reports14691113 provide information on clinical aspects of use of pasteurized bone autograft in terms of bone regeneration, bone union, breakage, wear, infection complications, etc. They have not objectively validated the oncologic safety of pasteurisation as an efficacious method of ensuring tumour kill. Since an individual in vitro experiment has its own read-out system and addresses different aspects compared to other techniques, we have employed various techniques to confirm the potential of pasteurization to eradicate tumour.
Previous studies617 performed histopathology to primarily demonstrate bone healing and union post-reimplantation of pasteurized autograft. Our in vivo work has shown histopathology data of bone tumours before and immediately after pasteurization treatment as a measure of efficacy for tumour eradication. The differences observed in change in necrosis in all samples can be attributed to individual variation since conventional high grade osteosarcomas are very heterogenous tumours. Additionally, certain areas of tumour also undergo spontaneous necrosis in vivo. Similar variations in graft reunion data have been observed earlier417.
In our study, cell viability reduction and apoptosis induction were measured using sensitive and precise method of flow cytometry. Cell death-related PI positivity was increased significantly from 15 to 75 per cent. In surviving cells, proliferation was further dropped by 22.6 per cent. Plaat et al17 observed 57 per cent drop in mitoses and 40 per cent reduction in cell proliferation and more than double apoptosis of tumour cells post hyperthermic isolated limb perfusion with tumour necrosis factor-alpha and melphalan (HILP-TM) treatment during reunion of treated autograft. However, their samples were paraffin-embedded sections as against our live culture system which is more sensitive to variations. They hypothesized that the remnants of killed tumour cells within pasteurized tumour segment can evoke immunity in treated patient. Similar observation was also reported by Kawano et al18 that cell constituents remaining after liquid nitrogen-treated bone tumour could stimulate effector T cell responses through activation of dendritic cell activity.
Efficacy of pasteurization to affect colony formation potential could be confirmed in two of four tumor samples as this potential was significantly abolished in their pasteurized counterpart. Other two samples failed to show this potential, so they were not considered for this evaluation.
It was interesting to note that though sample no. 3 had marginal change in necrosis observed by histopathology, this tumour cells exhibited colony forming potential and also presence of aneuploid population which was significantly abrogated after pasteurization. The non-pasteurized tissue of this sample further demonstrated tumour forming potential in in vivo study and exhibited presence of human DNA, cytokine in mouse organs/serum. The efficacy of pasteurization was evident in post-pasteurization mice serum and lung tissues and failure to form subcutaneous tumours. In sample no. 4 which had also exhibited marginal change in necrosis, demonstrated total abrogation of growth upon culture and 100 per cent efficiency in reducing tumour potential in vivo (3/3 tumour take before and 0/3 tumour take) post-pasteurization. In this sample, high necrosis due to chemotherapy prior to surgery could be the reason for removal of aneuploid cell population. Thus this sample had only diploid cell population in tumour tissue before and after pasteurization. Similar tumour heterogeneity and variations in response to different treatments have also been reported by others419.
In the current study of evaluating efficacy of pasteurization, we tried to pre-clinically mimic the situation by reimplanting pasteurized bone tissue into immunedeficient NOD-SCID mice and compare its tumorigenic potential with that of unpasteurized tumour tissue. We looked for tumour growth to check if in the similar clinical situation, tumour would recur. Pasteurization process had sigificantly eradicated tumour so that pasteurized human tumour tissues failed to form xenograft tumours while non-pasteurized bone tumour tissues (7 of 12) gave rise to tumour at the site of transplantation. Since osteosarcomas are known to give distant metastases, both xenograft tumour site as well as distant site (lung for metastasis) were assessed at cellular and molecular level by analyzing presence of malignant human cells, human housekeeping gene DNA, human protein markers and human inflammatory secreted cytokines in mice.
Presence of human cells was evaluated by immunohistochemistry of paraffin sections in xenograft tumours as well as lungs of mice. Lungs of mice transplanted with pasteurized bone tissue were also analysed for the presence of human nuclei, human bone-specific protein osteopontin, to check if malignant cells survived and metastasized to lungs. Absence of human cytokine IL-6 corroborated the IHC data. IL-6 levels have also been shown to be a reliable marker of the magnitude of systemic inflammation20. IL-6 has been implicated as an autocrine promoter of cancer growth for several human cancers. IL-6 overexpression has been reported in HER2+ breast cancer, liver cancer, biliary tract cancer, colorectal cancer, multiple myeloma, prostate cancer, ovarian cancer and gliomas and has been therapeutic target in many cancers21222324. It was observed that increased levels of IL-6 were secreted by xenograft tumours at various passages while pasteurized tumour did not show secretory human IL-6 in mice indicating that pasteurized graft did not have viable malignant tumour cells.
Chemokine CXCL1 (KC) is a member of IL-8 cytokine family and demonstrates structural and functional homology to IL-8. IL-8 is a major mediator involved in neutrophil recruitment and neutrophil dependent tissue damage1320. Mouse soluble KC/IL-8 levels were increased upto three months in mice transplanted with tumour (before and after pasteurization) compared to non-transplanted healthy mice indicating varied levels of inflammation in these mice. Ninomiya et al25 have also reported increased KC levels in NOD-SCID mice bearing adult T-cell leukaemia before and after all trans retinoic acid (ATRA) treatment. They also related increased KC levels to respiratory distress.
Similarly, mouse KC/IL-8 in mouse serum could be due to any inflammatory condition prevailing. However, human IL-6 in mouse serum may be considered as a potential marker for bone tumour growth. In our study we observed that human IL-6 levels in mice bearing xenografted tumours correlated with tumour volume. Bruscia et al24 have also shown that macrophages produced increased levels of cytokines IL-6 and KC/ IL-8 during chronic inflammatory condition of lung which could be a potential therapeutic target.
Rat26 and dog models27 have been used to demonstrate efficacy of pasteurization or microwave oven heating treatment, respectively, in healing and biomechanical strengthening of autograft. However, none of these studies addressed tumour eradication through treatment.
Thus, our study has provided a strong basis to establish the efficacy of pasteurization in ensuring tumour eradication. Given that pasteurized bone has been shown to have better biologic potential and strength than other tumour purging methods it can serve as a cost-effective alternative to allografts and extracorporeal radiation during limb salvage surgery. The clinical success of this non-invasive, easy to perform, spatially compatible and cost-effective process will ultimately depend on multi-centre study with large sample size, inclusion of appropriate controls and extended period of data collection.
Acknowledgment
Authors thank Dr S.V. Chiplunkar, Director, ACTREC, for critical reading and providing suggestions to improve the manuscript. The authors acknowledge funding from Terry-Fox Foundation through Institutional Review Board, Tata Memorial Centre, Mumbai Grant No 489. The second author (P.T) was also supported from this grant.
References
- Reconstruction with pasteurized autograft for primary malignant bone tumor of distal tibia. Bull Cancer. 2012;99:87-91.
- [Google Scholar]
- Extracorporeal irradiated tumor bone: a reconstruction option in diaphyseal Ewing's sarcomas. Indian J Orthop. 2010;44:390-6.
- [Google Scholar]
- Sarcomas of the bone. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer principles and practice of oncology. Philadelphia: Lippincott Williams and Wilkins; 2001. p. :1891-936.
- [Google Scholar]
- Evaluation of long-term outcomes of pasteurized autografts in limb salvage surgeries for bone and soft tissue sarcomas. Arch Orthop Trauma Surg. 2012;132:1685-95.
- [Google Scholar]
- Preservation and biodegradation of the morphogenetic property of bone matrix. J Theor Biol. 1973;38:155-67.
- [Google Scholar]
- Pasteurized autologous bone graft in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:258-66.
- [Google Scholar]
- Extracorporeal irradiated autogenous osteochondral graft: a histological study. J Bone Joint Surg Br. 2005;87:1006-11.
- [Google Scholar]
- Radiographic analysis of pasteurized autologous bone graft. Skeletal Radiol. 2003;32:454-61.
- [Google Scholar]
- Pasteurized autograft for intercalary reconstruction: an alternative to allograft. Clin Orthop Relat Res. 2007;456:203-10.
- [Google Scholar]
- Reconstruction with pasteurized autograft for distal tibial tumor. Arch Orthop Trauma Surg. 2008;128:159-65.
- [Google Scholar]
- Histological findings in a human autogenous pasteurized bone graft. Anticancer Res. 2004;24:1893-6.
- [Google Scholar]
- The comparison of the negative effect of autoclaving and pasteurization on bone healing. Acta Orthop Traumatol Turc. 2010;44:322-7.
- [Google Scholar]
- Which is the best method of sterilization of tumour bone for reimplantation? A biomechanical and histopathological study. Biomed Eng Online. 2010;9:48.
- [Google Scholar]
- Inhibition of Aurora-B supresses osteosarcoma cell migration and invasion. Exp Ther Med. 2014;7:560-4.
- [Google Scholar]
- On the thermal death point of sarcoma and normamononuclear cells (Walker rat tumor No. 1) Archiv f experimentelle Zellforschung. 1928;7:243-8.
- [Google Scholar]
- Destruction of solid tumors by heating with radio-frequency energy. Radio Sci. 1979;14:285-95.
- [Google Scholar]
- Hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan in patients with locally advanced soft tissue sarcomas: treatment response and clinical outcome related to changes in proliferation and apoptosis. Clin Cancer Res. 1999;5:1650-7.
- [Google Scholar]
- Cryoimmunologic antitumor effects enhanced by dendritic cells in osteosarcoma. Clin Orthop Relat Res. 2010;468:1373-83.
- [Google Scholar]
- Limb salvage in osteosarcoma using autoclaved tumor-bearing bone. World J Surg Oncol. 2012;10:105.
- [Google Scholar]
- Correlation of measurable serum markers of inflammation with lung levels following bilateral femur fracture in a rat model. J Inflamm Res 2010 2010:105-14.
- [Google Scholar]
- HER2 overexpression elicits a proinflammatory IL-6 autocrine signaling loop that is critical for tumorigenesis. Cancer Res. 2011;71:4380-91.
- [Google Scholar]
- Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083-96.
- [Google Scholar]
- Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator-/- mice. Am J Respir Cell Mol Biol. 2009;40:295-304.
- [Google Scholar]
- Retinoic acid syndrome in NOD/scid mice induced by injecting an acute promyelocytic leukemia cell line. Leukemia. 2004;18:442-8.
- [Google Scholar]
- Establishment of an animal model of a pasteurized bone graft, with a preliminary analysis of muscle coverage or FGF-2 administration to the graft. J Orthop Surg Res. 2009;4:31.
- [Google Scholar]
- The healing process of intracorporeally and in situ devitalized distal femur by microwave in a dog model and its mechanical properties in vitro. PLoS One. 2012;7:e30505.
- [Google Scholar]






