Translate this page into:
p40 & thyroid transcription factor-1 immunohistochemistry: A useful panel to characterize non-small cell lung carcinoma-not otherwise specified (NSCLC-NOS) category
Reprint requests: Dr. Deepali Jain, Associate Professor, Department of Pathology, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: deepalijain76@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Accurate histopathological subtyping of non-small cell lung carcinoma (NSCLC) is essential for targeted therapeutic agents. Immunohistochemistry (IHC) is helpful in identification of different tumour subtypes. In this study two marker approaches, one each for glandular and squamous cell differentiation was applied to maximize the proportion of accurately subtyped NSCLC not otherwise specified (NOS) tumours on small biopsy samples.
Methods:
Two hundred and sixty three consecutive lung biopsies of primary lung carcinoma were prospectively studied. These were subtyped first morphologically and then by IHC for p40 and thyroid transcription factor-1 (TTF-1). The diagnosis of NSCLC-NOS before and after addition of IHC was evaluated. Results were correlated and validated with morphologically proven cases and matched surgical specimens.
Results:
Based on morphology, only 140 of the 263 (53.2%) cases of NSCLC were characterized, whereas 123 (46.7%) were classified as NSCLC-NOS type. With addition of IHC (p40 and TTF-1), the latter category reduced to 14.4 per cent and a sum of 225 (85.5%) cases were accurately subtyped into squamous cell carcinoma, adenocarcinoma and adenosquamous carcinoma. p40 showed 100 per cent sensitivity and specificity for squamous differentiation whereas TTF-1 showed sensitivity of 85.3 per cent and specificity of 98.1 per cent. Ninety per cent correlation of morphologic subtypes was achieved with matched resected specimens.
Interpretation & conclusions:
Our results showed that an approach of using only a two-antibody panel (p40 and TTF-1) might help in reduction of diagnostic category of NSCLC-NOS significantly and contribute in saving tissue for future molecular testing.
Keywords
Immunohistochemistry
non-small cell lung cancer/carcinoma
NSCLC-not otherwise specified
p40
small biopsy
thyroid transcription factor-1
Non-small cell lung carcinomas (NSCLC) account for 80 per cent of all lung cancers1. The developments in the chemotherapy and targeted therapy against specific molecular alterations have necessitated precise subclassification of NSCLC which comprise adenocarcinomas (ADC) and squamous cell carcinomas (SQC) predominantly23. Most patients of lung cancer are diagnosed at a late stage precluding surgical resection. Hence, small biopsies or cytology remains the mainstay for accurate diagnosis. Moreover, preserving tissue for molecular studies is important because of its therapeutic implications4. Earlier studies have described the usefulness of immunohistochemistry (IHC) panels consisting of various markers such as thyroid transcription factor (TTF-1), napsin-A and cytokeratin 7 (CK7) for ADC and p63, CK5/6, CK34bE12, desmoglein-3 or desmocollin for SQC for subtyping of NSCLC5678. Others have recommended the use of a limited panel of immunohistochemical markers comprising one squamous marker such as p40 and one marker of glandular differentiation such as TTF-19. p40 is an isoform of p63 and represents the non-transactivating domain (deltaNp63). It is commonly found in basal layers of stratified epithelium and some glandular epithelium1011. p40 has been shown to be superior to the commonly used antibody p63 (clone 4A4) for squamous differentiation as p63 can be found in some lung ADCs and even large cell lymphomas, making it less specific11. The present study was undertaken to evaluate the utility of two marker approach one each for squamous cell (p40) and ADC (TTF-1) for accurate characterization of NSCLCs. In addition, for validation purposes of these two immunostains on small biopsies, IHC was also performed in morphologically identifiable SQC and ADC and results were compared.
Material & Methods
This prospective study was conducted in the department of Pathology, All India Institute of Medical Sciences, New Delhi, India, and included a series of 263 consecutive lung biopsies (January 2013 to April 2014) from patients of suspected primary lung cancer attending the departments of Pulmonary Medicine and Sleep Disorders and Medical Oncology. Of these, 145 were computed tomography-guided Tru-cut biopsies and 118 were obtained bronchoscopically. Biopsies having inadequate tumour tissue for immunohistochemical evaluation were excluded. None of these patients had any clinicoradiological evidence of primary cancer elsewhere. All biopsies were stained by standard haematoxylin and eosin stain and extra sections were cut on adhesive coated slides for IHC to prevent loss of tissue due to repeated facing of the block. Corresponding resection specimens were available for correlation of morphology and immunohistochemical results in 20 patients. The tumours were initially evaluated by morphological examination. The International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) classification criteria12 were used to determine histological subtypes of NSCLC. Small cell carcinomas, other neuroendocrine tumours and large cell neuroendocrine carcinomas were excluded on the basis of classical morphological features.
The study protocol was approved by the institutional ethics committee and written informed consent was obtained from all patients.
Morphological examination: All NSCLC were diagnosed as SQC when characteristic intercellular bridges and keratin pearls were present. Adenocarcinomas were diagnosed when there was a definite evidence of glandular differentiation and/or intracellular mucin vacuoles. Diagnosis of adenosquamous carcinoma was made when foci of both squamous and glandular differentiation were identified. All other cases showing solid growth pattern or lacking any definite differentiation were classified as NSCLC-not otherwise specified (NOS) by morphology. Cases with morphological features such as organoid pattern of growth suggestive of large cell neuroendocrine carcinomas were excluded.
Immunohistochemistry (IHC): IHC for p40 and TTF-1 was performed in all resected specimens and biopsies. The latter consisted of two groups including morphologically identifiable NSCLC and NSCLC-NOS. The cases were designated as NSCLC favour ADC if the immune profile was p40−/TTF1+, NSCLC favour SQC if p40+/TTF1− and NSCLC favour adenosquamous carcinoma if p40+/TTF1+ in different tumour cells. TTF-1 (mouse anti-human TTF-1 monoclonal antibody, clone 8G7G3/1 from Spring Bioscience Pleasanton, California) in 1:50 dilution and p40 (PC373 anti-p40 rabbit polyclonal antibody, from Calbiochem, Darmstadt, Germany) in 1:3000 dilutions were used. Briefly, after blocking endogenous peroxidase activity in 0.3 per cent hydrogen peroxidase and methanol solution for 15 min, deparaffinized sections were reacted for 30 min at room temperature with primary antibody. Slides were then incubated in a detection kit (EnVision plus HRP, DakoCytomation, Glostrup, Denmark) according to manufacturer's instructions, developing peroxidase activity with 3-3’-diaminobenzidine. Antigen retrieval was performed using citric acid (p H 6.0). Finally, slides were counterstained with haematoxylin, dehydrated and mounted.
Immunoreactivity was rendered semi- quantitatively on a scale from 0 to 3+, which was calculated as follows: percentage positivity of cells was graded as 0, 1 (1-25%), 2 (25-50%) and 3 (50-100%) and intensity graded as 0 (no staining), 1 (weak), 2 (moderate) and 3 (strong). The score was added and calculated on a scale of 0-9 (0-1=0, 2-4=1+, 5-7=2+, 8-9=3+). Care was taken not to interpret entrapped normal bronchial epithelium or pneumocytes as positive for tumour cell staining. Morphological assessment and IHC interpretation were performed independently by two pathologists at different time.
Statistical analysis: McNemar's test was performed to assess the significance of the correlation in the diagnosis of NSCLC-NOS before and after addition of IHC. The specificity and sensitivity of IHC markers was calculated and reported. GraphPad Prism software (https://www.graphpad.com/scientific-software/prism/) was used to quantify agreement with kappa.
Results
The median age of the patients was 58 yr with an interquartile range of 50-65 years. Male to female ratio was 3:1. Seventy per cent of the tumours were ADC in females whereas ADC constituted 45.9 per cent of all tumours in males. Males had a higher percentage of SQC and NSCLC-NOS as compared to females. All adenosquamous carcinomas were found in males.
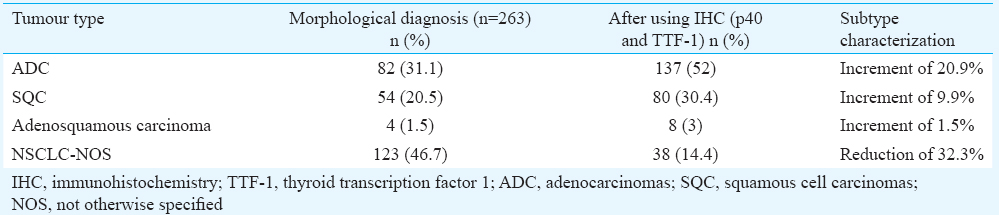
Based on the morphological analysis, 54 (20.5%) cases could be classified as SQC, 82 (31.1%) as ADC and four (1.5%) as adenosquamous carcinoma. The remaining 123 (46.7%) cases could not be further subtyped and were labelled as NSCLC-NOS.
Morphological analysis
Squamous cell carcinoma (SQC): Of the 54 morphologically identified SQC, 25 were well differentiated and 29 were moderately differentiated. One case revealed pseudo-glandular areas due to acantholysis. Seven cases had corresponding resection specimens, all of which turned out to be morphologically differentiated SQC.
Adenocarcinomas (ADC): Eighty two morphologically identified ADCs showed focal glandular differentiation and intracytoplasmic mucin. Five of these had corresponding resection and turned out to be well to moderately differentiated ADC.
Adenosquamous carcinoma: Four of the 263 cases had definite squamous and glandular areas on morphology and were diagnosed as adenosquamous carcinoma. None of these had corresponding resection specimen.
Immunohistochemical analysis
p40: All morphologically identified SQC were p40 positive (Fig. 1A and B). All cases showed 2+ to 3+ positivity except for two cases, which showed 1+ positivity. The staining intensity was strong in all cases. One case showing pseudoglandular architecture revealed 3+ positivity of p40 with no staining for TTF-1 (Fig. 1C and D).
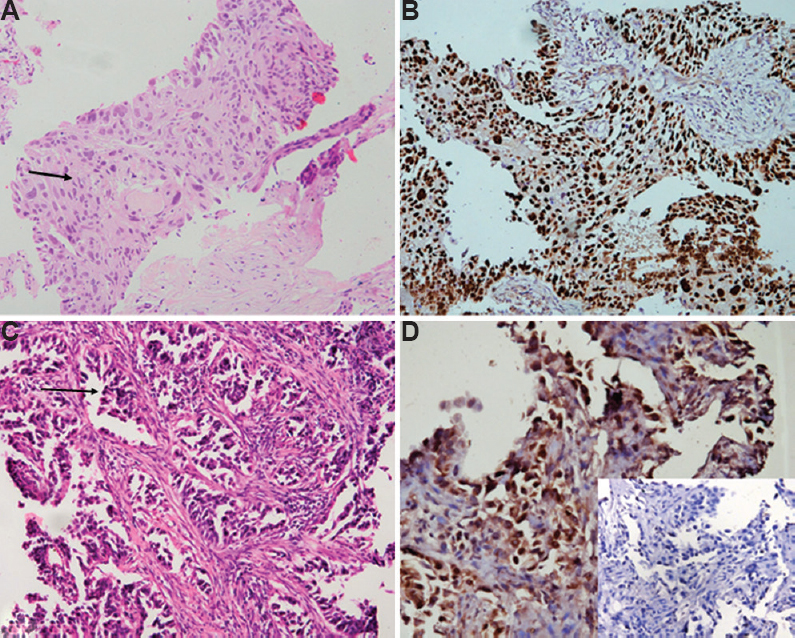
- (A) Morphologically differentiated squamous cell carcinomas with intercellular bridges and intracellular keratinization (arrow) (H & E, ×400). (B) p40 immunostain shows strong (3+) and diffuse nuclear positivity. (C) A case of squamous cell carcinomas with pseudoglandular pattern and acantholysis (arrow) (H & E, ×400). (D) p40 shows nuclear positivity. Inset shows negative TTF-1 stain.
Thyroid transcription factor-1 (TTF-1): Of the 82 morphologically diagnosed ADC, TTF-1 was positive (2+ to 3+) in 70 cases (85.3%) (Fig. 2A and B) whereas negative in 12 (14.6%) cases. TTF-1 was positive (3+) in one (1.8%) SQC along with p40 in the same tumour cells (sensitivity 85.3% and specificity 98.1%).
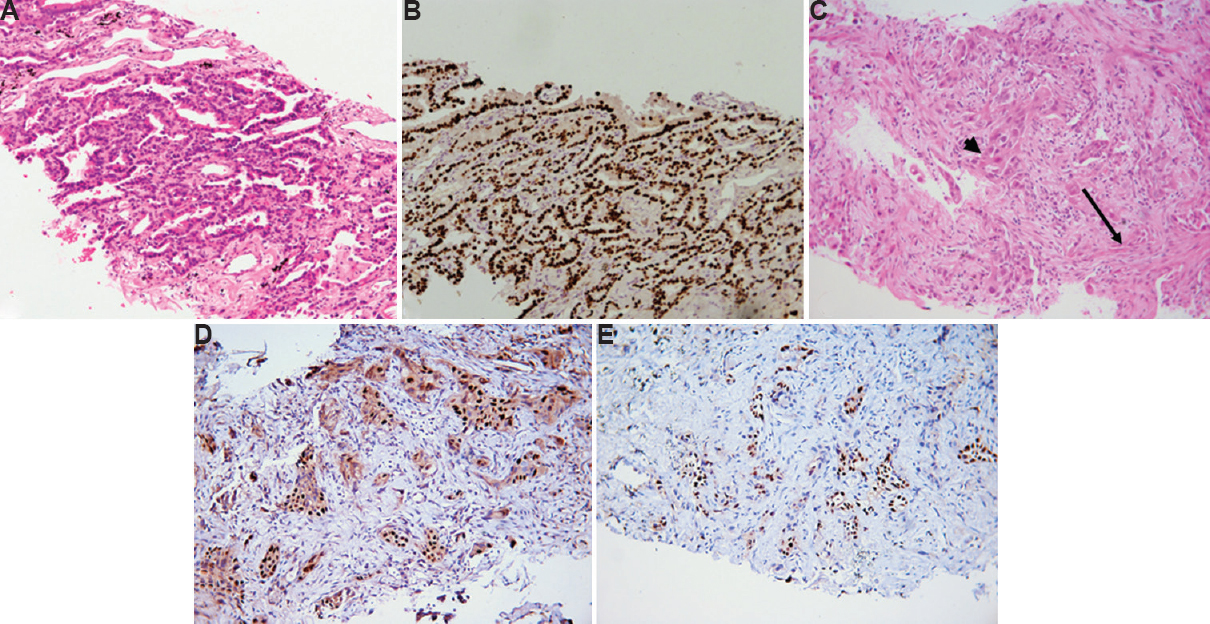
- (A) Morphologically differentiated adenocarcinomas with lepidic histologic pattern (H & E, ×400). (B) Thyroid transcription factor-1 immunostain shows strong (3+) and diffuse nuclear positivity. (C) Adenosquamous carcinoma with glandular (arrow) and squamous differentiation (arrowhead) (H & E, ×400). (D) p40 and (E) thyroid transcription factor-1 show nuclear positivity in different cell populations of adenosquamous carcinoma.
Adenosquamous carcinoma and immunohistochemistry: Amongst adenosquamous carcinomas, all four cases exhibited focal p40 and TTF-1 expression in different tumour cell populations (Fig. 2C-E).
NSCLC-NOS: One hundred and twenty three cases did not show any morphological differentiation (Fig. 3A-D) (Table I). These were classified on the basis of IHC. Detailed immunophenotypic characterization is shown in Table II. IHC was negative for both the markers in 38 (14.4%) cases, which remained NSCLC-NOS and could not be subtyped further.
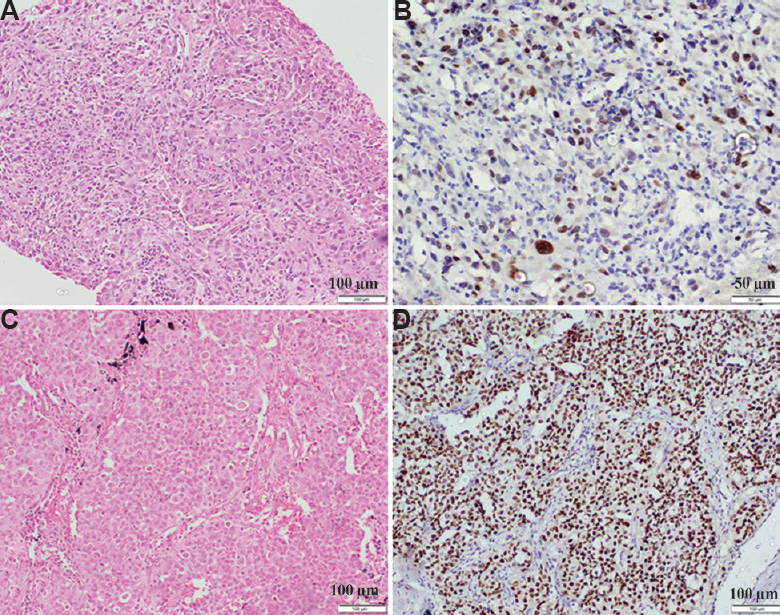
- (A) NSCLC-not otherwise specified without clear-cut morphologic differentiation (H & E, ×400). (B) p40 shows nuclear positivity in the same case indicating squamous lineage. (C) Another case of NSCLC not otherwise specified on morphology which is arranged in solid islands (H & E, ×400). (D) thyroid transcription factor-1 shows diffuse nuclear positivity suggesting diagnosis of adenocarcinoma.
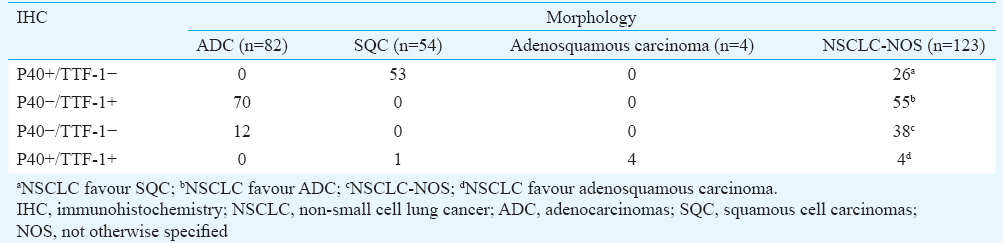
As a result, IHC for two specific markers when used adjunctively with morphology was able to correctly classify 225 of the 263 (85.5%) cases of NSCLC (P<0.001) (Table III).

Resection specimens: Corresponding resection specimens were available for 20 cases. Original diagnosis and immunohistochemical profile on small biopsies correlated well with final diagnosis in 18 of the 20 cases. One case classified as ADC on biopsy turned out to be adenosquamous on the surgical specimen while other cases classified as NSCLC-NOS was sarcomatoid carcinoma with CK, vimentin and focal TTF-1 positivity (Table IV).
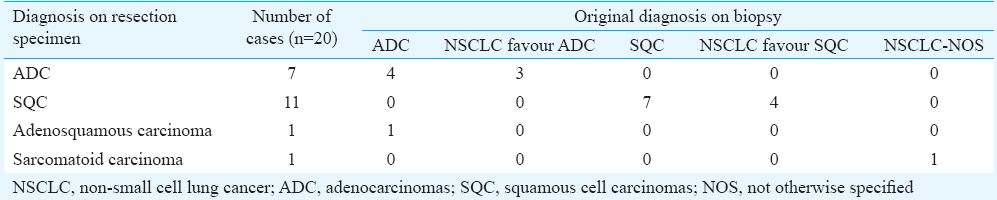
Of the seven ADCs on resection specimens; four were acinar predominant and three were lepidic predominant. SQCs were mostly keratinizing (6/11; 54.5%) type. No basaloid variant was recognized. Sarcomatoid carcinoma was of pleomorphic subtype due to focal glandular differentiation (TTF-1 positivity) and predominantly spindle cell areas. On biopsy, this case did not show spindle cells whereas TTF-1 and p40 negative solid tumour cell fragments were observed, thus labelled as NSCLC-NOS.
Discussion
This study was conducted with the aim of studying the effectiveness of a minimal panel of antibodies comprising p40 and TTF-1 in subclassification of NSCLC. Morphology alone is not very sensitive to precisely classify NSCLC into squamous or ADC in small biopsies. The error rate in classifying NSCLC into appropriate histological type is high in small biopsies because of sampling of solid areas of the tumour lacking morphologic differentiation13. In one such study, 10 per cent of SQC, 14 per cent of ADC and 50 per cent of large cell carcinomas were misclassified on bronchial biopsies14. The frequency of correct diagnosis of NSCLCs on small biopsies by light microscopy varies from 67 to 84 per cent15. We could classify only 53 per cent of tumours by morphology confidently which were further confirmed by IHC. The remaining 46.7 per cent were classified as NSCLC-NOS by morphology and further subtyped with the aid of IHC reducing the diagnosis of NSCLC-NOS significantly.
Using a panel of TTF-1, p63, CK5/6 and PAS (periodic acid-Schiff)-diastase, NSCLCs could be characterized in 65 per cent of cases16. A cocktail of nuclear and cytoplasmic stains, such as TTF-1/napsin-A and p63/CK5/6 was also suggested as a useful marker combination5. A limitation of availability of adequate material in a small biopsy precludes the usage of an extensive panel of immunohistochemical markers to accurately subtype NSCLCs, especially when tissue needs to be saved for molecular studies. Hence, it has been proposed to use one specific marker each for squamous and glandular differentiation4912. p40 has recently entered the diagnostic milieu of NSCLCs with only a few studies evaluating its role. All these studies considered p40 a single best marker for identifying SQC917181920. Many studies have utilized three or more markers for subclassification and have found a similar diagnostic rate as the present study where only two markers have been used202122. There are only a few studies exploring the ‘two marker’ minimalistic approach through coordinated use of single marker including p40 and TTF-1 for NSCLC subtyping in small biopsies9.
p40 is equivalent to p63 in terms of sensitivity but is superior in terms of specificity as it is negative in p63 positive ADC1123. p40 was positive in all morphologically differentiated SQC in our study and negative in all ADC. p40 also decorates the squamous component of adenosquamous carcinoma24. There were eight cases of adenosquamous carcinoma in our study all of which were focally positive for p40 defining the squamous component. Four of these cases were diagnosed in retrospect after IHC revealed focal p40 positivity.
TTF-1 is an established marker for lung ADC. However, its recorded sensitivity varies from 70 to 96 per cent825. TTF-1 was observed as a sensitive and specific marker (sensitivity 85.3% and specificity 98.1%) for glandular differentiation.
The coexistence of glandular and squamous traits within the same tumour cells are described in pulmonary26 and oesophageal27 carcinomas as a possibility of ‘amphicrine’ bi-phenotypic tumours. We found one morphologically differentiated SQC, in which p40 and TTF-1 were positive in the same tumour cells.
Negativity of TTF-1 in the absence of p40 reliably excludes SQC and may imply a poorly differentiated ADC. An unsuspected metastatic carcinoma, however, needs to be excluded in such cases by careful clinical investigation. Pan-CK stain should be performed to confirm the epithelial origin if morphologically tumour appears non-epithelioid.
Coordinated application of p40/TTF-1 immunopanel has been found to be useful in our study to significantly lower down the rate of NSCLC-NOS in small biopsies, and this panel was diagnostic in 85.5 per cent of cases. Amongst NSCLC-NOS category itself, this panel was helpful in diagnosing 70 per cent of tumours. The diagnosis on resected specimens correlated well with the diagnosis on biopsies in 90 per cent cases. One adenosquamous carcinoma and one sarcomatoid carcinoma escaped recognition probably because of sampling error.
In this study, CK7 was used as an additional stain for the 38 cases, which remained NSCLC-NOS after application of p40/TTF-1. Ten cases showed positivity for CK7. This was not included in the study as it was intended to evaluate the role of two-marker approach. The only limitation of the study was unavailability of matched resection specimens in all the cases. However, due to advanced stage of the disease at the time of presentation in lung cancer, resection was not found in most of the cases.
In conclusion, NSCLC characterization was performed using a combination of morphology and IHC. The two-antibody panel approach may contribute to accurate subtyping of NSCLC in small biopsies and a reduction in the NSCLC-NOS diagnostic category.
Acknowledgment
This work was supported by intramural grant of All India Institute of Medical Sciences, New Delhi (Grant No: A132).
Conflicts of Interest: None.
References
- Clinical features of 5,628 primary lung cancer patients: Experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452-62.
- [Google Scholar]
- Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129-39.
- [Google Scholar]
- Evaluation of epidermal growth factor receptor mutations based on mutation specific immunohistochemistry in non-small cell lung cancer: A preliminary study. Indian J Med Res. 2016;143:308-14.
- [Google Scholar]
- Diagnosis of lung cancer in small biopsies and cytology: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137:668-84.
- [Google Scholar]
- Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: Utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am J Surg Pathol. 2011;35:15-25.
- [Google Scholar]
- Tissue-sparing application of the newly proposed IASLC/ATS/ERS classification of adenocarcinoma of the lung shows practical diagnostic and prognostic impact. Am J Clin Pathol. 2012;137:946-56.
- [Google Scholar]
- The role of desmoglein-3 in the diagnosis of squamous cell carcinoma of the lung. Am J Pathol. 2009;174:1629-37.
- [Google Scholar]
- Analysis of clinical characteristics and differential diagnosis of the lung biopsy specimens in 99 adenocarcinoma cases and 111 squamous cell carcinoma cases: Utility of an immunohistochemical panel containing CK5/6, CK34ßE12, p63, CK7 and TTF-1. Pathol Res Pract. 2014;210:680-5.
- [Google Scholar]
- ΔNp63 (p40) and thyroid transcription factor-1 immunoreactivity on small biopsies or cellblocks for typing non-small cell lung cancer: A novel two-hit, sparing-material approach. J Thorac Oncol. 2012;7:281-90.
- [Google Scholar]
- p40: A p63 isoform useful for lung cancer diagnosis - A review of the physiological and pathological role of p63. Acta Cytol. 2013;57:1-8.
- [Google Scholar]
- p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405-15.
- [Google Scholar]
- International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-85.
- [Google Scholar]
- Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: A paradigm shift. J Thorac Oncol. 2010;5:411-4.
- [Google Scholar]
- Cell type accuracy of bronchial biopsy specimens in primary lung cancer. Chest. 1996;109:1199-203.
- [Google Scholar]
- Immunhistochemistry by means of widely agreed-upon markers (cytokeratins 5/6 and 7, p63, thyroid transcription factor-1, and vimentin) on small biopsies of non-small cell lung cancer effectively parallels the corresponding profiling and eventual diagnoses on surgical specimens. J Thorac Oncol. 2011;6:1039-49.
- [Google Scholar]
- Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol. 2010;5:436-41.
- [Google Scholar]
- Subtyping non-small cell lung cancer: Relevant issues and operative recommendations for the best pathology practice. Int J Surg Pathol. 2013;21:326-36.
- [Google Scholar]
- Utility and pattern of positivity of p40 in the diagnosis of squamous cell carcinoma of the lung by cytology: The first study on fine needle aspiration smears. Cytopathology. 2014;25:330-5.
- [Google Scholar]
- p40 is the best marker for diagnosing pulmonary squamous cell carcinoma: Comparison with p63, cytokeratin 5/6, desmocollin-3, and sox2. Appl Immunohistochem Mol Morphol. 2014;22:377-82.
- [Google Scholar]
- A comprehensive immunohistochemistry algorithm for the histological subtyping of small biopsies obtained from non-small cell lung cancers. Histopathology. 2014;65:868-78.
- [Google Scholar]
- ΔNp63, CK5/6, TTF-1 and napsin A, a reliable panel to subtype non-small cell lung cancer in biopsy specimens. Int J Clin Exp Pathol. 2014;7:4247-53.
- [Google Scholar]
- The utility of a novel triple marker (combination of TTF1, napsin A, and p40) in the subclassification of non-small cell lung cancer. Hum Pathol. 2014;45:926-34.
- [Google Scholar]
- Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. 2011;24:1348-59.
- [Google Scholar]
- Immunohistochemical staining with deltaNp63 is useful for distinguishing the squamous cell component of adenosquamous cell carcinoma of the lung. Anticancer Res. 2010;30:4717-20.
- [Google Scholar]
- Tissue-preserving antibody cocktails to differentiate primary squamous cell carcinoma, adenocarcinoma, and small cell carcinoma of lung. Arch Pathol Lab Med. 2013;137:1274-81.
- [Google Scholar]
- Pulmonary smell cell carcinoma showing tripartite differentiation in individual cells. Hum Pathol. 1981;12:286-94.
- [Google Scholar]
- Composite tumor of the esophagus with tripartite differentiation. Dig Dis Sci. 1997;42:1041-6.
- [Google Scholar]






