Translate this page into:
Oral metronomic chemotherapy for recurrent & refractory epithelial ovarian cancer: A retrospective analysis
For correspondence: Dr Lalit Kumar, Department of Medical Oncology, Dr. B.R. Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: lalitaiims@yahoo.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Advanced epithelial ovarian cancer (EOC) is associated with dismal outcome and progression-free survival (PFS) shortens with each subsequent relapse. For patients with recurrent and platinum refractory disease, therapeutic options are limited. Oral metronomic therapy (OMT) is associated with symptomatic relief and stable response in a significant proportion of patients. We retrospectively evaluated the outcome of patients with EOC treated with OMT at a tertiary care hospital in north India.
Methods:
Between January 2011 to December 2017, 36 EOC patients received OMT. Patients’ median age was 50 yr (range, 38-81 yr) and they had received a median of two lines of prior chemotherapy. OMT regimen included a combination of cyclophosphamide, etoposide (VP-16) and celecoxib with or without pazopanib along with supportive care. Response rates and outcomes were ascertained using the Gynecological Cancer Intergroup Guidelines. The toxicity was graded according to the Common Terminology Criteria for Adverse Events v.4.03.
Results:
The median CA-125 before initiating OMT was 160 U/ml (range, 42.23-5330 U/ml). The median interval between last chemotherapy and starting OMT regimen was 159 days (range, 1-1211 days). The overall response rate was 50 per cent. The median progression-free survival (PFS) was 8.2 months [95% confidence interval (CI): 5.03-10.33], and the median overall survival was 38 months (95% CI: 25.6-NR). Patients who received two lines of chemotherapy before OMT (P=0.052) and those who received pazopanib-based OMT (P=0.0513) had better PFS.
Interpretation & conclusions:
For patients with relapse and refractory EOC, OMT could be a reasonable option. A combination of oral etoposide (VP-16) and pazopanib needs further evaluation in a large number of patients in a randomized trial.
Keywords
EOC
oral metronomic therapy
progression free survival
recurrent ovarian cancer
refractory
survival
toxicity
VP-16
Advanced epithelial ovarian cancer (EOC) is associated with dismal outcomes despite initial good response to chemotherapy. Relapse is the major cause of treatment failure1. Patients with platinum-sensitive disease (platinum-free interval from the end of primary chemotherapy >6 months) are treated with paclitaxel- and carboplatin-based regimen. For those with platinum-free interval (PFI) less than or equal to six months (platinum-resistant disease), options remain limited2. Progression-free survival (PFS) shortens with each subsequent relapse. As a result, after three or four relapses, treatment options are only a few. Metronomic chemotherapy using low-dose cyclophosphamide, etoposide (VP-16), hormonal agents and multikinase inhibitors, e.g., pazopanib has been used for these refractory/recurrent subset of patients with good symptom control and stable disease (SD) in some patients34. There are limited published data on the use of metronomic therapy in recurrent and platinum refractory advanced EOC. Here, we report outcome of 36 patients who had recurrent/refractory EOC and were treated with metronomic approach at a tertiary care hospital in north India.
Material & Methods
Between January 2011 and December 2017, 36 patients were identified who had received oral metronomic therapy (OMT); all patients were symptomatic and had clinical and radiological evidence of disease. All patients were registered in the gynaecological tumour clinic and were seen jointly by medical oncology and gynaecologic oncologist and surgical oncologist in the clinic at the Institute Rotary Cancer Hospital, All India Institute of Medical Sciences (AIIMS), New Delhi, India. Patients were included in this study if they met the following criteria: (i) histologically proven EOC (staged according to FIGO staging); (ii) age >18 yr; (iii) previously treated with at least two lines of platinum-based chemotherapy or who were platinum-resistant/refractory; (iv) adequate organ functions, i.e., haemogram, liver and renal functions; and (v) able to swallow and retain oral medication. The study protocol was approved by the Institutional Ethics Committee. Primary objective of this study was PFS, and secondary outcomes included response rate, toxicity and identification of prognostic factors. Thirty six patients met eligibility criteria and were included for this analysis. The baseline characteristics of the patients are shown in Table I. Demographic details including age, Eastern Cooperative Oncology Group (ECOG) performance status, stage, histopathologic subtype, serum cancer antigen (CA)-125, details of prior treatment and response and major organ toxicities were recorded. Twenty five per cent (n=9) patients had comorbidities at diagnosis: diabetes mellitus in 8.3 per cent (n=3), hypertension in 13.9 per cent (n=5) and 11.1 per cent (n=4) of patients had hypothyroidism and were on replacement therapy. Details of OMT are given in Table II.
| Characteristics | n (%) |
|---|---|
| Baseline | |
| Median age (yr) | 50 (28-81) |
| Comorbidities | 9 (25) |
| Serum CA-125 (U/ml) | 900 (12.8-7349.5)† |
| Stage | |
| I | 5 (13.9) |
| IA | 3 (8.3) |
| IC | 2 (5.6) |
| III | 26 (72.2) |
| III B | 3 (8.3) |
| III C | 23 (63.9) |
| IV | 5 (13.9) |
| Initial treatment received | |
| Neoadjuvant | 18 (50) |
| Adjuvant | 31 (86.1) |
| Interval debulking surgery | |
| Suboptimal | 15 (41.6) |
| Optimal | 19 (52.8) |
| No surgery | 2 (5.6) |
| Response to primary therapy | |
| CR | 30 (83.3) |
| PR | 5 (13.9) |
| PD | 1 (2.8) |
| At OMT initiation | |
| Median age (yr) | 52 (33-81)† |
| ECOG PS | |
| 1 | 10 (27.8) |
| 2 | 25 (69.4) |
| 3 | 1 (2.8) |
| Median CA-125 (U/ml) | 160 (42.23-5330)† |
| Lab parameters | |
| Median haemoglobin (g/dl) | 10.6 (8.2-13.8)† |
| Median albumin (g/dl) | 3.8 (1.9-4.9)† |
| Clinical presentation | |
| Ascites | 25 (69.4) |
| Pleural effusion | 8 (22.0) |
| Others | 3 (8.3) |
| Previous lines of chemotherapy | |
| 1 | 1 (2.8) |
| 2 | 23 (63.9) |
| 3 | 9 (25.0) |
| 4 | 3 (8.3) |
| Histology | |
| High-grade serous adenocarcinoma | 34 (94.4) |
| Endometrioid | 1 (2.8) |
| Clear cell | 1 (2.8) |
†Values in parentheses are range. OMT, oral metronomic therapy; CR, complete response; PR, partial response; PD, progressive disease; ECOG PS, Eastern Cooperative Oncology Group performance status; CA-125, cancer antigen-125
| Serial number | Regimens used | Dose (mg) | Number of patients (%) |
|---|---|---|---|
| 1 | Pazopanib-containing regimens | 13 (36.1) | |
| 1A | VP16 CTx Pazopanib |
VP16=50 CTx=50 Pazopanib=200 |
4 (11.1) |
| 1B | VP16 CTx Pazopanib |
VP16=50 CTx=50 Pazopanib=400 |
7 (19.4) |
| 1C | Pazopanib weekly paclitaxel | Pazopanib=800 Paclitaxel=80 mg/m2 |
2 (5.6) |
| 2 | Non-pazopanib-containing regimens | 23 (63.9) | |
| 2A | Single-agent VP16 | VP16=50 | 6 (16.7) |
| 2B | Vp16+CTx+celecoxib | VP16=50 CTx=50 Celecoxib=200 |
4 (11.1) |
| 2C | VP16 CTx |
VP16=50 CTx=50 |
13 (36.1) |
| Total | 36 (100.0) | ||
CTx, cyclophosphamide; VP-16, etoposide
Response criteria: Response rates and outcomes were ascertained using serological criteria [Gynecological Cancer Intergroup (GCIG) guidelines] post three cycles and six cycles as well as RECIST 1.1 response criteria5. CA-125 response was defined as ≥50 per cent decrease from the baseline CA-125 level and confirmed ≥21 days after initial evaluation (baseline was defined as the higher value of 2 pre-treatment CA-125 assessments). If there were clinical signs and symptoms of progression, CA-125 was done and one more value repeated after 28 days to document clinical as well as serological progression. Progressive disease (PD) was defined as a CA-125 increase ≥100 per cent from nadir value; nadir was defined as the lowest CA-125 level until current assessment. SD was defined as changes in CA-125 not qualifying as either PD or response. The current status of patients was obtained from records and updated telephonically. The toxicity was graded using the Common Terminology Criteria for Adverse Event v.4.036.
Serum CA-125 estimation: CA-125 was done by the Abbott ARCHITECT CA125 II assay (Abbott Laboratories, Abbott Park, IL, USA), which is a chemiluminescent microparticle immunoassay (CMIA)7. This assay is a two-step immunoassay to determine the presence of OC 125 (CA-125)-defined antigen in human serum and plasma using CMIA technology with flexible assay protocol, referred as Chemiflex. In the first step, sample and OC 125-coated paramagnetic microparticles are combined. OC 125-defined antigen present in the sample binds to the OC 125 microparticle. After washing, M11 acridinium-labelled conjugate is added in the second step. Pre-trigger and trigger solutions are then added to the reaction mixture. The resulting chemiluminescent reaction is measured as relative light units (RLUs). A direct relationship exists between the amount of OC 125-defined antigen in the sample and RLUs detected by Abbott ARCHITECT system.
Dose modification: For grades 3 and 4 neutropenia, oral etoposide was reduced to 10 (day 1-10) from 14 days (day 1-14) (25-30% reduction). Pazopanib was reduced to 200 mg for patients with liver or skin toxicity. Re-escalation was not done. OMT was stopped in case of progressive disease (Table III).
| Variables | n (%) |
|---|---|
| Median follow up (95% CI) (months) | 12.2 (7.1-17)† |
| Progression | 21 (55.26) |
| Interval from the last chemotherapy (days) | 159 (1-1211)† |
| ORR (%) | 19 (50) |
| Dose modification: Pazopanib (200 mg) | 2 (5.6) |
| VP-16 (10 days) | 6 (16.6) |
| Treatment interruption | 7 (19.4) |
| Progression-free survival (95% CI) (months) | 8.2 (5.03-10.33)† |
| OS (95% CI) (months) | 38 (25.6-NR) |
†Values in parentheses are range. ORR, overall response rate; CI, confidence interval; OS, overall survival; NR, not reached
Statistical analysis: Survival analysis was done using Kaplan-Meier method. Comparison of survival was done by log-rank test. Univariate and multivariate analyses (Cox proportional hazards model) were done to determine the prognostic factors. STATA v13 (StataCorp LLC, TX, USA) was used for statistical analysis. The median follow up for the whole group was 12.2 months (95% CI: 7.1-17).
Results
Patient characteristics
At diagnosis: The median age of patients was 50 yr, ranging from 30 to 81 yr. The most common sites of disease were abdominopelvic mass, nodal disease and ascites. The median serum CA-125 at diagnosis was 900 U/ml (range, 12.8-7349.5 U/ml). At baseline, most patients had advanced stage, International Federation of Gynecology and Obstetrics (FIGO) stage 3 (72.2%, n=26) and stage 4 (13.9%, n=5). The most common histopathology subtype was serous cystadenocarcinoma (94.4%, n=34). Other subtypes were clear cell and endometrioid in one case each (2.8%). All patients had received paclitaxel plus carboplatinum-based chemotherapy; this included neoadjuvant in 18 (50%) patients. Nineteen (52.8%) patients had optimal debulking (Table I). Thirty (83.3%) patients achieved complete response (CR), five (13.9%) had partial response and one (2.8%) patient had platinum refractory disease.
At oral metronomic therapy (OMT) initiation: The median age at OMT initiation was 52 yr (range, 33-81 yr). Twenty five (69.4%) patients had ECOG performance status (PS) 2, 10 patients had PS 1 (27.8%) and one (2.8%) patient had PS 3. Sites of disease were ascites and pleural effusion in 69.4 per cent (n=25) and 22.2 per cent (n=8) patients, respectively. The mean body mass index (BMI) of patients was 25.4 kg/m2. The median haemoglobin was 10.6 g/dl (range, 8.2-13.8 g/dl), and the median serum albumin was 3.8 g/dl (range, 1.9-4.9 g/dl). Patients had received two lines (63.9%, n=23) or three (25%, n=9) lines of chemotherapy before starting OMT; three (8.3%) patients had received four lines. Only one (2.8%) patient was started on OMT in a platinum refractory setting after exposure to single line of paclitaxel, carboplatin. The median CA-125 before initiating OMT was 160 U/ml (range, 42.23-5330 U/ml). The median interval between last chemotherapy and starting OMT regimen was 159 days (range, 1-1211 days).
Metronomic regimens: Patients received the following 6 different OMT combination regimes (Table II), including VP-16- cyclophosphamide (36.1%, n=13), VP-16-cyclophosphamide-pazopanib 200 mg/day (11.1%, n=4), VP-16-cyclophosphamide-pazopanib 400 mg/day (19.4%, n=7), single-agent VP-16 (16.7%, n=6), VP-16-cyclophosphamide-celecoxib (11.1%, n=4), pazopanib 400 mg/day-weekly paclitaxel (5.6%, n=2). Thus, 63.9% (n=23) of patients had received non-pazopanib-containing regimens and 36.1 per cent (n=13) of patients received the pazopanib-based OMT.
Treatment response and outcomes: The overall response rate was 50 per cent (n=18). Of the 36 patients, 55.5 per cent (n=20) progressed while on OMT. The median PFS was 8.2 months (95% CI: 5.03-10.33) and the median overall survival (OS) was 38 months (95% CI: 25.6-NR) (Figs 1 and 2). There was no significant difference in OS as regards to the number of previous lines of chemotherapy received and the type of OMT received (non-pazopanib versus pazopanib containing). There was a trend for better PFS for patients who received two or more previous lines of chemotherapy (P=0.052) and type of OMT (non-pazopanib versus pazopanib containing) (P=0.0513) (Fig. 3A and B).
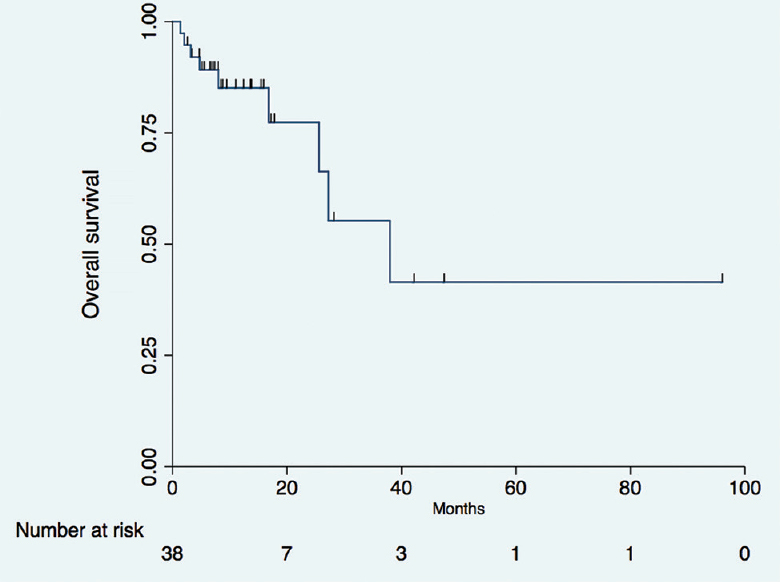
- Overall survival of patients who received oral metronomic therapy.
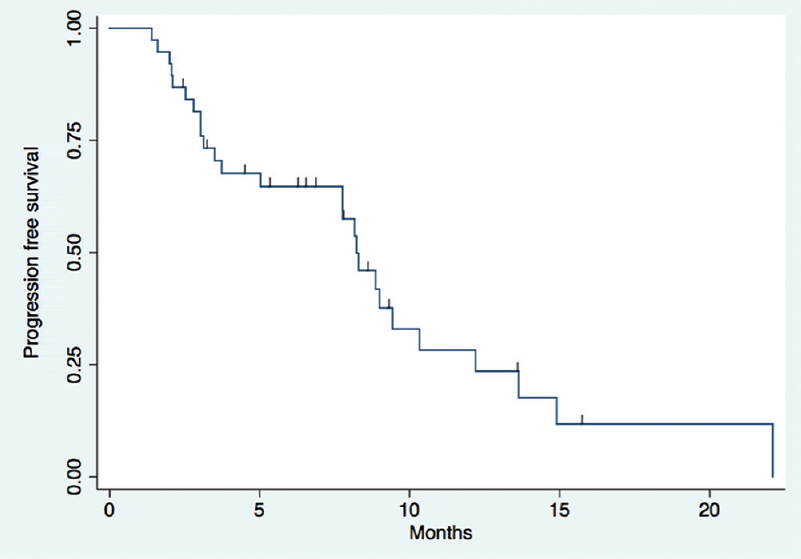
- Progression-free survival of patients who received oral metronomic therapy.

- Progression-free survival: (A) pazopanib versus non-pazopanib containing regimen; (B) Number of previous lines of chemotherapy received
Factors affecting survival: There was a significant difference in PFS [hazard ratio (HR)=1.01, P=0.04] between two or more previous lines of chemotherapy received on univariate analysis, but not on multivariate analysis. When patients were stratified on the basis of different OMT regimens, there was no significant difference in PFS (HR=0.74, P=0.516) or OS (HR=1.04, P=0.95) among the non-pazopanib versus pazopanib-containing regimens groups. For patients who achieved objective response, there was significant difference in survival for both PFS (HR=0.43, P=0.043) and OS (HR=0.10, P=0.038) (Table IV).
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| PFS | OS | PFS | OS | |
| Lines of therapy (≤2 versus >2 lines) | ||||
| HR | 1.01 | 0.4032119 | 1.68 | 0.37 |
| P | 0.965 | 0.266 | 0.31 | 0.30 |
| 95% CI | 0.43-2.39 | 0.081-1.99 | 0.60-4.72 | 0.059-2.40 |
| Regimen (non-pazopanib versus pazopanib) | ||||
| HR | 0.74 | 1.04 | 0.55 | 1.77 |
| P | 0.516 | 0.95 | 0.27 | 0.51 |
| 95% CI | 0.31-1.78 | 0.26-4.16 | 0.18-1.60 | 0.31-10.16 |
| ORR (responders versus non-responders) | ||||
| HR | 0.43 | 0.10 | 0.609855 | 0.19 |
| P | 0.043 | 0.038 | 0.481 | 0.264 |
| 95% CI | 0.19-0.97 | 0.013-0.88 | 0.15-2.41 | 0.01-3.49961 |
PFS, progression-free survival; HR, hazard ratio
Toxicities: OMT was well tolerated. Grades 3 and 4 toxicities included mucositis (13.9%, n=5), nausea (8.3%, n=3), vomiting (8.3%, n=3), liver functions derangement (8.3%, n=3), hypertension (5.6%, n=2), diarrhoea (5.6%, n=2), hand-foot syndrome (2.8%, n=1), fatigue (2.8%, n=1) and neutropenia (2.8%, n=1).
Current status: Nine (25%) patients died of progressive disease. Of the remaining 27 patients, 10 (27.8%) were alive on metronomic, two (5.6%) on best supportive care and 11 (30.5%) patients were on systemic chemotherapy. Four (11.1%) patients were lost to follow up.
Discussion
EOC is the second most common gynaecological cancer and is a leading cause of death68. With current approach of surgical debulking and chemotherapy, about 70-80 per cent of patients attain clinical CR. However, majorities (70-80%) of patients eventually relapse and die of the progressive disease2. With subsequent relapses and multiple lines of chemotherapy received, patients develop chemorefractory disease, increased toxicities to intravenous chemotherapy; this has a bearing on the quality of life of the patients and economical burden for patients and their families.
In recent years, there has been growing interest in the use of ‘metronomic-low dose, continuous’ therapy for these patients who have been exposed to multiple lines of therapy and have refractory/progressive disease. Seminal work done over the past decade has suggested that main mechanisms behind metronomic therapy are its anti-angiogenesis and effect on T regulatory cells910. Angiogenesis has been established as a hallmark of tumour development, growth and metastasis; EOC is particularly sensitive to anti-angiogenic therapy411. OMT possibly also works through the regulation of tumour microenvironment12.
OMT is yet to carve out its niche in the ovarian carcinoma management spectrum. Large studies like the AGO OVAR trial13 have established the role of oral drugs such as pazopanib in maintenance therapy. However, its role as a therapeutic agent in refractory and recurrent setting has not been evaluated14. Majority of patients in our study had advanced-stage cancers and presented with poor performance status (ECOG PS 2/3) with a low BMI and nutritional status (median serum albumin 3.8 g/dl). About one-fourth (n=9) had comorbidities.
Different OMT regimens were used in our study based on their efficacy in earlier reports; drugs such as cyclophosphamide, etoposide, pazopanib and celecoxib have been studied for their antiangiogenic and metronomic potential15161718. Pazopanib is a multikinase inhibitor. It inhibits angiogenesis signalling pathway via ATP-competitive inhibition of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2 and VEGFR-319. The MITO 11 trial had evaluated weekly paclitaxel and pazopanib versus paclitaxel in relapsed EOC. The median PFS was superior in combination arm; 6.35 (95% CI: 5·36-11.02) versus 3.49 months (95% CI: 2.01-5.66), HR 0.42 (95% CI: 0.25-0·69); P=0.000220. Several phase-I studies have been conducted to identify the optimal dose of pazopanib192122. Higher dose of pazopanib was associated with high incidence of hypertension AGO OVAR trial leading to dose modification in 33.3 per cent of patients14 and other toxicities in two doses of pazopanib (200-400 mg/day). Cyclophosphamide has been established as a metronomic agent over the past decade18. A few studies have evaluated cyclophosphamide as a single agent in recurrent carcinoma ovary1718. PACOVAR study which used a combination of pazopanib with cyclophosphamide demonstrated a median PFS and OS of 6.7 and 15.2 months, respectively23 (Table V).
| Author (reference) | Year | n | Agents used | Response rates (%) | Survival | Adverse events (%) |
|---|---|---|---|---|---|---|
| Beck and Boyes24 | 1968 | 78 | CTx (50-150 mg per day) | 48 | Responders: 20 months Non-responders: 13 months | Leucopenia (19) Alopecia (10) |
| Markman et al25 | 1992 | 18 | VP-16: 50 mg/day q20 days | 4/18 patients | NA | Neutropenia (11) Nausea (6) |
| Friedlander et al19 | 2010 | 36 | Pazopanib 800 mg/day | 31 | Six-month PFS: 17% (95% CI: 6-33) | Liver enzymes elevation: Grade 3 (8) Peripheral oedema grade 4 (2.8) |
| Eichbaum et al23 | 2011 | 16 | Pazopanib 600 mg CTx 50 mg | - | Median PFS; 6.7 months OS: 15.2 months | Hypertension, sepsis, vomiting, ileus and fatigue |
| Ferrandina et al17 | 2014 | 54 | CTx 50 mg daily | 20.4 | Median PFS: Four months Median OS: 13 months | 1 patient experienced grade 3 anaemia |
| Pignata et al20 | 2015 | 74 | Weekly paclitaxel 80 mg/m2 with or without pazopanib 800 mg daily | 25 (95% CI: 12-42) patients in the paclitaxel only group versus 56 (95% CI: 38-72) in the paclitaxel and pazopanib group (P=0.008) | Median PFS: 6.4 months in the pazopanib group (95% CI: 5·36-11·02) versus 3·49 months in the paclitaxel group (2.01-5.66) | Neutropenia: 30 in the pazopanib group versus 3 in the paclitaxel group Leucopenia: 11 versus 3 Fatigue: 11 versus 6 Hypertension: 8 versus 0 |
| Handolias et al26 | 2016 | 23 | CTx 150 mg/day for 14 days | 44 | PFS: Four months Six-month PFS: 35 (17-54 CI) OS: Eight months Six-month OS: 65 (42-81 CI) | No grade 3 or 4 haematological or non-haematological toxicity |
| Wong et al27 | 2017 | 20 | CTx 50 mg daily | 25 | Median PFS: 15 wk (range, 5-60 wk) | Grade 2-3 myelosuppression |
| Lan et al28 | 2018 | 35 | Apatinib 500 mg daily VP-16: 50 mg day 1-14 | 54 (95% CI: 36.6-71.2) | PFS: 8.1 months (95% CI: 2.8-13.4) | Neutropenia: 50 Fatigue: 32 Anaemia: 29 Mucositis: 24 |
| Present study | 2019 | 36 | Pazopanib 200 mg/400 mg CTx 50 mg Etoposide 50 mg | 50 | PFS: 8.2 months (95% CI: 5.03-10.33) | Mucositis (13.9, n=5), nausea (8.3, n=3), vomiting (8.3, n=3), liver functions derangement (8.3, n=3) |
NA, not available
The successive decline in PFS with succeeding chemotherapy regimens is a well-known phenomenon in carcinoma ovary. Exploring newer endpoints pertaining to oral metronomic regimens may be the way ahead1. Outcomes such as ‘chemotherapy-free interval’ or interval from the last chemotherapy while on OMT provide us a broad overview of prolongation of survival vis-a-vis maintaining quality of life. Artificial prolongation of platinum-free interval (in hope to improve platinum sensitivity) in patients receiving alternate regimens has been studied extensively and no benefit was displayed in a meta-analysis by Pignata et al29. This real-world conundrum might be further validated in prospective studies if a subset of patients who responded better to the OMT initially had improved survival. A study done in rural Indian setting has tried to address these concerns30.
The OMT regimens used in our study were generally well tolerated with low grades 3 and 4 adverse events. The most common adverse effect was oral mucositis. In patients who are in poor general condition and do not consent for intravenous therapy, offering an oral regimen with low toxicity might be prudent. For response evaluation, the GCIG criteria of serological response and progression was used5. RECIST criteria were not used in our study. Our study showed no significant difference in survival as per the number of previous lines of chemotherapy received. In all the studies on metronomic therapy, data available are largely heterogeneous. Majority of the data are retrospective in nature. Doses used in various studies are usually arbitrary and based on physician's discretion. Achieving a standard dose for each oral drug might be difficult as there are various permutations and combination of drugs based on the tolerability of the patients.
The limitations of our study were its retrospective nature, small sample size, wide heterogeneity among patient's groups and therefore, confounding the comparison between the groups. This setting is not uncommon in real-world practice, and based on our results, oral therapy can be utilized as an option in platinum refractory/recurrent EOC. Newer endpoints such as chemotherapy-free interval can be extrapolated and explored in prospective studies.
In conclusion, the results of the present study suggested that OMT could be an option in patients with relapse/refractory EOC. In future, randomized trials on metronomic chemotherapy need to be done in a large number of relapsed/refractory EOC patients.
Acknowledgment
Authors acknowledge the help of technical staff, department of Reproductive Biology, All India Institute of Medical Sciences, New Delhi, for estimating serum CA-125 for the patients included in the study.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Survival following the documentation of platinum and taxane resistance in ovarian cancer: A single institution experience involving multiple phase 2 clinical trials. Gynecol Oncol. 2004;93:699-701.
- [Google Scholar]
- The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23:2605-12.
- [Google Scholar]
- Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194-204.
- [Google Scholar]
- Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007;67:281-8.
- [Google Scholar]
- Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419-23.
- [Google Scholar]
- Common Terminology Criteria for Adverse Events v4.0 (CTCAE). Available from: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- ARCHITECT system. CA 125 II. Available from: http://www.ilexmedical.com/files/PDF/CA125_ARC.pdf
- Fact Sheets by Cancer. International Agency for Research on Cancer. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423-36.
- [Google Scholar]
- Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273-86.
- [Google Scholar]
- Platinum-sensitive recurrence in ovarian cancer: The role of tumor microenvironment. Front Oncol. 2013;3:251.
- [Google Scholar]
- Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16:561-8.
- [Google Scholar]
- Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol. 2014;32:3374-82.
- [Google Scholar]
- Prolonged progression-free survival with maintenance metronomic oral cyclophosphamide and etoposide treatment in macroscopic residual disease or recurrent/advanced stage ovarian cancer. J BUON. 2014;19:980-4.
- [Google Scholar]
- Metronomic oral cyclophosphamide (MOC) in the salvage therapy of heavily treated recurrent ovarian cancer patients: A retrospective, multicenter study. BMC Cancer. 2014;14:947.
- [Google Scholar]
- Cyclophosphamide-based metronomic chemotherapy: After 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol. 2012;82:40-50.
- [Google Scholar]
- A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119:32-7.
- [Google Scholar]
- Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): A randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16:561-8.
- [Google Scholar]
- A Phase I, dose-escalation trial of pazopanib in combination with cisplatin in patients with advanced solid tumors: A UNICANCER study. Oncol Ther. 2016;4:211-23.
- [Google Scholar]
- Phase I study of pazopanib in combination with paclitaxel and carboplatin given every 21 days in patients with advanced solid tumors. Mol Cancer Ther. 2012;11:1820-8.
- [Google Scholar]
- The PACOVAR-trial: A phase I/II study of pazopanib (GW786034) and cyclophosphamide in patients with platinum-resistant recurrent, pre-treated ovarian cancer. BMC Cancer. 2011;11:453.
- [Google Scholar]
- Treatment of 126 cases of advanced ovarian carcinoma with cyclophosphamide. Can Med Assoc J. 1968;98:539-41.
- [Google Scholar]
- Phase 2 trial of chronic low-dose oral etoposide as salvage therapy of platinum-refractory ovarian cancer. J Cancer Res Clin Oncol. 1992;119:55-7.
- [Google Scholar]
- Oral cyclophosphamide in recurrent ovarian cancer. Asia Pac J Clin Oncol. 2016;12:e154-60.
- [Google Scholar]
- Continuous oral cyclophosphamide as salvage or maintenance therapy in ovarian, primary peritoneal, and fallopian tube cancers: A retrospective, single institute study. Taiwan J Obstet Gynecol. 2017;56:302-5.
- [Google Scholar]
- Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): A phase 2, single-arm, prospective study. Lancet Oncol. 2018;19:1239-46.
- [Google Scholar]
- Randomized controlled trial testing the efficacy of platinum-free interval prolongation in advanced ovarian cancer: The MITO-8, MaNGO, BGOG-Ov1, AGO-Ovar2.16, ENGOT-Ov1, GCIG study. J Clin Oncol. 2017;35:3347-53.
- [Google Scholar]
- Outcomes of advanced epithelial ovarian cancer with integration of metronomic chemotherapy: An Indian rural cancer centre experience. South Asian J Cancer. 2016;5:59-62.
- [Google Scholar]






