Translate this page into:
Oestrogen receptor Rsa I gene polymorphism in osteoporosis periodontitis patients with or without dental fluorosis
For correspondence: Dr K.L. Vandana, Department of Periodontics, College of Dental Sciences, Davangere 577 004, Karnataka, India e-mail: vanrajs@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
There is a paucity of information on association between dental fluorosis, osteoporosis and periodontitis. The aim of this pilot study was to evaluate oestrogen receptor (ER) Rsa 1 gene polymorphism in osteoporosis periodontitis patients with and without dental fluorosis.
Methods:
Twenty one primary osteoporotic patients suffering from periodontitis with dental fluorosis and 20 primary osteoporotic patients suffering from periodontitis without dental fluorosis participated in this study. Periodontitis was diagnosed based on age, gender T-scores using clinical parameters such as plaque scores, gingival bleeding scores and probing pocket depth, clinical attachment level (CAL) and severity of dental fluorosis. DNA was genotyped at the RsaI RFLP (in exon 5) inside the ER gene to study ER Rsa I gene polymorphism in osteoporosis periodontitis patients with and without dental fluorosis.
Results:
Patients with dental fluorosis had higher degree of osteoporosis than those without fluorosis. CAL was significantly higher (P<0.05) in those with dental fluorosis compared with those without. Rr heterozygote (21.95%) was observed in patients without fluorosis whereas RR mutant homozygote was absent in both the groups. Rr wild homozygote type was seen more in the patients with fluorosis (51.21%). Significant differences were found in distribution of these genotypes between patients with and without dental fluorosis.
Interpretation & conclusions:
This preliminary study showed the presence of ER I gene polymorphism in osteoporosis periodontitis patients without dental fluorosis. Further studies with large sample size are needed to confirm the association shown in this preliminary study.
Keywords
Dental fluorosis
oestrogen receptor gene polymorphism
osteoporosis
periodontitis
Periodontitis is a rare chronic infectious disorders caused primarily by bacteria. The various risk factors associated with periodontal diseases are local factors, host response factors, genetic factors and systemic factors, e.g. osteoporosis, race ethnicity and geographic region1. Oestrogen receptor (ER) gene polymorphism has also been shown to be associated with low bone mineral density (BMD), i.e. osteoporosis and chronic periodontitis2 as well as fluorosis3. Although some research groups have investigated possible association between osteoporosis and endemic fluorosis34, the occurrence of periodontitis in endemic fluoride areas567, possibly links between osteoporosis and periodontal disease8 and association of ER gene polymorphism and osteoporosis resulting in low bone mass9 with the occurrence of periodontitis2, but there are not much literature available on association between osteoporosis, periodontitis and dental fluorosis. This study was undertaken to assess the ER Rsa I gene polymorphism in osteoporotic periodontitis patients with and without dental fluorosis.
Material & Methods
Forty one consecutive primary osteoporotic patients (BMD score ≥2.5)10 diagnosed with chronic periodontitis [community periodontal index (CPI), WHO 1997]11 in the age group of 35-70 yr of both sexes who fulfilled inclusion criteria participated in this pilot study. These patients were attending the Outpatient Department of Periodontics, College of Dental Sciences, Davangere, India, during 2014-2015. Patients with dental fluorosis were selected based on the following criteria512: each patient had (i) lived in the endemic water fluoride area for more than 10 yr, (ii) consuming water with fluoride levels above 1.2 ppm (Davangere water fluoride levels: 0.2-2.41 mg/l)13, and (iii) mottled tooth enamel, indicating dental fluorosis. The control group included osteoporosis periodontitis patients without dental fluorosis. A written consent was obtained from the participants, and ethical clearance was obtained from the Institutional Review Board. Menopausal history was recorded from female patients. Patients suffering from secondary osteoporosis (e.g. primary hyperparathyroidism and kidney dysfunction) or systemic conditions predisposing osteoporotic states, e.g. diabetes mellitus, Cushing's disease14, skeletal fluorosis, pregnant or lactating women, smokers and alcoholics14 were excluded. Osteoporosis status was assessed using peripheral quantitative (calcaneal-heel) ultrasound. Based on the T-scores10, the patients were subcategorized as mild osteoporosis [−2.5 to −3.5 standard deviation (SD)], moderate (−3.5 to −4.5 SD) and severe osteoporosis (−4.5 and above). Dichotomous plaque and bleeding scores15, CPI16 and Jackson's fluorosis index16 were recorded.
Fasting venous blood (6 ml) was collected from each patients and DNA was extracted using whole blood genomic DNA kit (Merck, New Jersey, USA). DNA was genotyped at the following marker at 1082 in exon 5 inside the ER gene by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method using Rsa I enzyme. The forward primer (5’-TCTTTGCTTTCCCCAGGCTTT-3’) and the reverse primer (5’-ACCTGTCCA GAACAAGATCT-3’) (BioServe Biotechnologies India Pvt. Ltd., Hyderabad) were used in PCR to produce 156 base pair (bp) DNA fragment4. The PCR reaction amplification was conducted in reaction mixtures each containing 13 μl of distilled water, 2.5 μl of 10× buffer, 1.5 mM MgCl2, deoxynucleotide triphosphate (dNTP, 200 mM), 2.5 U of Taq DNA polymerase (Chromous Biotech, Bengaluru), 0.25 μM of each of primer (Bioserve Biotechnologies India Pvt. Ltd., Hyderabad) and 200 ng of genomic DNA. PCR amplification was performed in Veriti Thermal Cycler (Applied Biosystems, USA) with PCR conditions as follows: initial denaturation at 94°C for 5 min, 35 cycles of denaturation at 94°C for 20 sec, annealing at 53°C for 30 sec, extension at 72°C for 30 sec and final extension at 72°C for 5 min. After amplification, PCR products were digested with endonuclease enzyme Rsa I (FastDigest Rsa I, Thermo Scientific, USA) at 37°C for two and a half hours, and the samples were electrophoresed on 2 per cent agarose gels and stained with 0.5 μg/ml of ethidium bromide. Gels were visualized on a UV transilluminator (Gel doc system, Major Science, Saratoga, USA) under UV light and photographed. The absence and presence of the Rsa I restriction sites of the ER gene were designated as R and r alleles, respectively (Figure).
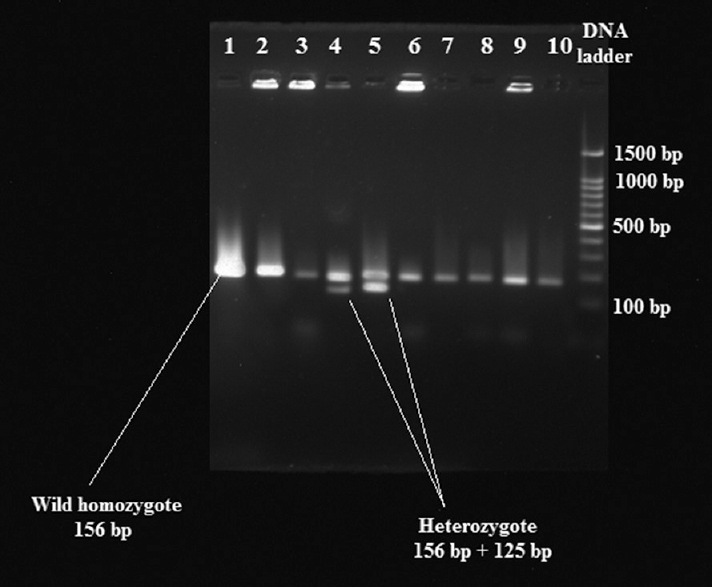
- Polymerase chain reaction-restriction fragment length polymorphism of oestrogen receptor fragments. Lanes 2, 6 and 9 heterozygote (Rr); lanes 1, 3, 4, 5, 7, 8 and 10 wild- type homozygote (rr); last lane, DNA ladder.
Ten millilitres of instant urine sample were collected from each patient for the determination of urinary fluoride levels (UFLs) by fluoride ion selective electrode (Thermo Orion model 96-09, Beverly, USA).
Statistical analysis: Mean and SD were calculated based on age and gender for clinical variables [dichotomous plaque index (PI) and gingival bleeding index (GBI)]; CPI-probing pocket depth and clinical attachment level (CPI-PPD and CPI-CAL) were analysed using SPSS 17.0 (Chicago, Illinois, USA). Distribution of gender, age, PI and GBI scores was compared using independent t test. UFLs were compared using Chi-square test. Distribution of genotypes among patients was evaluated using Fisher's extract probability test.
Results
The mean age ±SD of patients groups with and without dental fluorosis was 54±11.39 and 49.15±11.45 yr, respectively; the female-to-male ratio was 13:8 and 12:8, respectively, in the two groups. There was no significant difference in plaque and bleeding scores between the groups with and without dental fluorosis (Table I). The CPI-CAL score was significantly higher (P<0.05) patients with dental fluorosis as compared to without fluorosis group. The CPI shallow PPD of 4-5 mm was found to be 50 per cent in patients without dental fluorosis whereas deeper PPD>6 mm was seen in 62 per cent patients in fluorosis group. The CPI-CAL of 0-3 mm was highest in non-fluorosis group (70%) whereas the CPI-CALs of 4-5 and 6-8 mm were 48 and 10 per cent in fluorosis group, respectively. Based on fluorosis index, deeper PPD (>4-5 and >6 mm) was found in score B (50 and 31%, respectively).
| Clinical parameters | With dental fluorosis (n=21) | Without dental fluorosis (n=20) |
|---|---|---|
| Plaque scores (mean±SD) | 82.32±18.68 | 74.48±20.32 |
| Bleeding scores (mean±SD) | 75.95±21.09 | 67.46±16.82 |
| CPI-PPD (mean rank) | 22.19 | 19.75 |
| CPI-CAL (mean rank) | 24* | 17.85 |
*P<0.05 compared to group without dental fluorosis CPI-PPD, community periodontal index-probing pocket depth; CAL, clinical attachment level
The CPI-CAL (0-3 mm) was found to be highest in score B (44%), followed by score C (33%) and score D (22%). The CPI-CAL (4-5 mm) was found to be highest in score B (40%), followed by score D (30%), score C (20%) and score F (10%). The severe CPI-CAL (6-8 mm) was found to be highest in score C and score E (50%).
The CPI-CAL (0-3 mm) was maximum (66%) in mild osteoporosis, followed by CPI-CAL (4-5 mm) of 60 per cent in moderate osteoporosis and CPI-CAL (6-8 mm) of 50 per cent in moderate-to-severe osteoporosis. The CAL-PPD (4-5 and >6 mm) was maximum in moderate osteoporosis (62 and 61.53%, respectively). The UFLs were significantly (P<0.001) higher in patient group with dental fluorosis (1.17±0.14 mg/l) than in those without dental fluorosis (0.48±0.19 mg/l).
Oestrogen receptor (ER) Rsa I genotypes: For the Rsa I RFLP, a 156 bp DNA fragment was produced. Rr heterozygote (n=9) was observed in patients without dental fluorosis whereas RR mutant homozygote was absent in both the groups. The rr wild homozygote type occurred more in patients with dental fluorosis (n=21) than non-fluorosis group (n=11). Rr wild heterozygote was significantly more in non-fluorosis group than in fluorosis group. The frequency distribution of ER Rsa I genotype was rr=21 (100%), RR=0 (0%) and Rr=0 (0%) in fluorosis group and rr=11 (64%), Rr=9 (35.29%) and RR=0 (0%) in non-fluorosis group (Table II).
| Group | Rr (heterozygote) | rr (wild homozygote) | RR (homozygote) | Total |
|---|---|---|---|---|
| With dental fluorosis | 0 | 21 | 0 | 21 |
| Without dental fluorosis* | 9 | 11 | 0 | 20 |
| Total | 9 | 32 | 0 | 41 |
*P<0.05
Discussion
In the current pilot study, periodontal and osteoporosis status of patients with and without dental fluorosis was compared. Plaque scores, bleeding scores, PPD and CAL reported in the literature were useful in deciphering the occurrence of periodontitis and osteoporosis. However, a couple of studies have suggested an association between systemic osteoporosis and periodontal disease1718. Vishwanath et al 201119 showed weak but negative association between BMD and periodontal status. In contrast to the above findings, Pepelassi et al 201220 found higher bleeding scores in osteoporotic patients than in patients with normal bone mineral density.
Our study showed no association between BMD score of patients and PPD. Shen et al21 reported a significant relationship between PPD and osteoporosis at interproximal instead of the faciolingual sites. Ronderos et al22 also reported an association between osteoporosis and periodontal disease as measured by CAL.
The relationship between osteoporosis and periodontitis is difficult to assess as both diseases have multifactorial aetiology. Multiple systemic factors influence the progression of osteoporosis, including age, race, diet, gender, hormone therapy, smoking, fluorosis, genetic factors, exercise and body weight23. Several of these are also risk factors for severe periodontal disease. Genetic predisposition to systemic and periodontal bone loss may also be a factor, as well as environmental factors such as living in an fluorosis endemic area or lifestyle factors that predispose some people to both diseases.
Osteoporosis risk is modulated by genetic markers such as ESR1, including polymorphisms of the vitamin D receptor gene, the collagen I gene and several other candidate genes23. Studies have reported that there is the relationship between ER gene polymorphism and the occurrence of osteoporosis and other diseases. Tezal et al9 reported an association of ERα gene polymorphism with bone mass whereas others failed to find an association24. The differences among study population in terms of age and environmental may explain the inconsistency in results. A study conducted by Zhang et al2 indicated that ER-alpha may be a susceptible indicator for chronic periodontitis in female Han Chinese patients.
Oestrogen plays an important role in stimulating osteoblast activity and promoting the deposition of calcium and phosphate in bone4. Therefore, ER genetic polymorphism may have an impact on the combination of oestrogen and biological activity. Ba et al3 conducted a study on children aged 8-12 yr, born and raised in high fluoride areas and control areas of Henan province to explore the distribution of ER RsaI genotype in children in the areas with or without high fluoride and evaluated the relationship between ER RsaI gene polymorphism and dental fluorosis. They found that children carrying allele R of ER RsaI had a significantly increased risk of dental fluorosis compared to children carrying the allele r in endemic fluorosis areas3. Wang et al4 investigated relationship between ER gene RsaI polymorphisms and children's dental fluorosis status and found no correlation between the two.
In addition to the study of single genes or polymorphisms in isolation, it has been realized that both gene-gene and gene-environment interactions play an important role in influencing the variation of expression of complex traits such as periodontitis, dental fluorosis and osteoporosis within populations2’, 4. The main limitation of this study was small sample size. Thus, to confirm the role of ER RsaI gene polymorphism in osteoporotic and periodontitis patients with dental fluorosis, further study with larger sample size is required. Further, to ascertain the role of fluorosis in osteoporotic patients with periodontitis, further radiographical, histologic and biochemical (biochemical markers of bone destruction) osseous parameters need to be studied.
Acknowledgment:
Authors thank Dr Kishor Bhat and his team for the timely support and assistance.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Identification of genetic risk factors for periodontitis and possible mechanisms of action. J Clin Periodontol. 2005;32(Suppl 6):159-79.
- [Google Scholar]
- Correlation of estrogen receptor alpha gene polymorphisms and bone mineral density in Chinese women with chronic periodontitis. Chin Med J (Engl). 2010;123:3262-7.
- [Google Scholar]
- Study on the relationship between ER Rsa I gene polymorphism and children's dental fluorosis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:869-72.
- [Google Scholar]
- ERâ gene RsaI polymorphism and children's dental fluorosis. Life Sci J. 2010;7:51-5.
- [Google Scholar]
- Osteoporosis - An early radiographic sign of endemic fluorosis. Skeletal Radiol. 1986;15:350-3.
- [Google Scholar]
- Assessment of periodontal status in dental fluorosis subjects using community periodontal index of treatment needs. Indian J Dent Res. 2007;18:67-71.
- [Google Scholar]
- Fluorosis and periodontium: A report of our institutional studies. J Int Clin Dent Res Organ. 2014;6:7-15.
- [Google Scholar]
- Assessment of periodontal status of the patients with dental fluorosis in area with natural high levels of fluoride: A cross-sectional survey. Dent Hypotheses. 2013;4:127-30.
- [Google Scholar]
- The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492-8.
- [Google Scholar]
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1-29.
- [Google Scholar]
- Prevalence and severity of periodontitis in a high fluoride area in South Africa. Community Dent Oral Epidemiol. 1985;13:108-12.
- [Google Scholar]
- Endemic fluorosis of the skeleton: Radiographic features in 127 patients. AJR Am J Roentgenol. 1994;162:93-8.
- [Google Scholar]
- Government of India Ministry of Water Resources Central Ground Water Board. In: Ground Water Information Booklet Davanagere District, Karnataka, South Western Region Bangalore. 2012.
- [Google Scholar]
- Approach to the patient with secondary osteoporosis. Eur J Endocrinol. 2010;162:1009-20.
- [Google Scholar]
- Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229-35.
- [Google Scholar]
- Gingivitis and gingival recession in adults from high-fluoride and low-fluoride areas. Arch Oral Biol. 1972;17:1269-77.
- [Google Scholar]
- The strength of association between systemic postmenopausal osteoporosis and periodontal disease. Int J Prosthodont. 1996;9:479-83.
- [Google Scholar]
- Correlation of periodontal status and bone mineral density in postmenopausal women: A digital radiographic and quantitative ultrasound study. Indian J Dent Res. 2011;22:270-6.
- [Google Scholar]
- The relationship between osteoporosis and periodontitis in women aged 45-70 years. Oral Dis. 2012;18:353-9.
- [Google Scholar]
- Periodontal status in post-menopausal osteoporosis: A preliminary clinical study in Taiwanese women. J Chin Med Assoc. 2004;67:389-93.
- [Google Scholar]
- Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: Cross-sectional evaluation of US adults from NHANES III. J Clin Periodontol. 2000;27:778-86.
- [Google Scholar]
- Genetic control of susceptibility to osteoporosis. J Clin Endocrinol Metab. 2002;87:2460-6.
- [Google Scholar]
- No major effect of estrogen receptor gene polymorphisms on bone mineral density or bone loss in postmenopausal Danish women. Bone. 2000;26:111-6.
- [Google Scholar]






