Translate this page into:
Nucleic acid amplification test: Bridging the gap in blood safety & re-evaluation of blood screening for cryptic transfusion-transmitted infection among Indian donors
For correspondence: Dr Chand Wattal, Department of Clinical Microbiology & Immunology, Sir Ganga Ram Hospital, New Delhi 110 060, India e-mail: chandwattal@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Nucleic acid amplification test (NAT) in blood donor screening not only detects window period (WP) donors but also those with chronic occult infections which are negative by routine serological screening. This study was conducted to determine the time trend of NAT positivity and seroprevalence of transfusion-transmitted infections (TTIs) through a period of six years and evaluate the strength of NAT as a supplementary test in identifying the cryptic carriers in blood donor population.
Methods:
A total of 1,01,411 blood donations were screened between January 2011 and December 2016 by the ELISA and individual donor (ID) NAT Procleix Ultrio Plus Assay. Additional molecular and serological assays were done on the NAT yield samples to differentiate the type of cryptic carriers.
Results:
NAT yields comprised 0.05 per cent (50/101411) of the total samples tested with a yield rate of 1/2028. Hepatitis B virus (HBV) contributed to 80 per cent of the total NAT yields and the rest 20 per cent due to hepatitis C virus (HCV). Majority of HBV NAT yields (75%) were from chronic occult donors and 25 per cent were WP donors. Both HBV and HCV NAT yields had a wide range of viral count. There was no HIV NAT yield. A significant decline in the prevalence rate of TTIs through the study period of six years was observed.
Interpretation & conclusions:
The cryptic infections found in blood donors increase the risk of TTIs. Blood screening by both serology and NAT can reduce this threat.
Keywords
Blood donor screening
NAT
transfusion-transmitted infections
Transfusion-transmitted infections (TTIs) continue to be a major threat associated with blood transfusion in the developing nations. India has a high disease burden of viruses causing TTIs with a prevalence rate of 0.3, 2 and 2-8 per cent for human immunodeficiency virus (HIV), hepatitis C virus (HCV) and hepatitis B virus (HBV), respectively1. However, the seroprevalence for HIV, HCV and HBV among Indian blood donors ranges from 0.20 to 0.34, 0.34 to 1.09 and 0.75 to 2.61 per cent, respectively2. The implementation of nucleic-acid amplification test (NAT) screening has revealed the presence of cryptic infections caused by these viruses which are undetected serologically and are a potential threat of TTIs.
These cryptic TTIs are caused at varied stages of infection. Some are occult hepatitis B chronic carriers (OHB) defined as donors with exposure to HBV, indicated by positive HBV DNA. The other serological markers such as anti-HBc and/or anti-HBe are positive, but these OHB donors lack detectable hepatitis B surface antigen (HBsAg)3. Some of them harbour immunologically variant mutant virus which go undetected serologically3. Some are window period (WP) donors at an early stage of infection, viraemic but serologically negative. Then, there are immunosilent donors with prolonged serological WP hence undetected serologically.
Hence, zero risk with transfusion of blood and blood products is still an unattainable goal and ensuring blood safety is a challenging task. NAT as a supplement test to serology combines the advantage of direct and highly-specific detection of viral genomes (DNA and RNA) with sensitivity which is several times in magnitude greater than serology and can detect donors with cryptic infections. The objective of this study was thus to determine the seroprevalence and NAT positivity of HIV, HBV and HCV among blood donors during a six year period and to evaluate the impact of NAT in detecting different types of cryptic carriers in the donor population of a tertiary care hospital in north India.
Material & Methods
The study was carried out in the departments of Clinical Microbiology and Immunology and Transfusion Medicine, Sir Ganga Ram hospital (SGRH), New Delhi, India. The data reported here were collected over a period of six years from January 2011 to December 2016.
Ethical approval for the study was obtained from SGRH Institutional Review Board before the onset of the study (reference no. EC/07/14/688). The samples were collected as a part of the routine screening process in the blood bank with routine consent taken from the donors during blood screening.
A total of 101,411 blood donations were screened during the study period of six years. The samples collected were screened for three viruses viz., HIV, HBV and HCV serologically by ELISA (by Virostika HIV Ag-Ab, Hepanostika HBsAg ultra Biomerieux, France, Genedia HCV, Korea) using automated ELISA platform (DaVinci Quattro, Biomerieux, France) and by individual donation nucleic acid amplification test (ID-NAT) screening in the platform “Chiron Procleix ESAS (Enhanced Semi-Automated System) NAT System” (Novartis Diagnostics USA), using the Procleix Ultrio assay, which was subsequently replaced by Procleix Ultrio Plus assay from January 2014.
All the reactive samples were re-tested using fresh samples both by ELISA and ID-NAT. The Procleix ID-NAT India User algorithm was followed (Figure). All initially reactive (IR) samples were discriminated to HBV, HCV and HIV-1 as per the Procleix ID-NAT India User Algorithm. No follow-through was done on NAT NR (non-reactive) and seronegative samples which were reported as such. NAT was repeated thrice before reporting discordant reports which were the seronegative and NAT IR (NAT yield) or seropositive and NAT NR (sero-yield) samples.
One hundred blood donor samples with 90 negatives and 10 positives (including 2 NAT yields) were separately used as control samples which were sent for inter-laboratory comparison to a donor screening reference laboratory.
ID-NAT Procleix Ultrio Plus assay: The ID-NAT Procleix Ultrio Plus assay constitutes three steps which take place in a single tube. The three steps are as follows: sample preparation, HIV-RNA, HCV RNA and HBV DNA target amplification by transcription-mediated amplification and detection of the amplicon by hybridization protection assay (HPA) according to the Procleix® Ultrio Plus® Assay (www.fda.gov/media/112861/download).
A dual kinetic assay enables to differentiate between the signals of the internal control and combined HIV-1/HBV/HCV signals. The IR samples were further discriminated utilizing specific probe that help differentiating the specific viruses.
Additional serological tests were done on the NAT yield samples to detect and differentiate between the WP and OHB donors. Total anti-HBc, anti-HBe serology and anti-HBs serology were done using VIDAS automated enzyme-linked fluorescent assays (bioMerieux SA, France).
Reference method used for NAT yield samples: Real-time polymerase chain reaction was done on all NAT yield discriminated samples which further substantiated the NAT positivity and also determined the viral load using the Food and Drug Administration (FDA) approved quantitative COBAS Taqman v 2.0 HBV and HCV test using high pure (HP) system (Roche Molecular Systems, USA). The detection range for HBV assay was 29 - 1.1 × 108 IU/ml, counts between 6 and 29 was reported as below detection range (BDL) and <6 IU/ml was reported as a target not detected (TND). For HCV, the detection range was 25 - 3.9 × 108 IU/ml and <25 IU/ml was reported as a TND.
Statistical analysis: Statistical analysis was performed using the SPSS statistical package (version 17.0; SPSS Inc., Chicago, IL, USA). Prevalence rate of the TTIs was calculated and was further analyzed by studying the trend of increase and decrease over time. Time trend analysis from 2011 to 2016 was done by fitting the time trend function (y = aebt)5. Taking log on both sides log (y) = log (a)+bt, where b is the percentage growth rate per year. The growth rate was tested for its significance using t test. The yield rate was calculated as a ratio x/y, where x = 1 and y = total donations/NAT yields.
Results
A total of 101,411 donations were screened over a period of six years (2011-2016). The mean age of donor population was 33 ± 5 yr with a male: female ratio of 2.5:1 and the donations were drawn predominantly from replacement/voluntary family donors. A decreasing trend (in negative) of prevalence rate per year (denoted by ‘b’) of the total TTIs was observed through the period of six years (Table I). In 2011 HBV seroprevalence was 0.98 per cent and in 2016, it was 0.68 per cent (Table I). The decline in seroprevalence of HBV over a period of six years was significant (P=0.010). A significant decline in total seroprevalence of TTIs was also observed (P=0.001) (Table I). This decline was also observed in NAT screened samples (Table I).
| Parameters | Years, n (%) | b (%) | P | Overall prevalence rate, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2011 (n=12,543) | 2012 (n=16,592) | 2013 (n=17,704) | 2014 (n=17,494) | 2015 (n=18,225) | 2016 (n=18,853) | ||||
| HBV serology | 124 (0.98) | 134 (0.80) | 147 (0.83) | 136 (0.77) | 137 (0.75) | 130 (0.68) | (-)6 | 0.010 | 808 (0.79) |
| HCV serology | 20 (0.15) | 38 (0.22) | 30 (0.16) | 35 (0.20) | 28 (0.15) | 36 (0.19) | 0.70 | 0.87 | 187 (0.18) |
| HIV serology | 10 (0.07) | 14 (0.08) | 10 (0.05) | 12 (0.06) | 9 (0.04) | 11 (0.06) | (-)7.6 | 0.22 | 66 (0.065) |
| Total | 154 (1.2) | 186 (1.1) | 187 (1.05) | 183 (1.04) | 174 (0.95) | 177 (0.93) | (-)4.9 | 0.001 | 1061 (1.04) |
| HBV NAT | 123 (0.98) | 132 (0.79) | 143 (0.80) | 138 (0.78) | 142 (0.77) | 143 (0.75) | (-)4.1 | 0.05 | 821 (0.80) |
| HCV NAT | 18 (0.14) | 39 (0.23) | 26 (0.14) | 33 (0.18) | 21 (0.11) | 35 (0.18) | (-)2.0 | 0.78 | 172 (0.17) |
| HIV NAT | 10 (0.079) | 10 (0.06) | 9 (0.05) | 12 (0.05) | 9 (0.04) | 11 (0.06) | (-)7.4 | 0.20 | 61 (0.060) |
| Total | 151 (1.2) | 181 (1.09) | 178 (1.00) | 183 (1.04) | 172 (0.94) | 189 (1.0) | (-)3.8 | 0.041 | 1054 (1.03) |
HBV, hepatitis B virus; HCV, hepatitis C virus; NAT, nucleic acid amplification technique; HIV, human immunodeficiency virus; b, prevalence rate per year
A collective prevalence rate of TTIs in our study was 1.00 per cent (Table II). Individually, the TTI seroprevalence rate was 1.04 per cent and NAT positivity rate was 1.03 per cent, respectively (Table II). All NAT IR samples were discriminated. All were concordant positive discriminated and NAT yield discriminated. None were non-discriminated NAT yields or non-discriminated concordant positives as per the User algorithm (Figure). The total NAT NR seroyield donations comprised 0.056 per cent of the total donations (Table II) and constituted 5.3 per cent of the seroreactive samples (57/1061). These included 28 HBsAg positive samples, 24 anti-HCV and 5 HIV seropositive samples.
| TTI Yields | HIV-1 (%) | HBV (%) | HCV (%) | Total (%) | Prevalence rate of TTIs (%) |
|---|---|---|---|---|---|
| Total NAT reactive and seroreactive | 61 (0.06) | 780 (0.76) | 129+34=163 (0.160) | 1004 (1.00) | 1.00 |
| Seropositive TTIs | 66 (0.065) | 808 (0.79) | 187 (0.18) | 1061 (1.04) | 1.04 |
| NAT reactive | 61 (0.060) | 821 (0.80) | 172 (0.17) | 1054 (1.03) | 1.03 |
| NAT NR total sero-yields | 5 (0.006) | 28 (0.027) | 24 (0.023) | 57 (0.056) | 0.056 |
| NAT-yields (seronegative) | 0 | 40 (0.039) (1 co-infection with HCV) | 10 (0.009) | 50 (0.05) | 0.05 |
| NAT yield rate | - | 1/2535 | 1/10141 | 1/2028 | - |
TTIs, transfusion transmitted infections. Abbreviations are as given in Table I
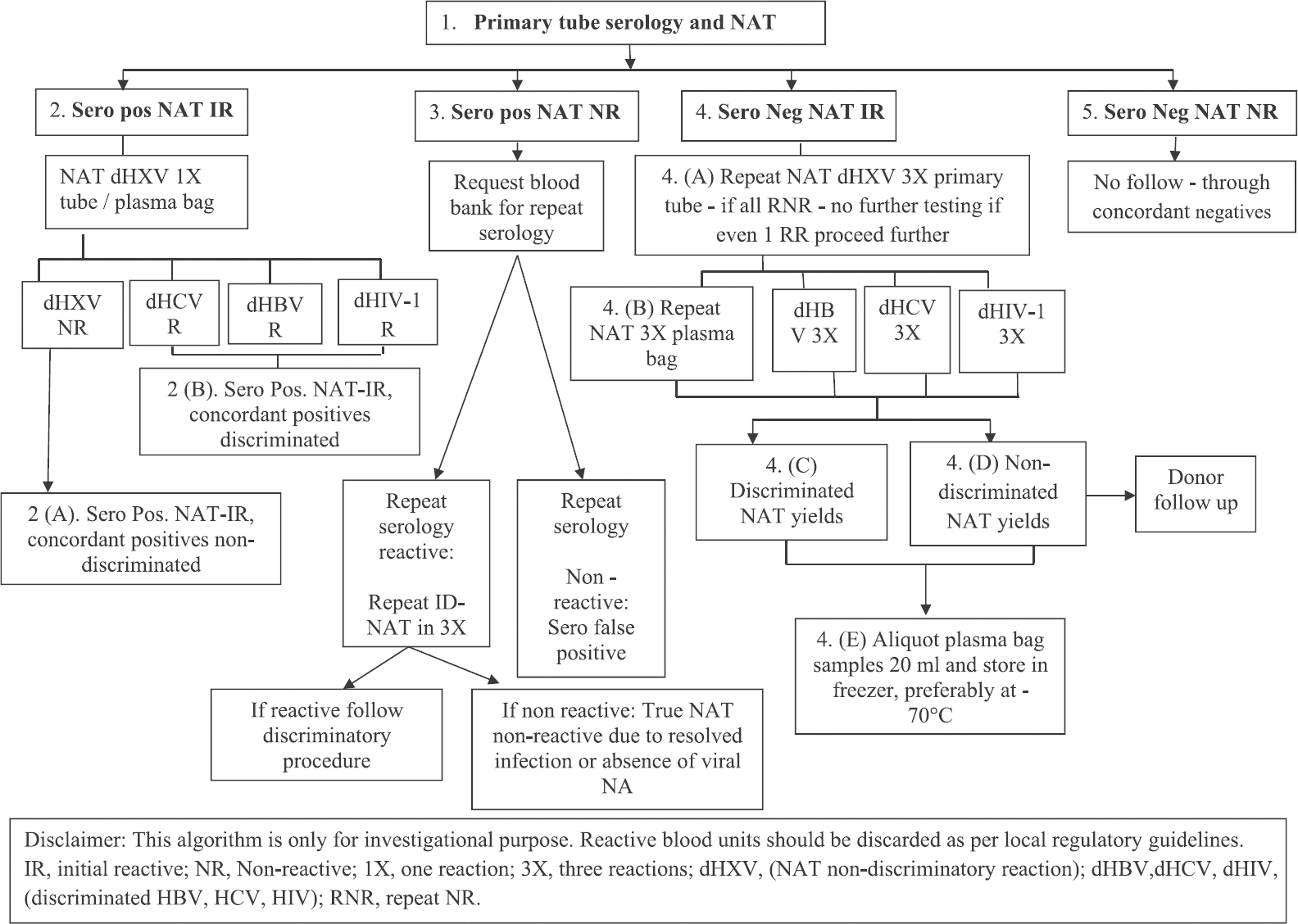
- Procleix ID-NAT India user algorithm. Source: Ref. 4.
The total seronegative NAT yields comprised 0.05 per cent of the total donations and constituted 4.7 per cent of the total NAT reactive samples (50/1054). Forty of the NAT yields (80%) were HBV DNA positive and rest were HCV RNA positive samples (Table II). The total NAT yield rate was 1 in 2028 donations (Table II). There was no HIV NAT yield. The virus-specific NAT yield rate was 1 per 2535 donations for HBV and 1 in 10,141 for HCV (Table II). Of the 40 HBV NAT yields, 10 were WP donors which were serologically inert with supplementary serological assays negative. Thirty were OHB chronic carriers of whom 24 were both total anti-HBc and anti-HBe positive and remaining six were only anti-HBc positive (Table III). These were all anti-HBs negative. There were only 10 HCV NAT yields and no HIV NAT yields. The results of all hundred control donor samples sent for interlaboratory comparison to a reference laboratory had a 99 per cent concordance with our report.
| NAT yield viruses (n=50) Other seromarkers | HBV DNA positive (HBsAg negative) samples (n=40) | HCV RNA positive (anti-HCV negative) samples (n=10) Window period | ||
|---|---|---|---|---|
| Seronegative (early window) | Anti-HBe and total anti-HBc positive OHB | Only total anti-HBc positive OHB | ||
| Viral counts: BDL 6-29 IU/ml | 4 | 10 | 5 | |
| <2×102 IU/ml | 6 | 10 | 1 | 3 |
| 200-103 IU/ml | - | 3 | - | 2 |
| >103 IU/ml | - | 1 (co-infection) | 5 | |
| Total | 10 | 24 | 6 | 10 |
OHB, occult hepatitis B; HBsAg, hepatitis B surface antigen; Anti-HBc, hepatitis B core antibody; Anti-HBe, hepatitis Be antibody; BDL, below detection range . Abbreviation are as given in Table I
Discussion
A decreasing trend in all the TTIs was observed in our study. A similar declining trend in seroprevalence was also observed by Makroo et al6. This could be attributed to a significant fall in HBV positivity which was the predominant TTI observed among the donors in our study. Our combined NAT yield rate of 1/2028 was close to that observed by Makroo et al7 and less than that reported by another study8 from Delhi. However, it was 20-100 times higher than that observed in developed nations9. The HBV donor yield rate in various parts of the world ranged from 1 in 350,000 to 610,000 in North America, 1 in 200,000 in Europe, 1 in 50,000 in Japan and <1 in 5000 in SE Asia9. We had an HBV yield rate of 1 in 2535.
With 10 per cent of the world's HBV population, the estimated residual risk of HBV TTI is significantly higher than the risk of either HIV-1 or HCV10. The introduction of NAT in blood donor screening helped to identify the occult TTIs in the donor population. The NAT yield in our study predominantly comprised the OHB donors. OHB is a disparate group of HBV infection with a low level of circulating HBV DNA in the absence of HBsAg, and the donor remains infective11. The prevalence of chronic OHB in India is 7.5-30 per cent as compared to 0.1-2.4 per cent in developed nations9. In our study, 75 per cent of the HBV NAT yields were of chronic OHB carriers who were anti-HBc and or anti-HBe positive. All were anti-HBs negative and were infective.
Although OHB carriers are usually characterized by very low-HBV DNA load in plasma (<200 IU/ml), in our study, four of the 30 OHB cases had counts more than 200 IU/ml. This was contrary of the 2008 Statements from Taormina expert meeting on occult hepatitis infection12 that had clarified the definition of OHB in establishing a threshold value of plasma HBV DNA <200 IU/ml. Those with plasma HBV DNA levels >200 IU/ml are considered as ‘false OHB’ and are usually due to infection by HBV variants with mutations in the S gene (escape mutants which needs further evaluation)12. Similar findings have been reported from Japanese Red Cross OHB donors with viral loads >103 IU/ml10. The viral counts in 91 per cent of OHB donor samples of European origin reported a high viral load with a maximum of 5640 IU/ml13. A Southeast Asian study detected a viral count of 3,670 IU/ ml in OHB blood donors14.
Although infectivity of OHB carriers is significantly lower than those of WP infections9, but breakthrough transmissions are known to occur irrespective of the viral counts and some have fatal outcomes15, especially in donors with no anti-HBs titres as was observed in our study. The highest viral load recorded in OHB donors in our study was 1280 IU/ml. This occult HBV donor had co-infection with HCV. Globally, the prevalence of HCV co-infected OHB carriers has been reported to be from 6.7 to 91 per cent16. HBsAg clearance is 2.5 times higher in HCV co-infected cases than HBV infection alone7. This could be another possible reason for HBsAg negativity irrespective of the high viral count.
About 8-18 per cent of donors in India are anti-HBc positive11, and the inclusion of this marker for TTI screening would result in attrition of a large number of donor samples, causing shrinkage of effective donor population11. Studies from India have reported only 12.4 per cent HBV DNA positive amongst anti-HBc positive donors17. Inclusion of NAT would be expensive, but a better alternative and will prevent wasteful discard of this useful resource.
Another important contribution of ID-NAT is reduction of the WP. The average WP during which immunological assays are unable to detect the anti-HIV, anti-HCV and HBsAg is estimated to be 19, 65 and 38 days, respectively. ID-NAT reduces it to an average of 5, 7 and 15.8 days for HIV, HCV and HBV, respectively1819. Sero-silent HCV infection due to prolonged WP, though a phenomenon in immuno-suppressed patients20 is a sporadic phenomenon among blood donors21. Genetic predispositions or defects affecting humoral responses against HCV have been suggested, and their reported prevalence among the blood donors is 0.0004-0.08 per cent21. All the HCV NAT yields in our study had a wide range of viral load from BDL to >1000 IU/ml. All these NAT yields would have been missed if only serological screening was used.
There was no HIV NAT yield in our study. From India, the reported NAT yield for HIV is as low as 0.016 per cent7, suggesting residual risks of TTI with HBV and HCV are comparatively more than HIV amongst blood donors. In our study, 0.027 per cent donors were HBV sero-yield donors which comprised 3.4 per cent of the total HBV seropositive donors. A prevalence rate of 30 per cent of HBV sero-yield carriers was reported by Pandey et al22. Further studies are needed to understand the infectivity of such carriers though 4-20 per cent of them have spontaneous viraemia after years of quiescence23. Five HIV sero-yield donors (0.006%), repeat non-reactive by NAT were detected in our study. Possibly, they could have been aviraemic donors at the time of donation and infectivity of this subgroup of patients have been documented24.
In India, serology continues to be the mainstay for donor screening. Although NAT testing is done in a few centres, but it is not a mandatory screening test for TTIs in India19. This study highlights the persistence of chronic OHB carriers with varied viral counts, as the predominant contributor of TTIs among blood donors. Though there was a significant decreasing trend of TTIs observed through the study period, the presence of such occult as well as WP infections among the seemingly healthy population of our country shows the benefit added, when NAT is combined with serology. The dual screening strategy adds a safety cover to the threat caused by cryptic TTIs.
Acknowledgment:
Authors acknowledge Ms. Parul Thakkar for her statistical help.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Individual donor-nucleic acid testing for human immunodeficiency virus-1, hepatitis C virus and hepatitis B virus and its role in blood safety. Asian J Transfus Sci. 2015;9:199-202.
- [Google Scholar]
- Seroprevalence of transfusion-transmissible infections among replacement and voluntary blood donors in a tertiary care hospital. Indian J Sex Transm Dis AIDS. 2017;38:101-2.
- [Google Scholar]
- Comparison of Procleix Ultrio Elite and Procleix Ultrio NAT assays for screening of transfusion transmitted infections among blood donors in India. Int J Microbiol 2016:2543156.
- [Google Scholar]
- A ten year analysis of multi-drug resistant blood stream infections caused by E. coli & K. pneumoniae in a tertiary care hospital. Indian J Med Res. 2012;135:907-12.
- [Google Scholar]
- Seroprevalence of infectious markers & their trends in blood donors in a hospital based blood bank in North India. Indian J Med Res. 2015;142:317-22.
- [Google Scholar]
- Multicenter evaluation of individual donor nucleic acid testing (NAT) for simultaneous detection of human immunodeficiency virus -1 & hepatitis B & C viruses in Indian blood donors. Indian J Med Res. 2008;127:140-7.
- [Google Scholar]
- Nucleic acid testing for blood banks: An experience from a tertiary care centre in New Delhi, India. Transfus Apher Sci. 2013;49:482-4.
- [Google Scholar]
- Occult hepatitis B virus infection: A covert operation. J Viral Hepat. 2010;17:1-5.
- [Google Scholar]
- Anti-HBc screening in Indian blood donors: Still an unresolved issue. World J Gastroenterol. 2008;14:5327-30.
- [Google Scholar]
- Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652-7.
- [Google Scholar]
- An overview of occult hepatitis B virus infection. World J Gastroenterol. 2011;17:1927-38.
- [Google Scholar]
- Occult hepatitis B infection in blood donors from South East Asia: Molecular characterisation and potential mechanisms of occurrence. Gut. 2012;61:1744-53.
- [Google Scholar]
- HBsAg non reactive HBV infection in blood donors: Transmission and pathogenecity. Med Virol. 2007;79:S32-6.
- [Google Scholar]
- Role of occult hepatitis B virus in chronic hepatitis C patients with flare of liver enzymes. Eur J Intern Med. 2011;22:187-90.
- [Google Scholar]
- Significance of anti-HBc screening of blood donors and its association with occult hepatitis B virus infection: Implications for blood transfusion. Indian J Med Res. 2010;132:312-7.
- [Google Scholar]
- Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion. 2009;49:1115-25.
- [Google Scholar]
- Utility of routine real time quantitative PCR monitoring of HCV infection in haemodialysis patients. Indian J Med Microbiol. 2015;33:106-11.
- [Google Scholar]
- Seronegative hepatitis C virus infection. Arch Immunol Ther Exp (Warsz). 2014;62:145-51.
- [Google Scholar]
- A comprehensive serological and supplemental evaluation of hepatitis B “seroyield” blood donors: A cross-sectional study from a tertiary healthcare center in India. Asian J Transfus Sci. 2015;9:189-94.
- [Google Scholar]
- Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376-84.
- [Google Scholar]
- Possible transmission of human immunodeficiency virus-1 infection from an Elite controller to a patient who progressed to acquired immunodeficiency syndrome: A case report. J Med Case Rep. 2012;6:291.
- [Google Scholar]






