Translate this page into:
Non invasive real-time monitoring of bacterial infection & therapeutic effect of anti-microbials in five mouse models
Reprint requests Dr Tarani Kanta Barman, Ranbaxy Research Laboratories, Department of Infectious Diseases, New Drug Discovery Research (R & D-3), #20, Sector-18, Udyog Vihar Industrial Area, Guragon 122 001, India tarani.barman.ud@dsin.co.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
In vivo imaging system has contributed significantly to the understanding of bacterial infection and efficacy of drugs in animal model. We report five rapid, reproducible, and non invasive murine pulmonary infection, skin and soft tissue infection, sepsis, and meningitis models using Xenogen bioluminescent strains and specialized in vivo imaging system (IVIS).
Methods:
The progression of bacterial infection in different target organs was evaluated by the photon intensity and target organ bacterial counts. Genetically engineered bioluminescent bacterial strains viz. Staphylococcus aureus Xen 8.1, 29 and 31; Streptococcus pneumoniae Xen 9 and 10 and Pseudomonas aeruginosa Xen-5 were used to induce different target organs infection and were validated with commercially available antibiotics.
Results:
The lower limit of detection of colony forming unit (cfu) was 1.7-log10 whereas the lower limit of detection of relative light unit (RLU) was 4.2-log10. Recovery of live bacteria from different target organs showed that the bioluminescent signal correlated to the live bacterial count.
Interpretation & conclusions:
This study demonstrated the real time monitoring and non-invasive analysis of progression of infection and pharmacological efficacy of drugs. These models may be useful for pre-clinical discovery of new antibiotics.
Keywords
Bacterial infection
bioluminescence
mouse model
new antibiotics
Evaluating in vivo efficacy of new chemical entities (NCE) for proof of concept (POC) usually performed in the animal infection models is of paramount importance in preclinical drug discovery before recommending it for clinical testing in human beings12. POC in animal model demonstrates the probability of the hypothesis34. The main limitations of animal modeling are the significant amount of time and large number of animals required for in vivo testing of a NCE. Live animal in situ bio-photonic imaging is a technique that has contributed significantly to the understanding of bacterial pathogenesis and efficacy of drugs against infections in vivo56. Genetically modified bacterial pathogens have been used to monitor the progression of infection in live animals5–12. In this technique the bacterial pathogens engineered to express bioluminescence can be readily monitored from outside of the living infected animal using specialized imaging equipment, enabling their growth, expansion and treatment efficacy completely without sacrificing the animals. Moreover, the same group of animals can be imaged at each time-point throughout the study reducing the total number of animals used. As each animal acts as its own control over time, the issues associated with animal-to-animal variation are also circumvented, thus improving the quality of the biostatistical data generated13. This enables investigators to observe disease profiles that could otherwise easily be missed using conventional methodologies89.
In this study we aimed to establish five rapid, non-invasive murine infection models with bioluminescent Staphylococcus aureus, Streptococcus pneumoniae and Pseudomonas aeruginosa using in vivo imaging system (IVIS) 100. The experimental protocol and validation of models with commercially available standard antimicrobial agents by visualizing the photon signals are presented here.
Material & Methods
Experimental animals: All animal studies were conducted after ethical approval from the Institutional Animal Ethics Committee (IAEC), Ranbaxy Research Laboratory, Gurgaon, Haryana, India and as per guidelines of the Committee for the Purpose of Control & Supervision of Experiments on Animals (CPCSEA), New Delhi, India. Swiss albino mice weighing 20 ± 2 g of either sex were used for the study. Animals were taken from the animal breeding and housing facility, Ranbaxy Research Laboratory, Gurgaon, India. Animals were taken 2-3 days prior to the start of experiments to acclimatize to the experimental environment (temperature 25°C±2°C and relative humidity 30-70%). Feed and water were provided ad libitum during the whole study. In all treated as well as untreated control groups, the experiments were performed taking five animals in each group.
Culture medium, antibiotics and bioluminescent bacterial strains: All bacteriological media used were purchased from Becton, Dickinson and Company, MD, USA. Linezolid (LNZ) was obtained from National Chemical Laboratory, Pune, India. Vancomycin was purchased from API manufacturing facility of Ranbaxy Research Laboratories. Ceftriaxone, levofloxacin, amoxicillin and telithromycin were purchased from commercial sources. All genetically engineered bioluminescent bacterial strains viz., Staphylococcus aureus Xen 8.1, 29 and 31; Streptococcus pneumoniae Xen 9 and 10 and Pseudomonas aeruginosa Xen 5 were purchased from Xenogen, California, USA (Caliper Life sciences, USA). In Vivo Imaging System-100 (IVIS-100) was purchased from Xenogen, California, USA and was installed in the optical imaging laboratory, Ranbaxy Research Laboratories, Gurgaon, India.
Imaging: The animals were imaged under IVIS-100 at regular time intervals to monitor the change in bioluminescence emitting from the different target organs. Isoflurane gas anaesthesia machine (XG-8 Gas Anaesthesia System, Xenogen USA) was used to anaesthetize mice prior and during imaging. The total photons emission in terms of relative light units (RLU) in the region of interest (ROI) with respect to exposure time, binning, f/stop was standardized.
In vitro susceptibility testing: Minimum inhibitory concentration ( MIC) of all antibacterial drugs used was determined in-house against all the bioluminescent bacterial pathogens using microbroth dilution methods14.
Rapid mouse protection model: S. aureus Xen 29 was grown in trypticase soy broth (TSB) kept in a shaker incubator (200 rpm) at 37°C for 2½ h. The optical density of 1:10 diluted culture was adjusted to 0.3 - 0.4 at 600 nm. The culture was mixed with equal volume of 5 per cent hog gastric mucin and 0.5 ml of the suspension was injected intraperitoneally into each mouse. Only single dose of treatment was given half an hour post infection with vancomycin (25 mg/kg) by subcutaneous (s.c.) route; linezolid (25 mg/kg) and telithromycin (50 mg/kg) by p.o. route. Animals were imaged at 3 and 6 h to examine growth and treatment effect. The concentration of antibiotics and the treatment regimens were selected on the basis of information already available15–18.
Mouse pulmonary infection model: Pulmonary infection was induced by S. pneumoniae Xen 10. The overnight grown culture was suspended in sterile saline and OD was adjusted to 0.3-0.4 at 560 nm which was further diluted with saline in 1:10 ratio and used as final inoculum. For this infection, a 20 G bent needle with 30° angle at the tip through which polyethelene tubing (outer diameter 0.61 mm and internal diameter 0.28 mm) could freely pass, was designed. Disposable 1 ml syringe was fixed at the one end of tubing and the other end was allowed to pass through the needle. This assembly was used for directly instilling the infection into the mouse lungs. Mice were initially anaesthetized in isoflurane gas anaesthesia machine. The needle was gently inserted into the trachea per os which was confirmed by feeling of tracheal ring with the tip of the needle. Once the needle was inside the trachea, it was kept fixed and polyethylene tubing was passed to the base of the lungs and 50 μl of culture (108-107 cfu/ml) was instilled into the lungs. The needle assembly was withdrawn immediately. After 1, 6, 8, 12 and 24 h all animals were imaged under IVIS and animals showing good bioluminescence at 1 h were considered for treatment protocol. Animals were treated with two doses of telithromycin (25 mg/kg, po) at 2 and 7 h post-infection. At the end of experiment animals were sacrificed and lungs were removed, aseptically homogenized in 1 ml normal saline and ten-folds serially diluted tissue homogenates were spotted on blood agar plate. Individual animals were evaluated separately for cfu determination. Then plates were incubated overnight at 37°C under 5 per cent CO2 and cfu was determined.
Mouse thigh infection (immunocompromised versus immunocompetent): Mice were rendered immunocompromised (neutrophil count <100/mm3) with two intraperitoneal injections of cyclophosphamide (Dubar India Limited, New Delhi) on days -4 and -1 prior to infection at 150 and 100 mg per kg body weight. Overnight grown culture of S. aureus Xen 8.1 was adjusted to 2.3 Mac Farlands. The suspension was then diluted with normal saline in 1:10 (v/v) and mixed with 5 per cent hog mucin in 1:1 proportion and 100 μl was injected intramuscularly in the thigh muscles of the mice. Similarly, the immunocompetent mice were also infected with 100 μl of this culture suspension. Before treatment all the mice were imaged under IVIS and those showing good photon signals were selected for the treatment. Two doses of linezolid (100 mg/kg) were administered orally at +2 h and +7 h post-infection to both compromised and competent mice. Animal were imaged at 1, 6, 12, and 24 h post-infections. At the end of 18 h after the last dose animals were sacrificed, thighs were excised out, homogenized in 1 ml normal saline and ten folds serially diluted tissue homogenates were spotted on tryptic soy agar (TSA) plates.
Mouse meningitis model: S. pneumoniae Xen-9 culture was grown on Columbia blood agar (CBA) plate in CO2 incubator at 37°C overnight. This culture was adjusted to 3.3 Mc Farland in sterile normal saline and 20 ml was injected intracranially to (on right orbital surface of the zygomatic bone) the pre-anaesthetized mice with isoflurane gas anaesthesia. After 1 h of infection, animals were imaged under IVIS-100 and those showing photon signals in brain region were selected for the study. Treatment was started 1 h post infection. The mice were treated with telithromycin (100 mg/kg), amoxycillin, vancomycin and ceftriaxone (50 mg/kg) twice daily for two days. Both telithromycin and amoxycillin were administered via oral route while ceftriaxone and vancomycin were injected by s.c. route. At 48 h after taking image, animals were sacrificed and brains were removed, aseptically homogenized in 1 ml normal saline and ten folds serially diluted tissue homogenates were spotted on blood agar plate. The plates were incubated and bacterial counts were enumerated as stated previously.
Murine burn wound infection model: Topical subcutaneous burn wound infection was established under general anaesthesia with ketamine xylazine mixture (5 and 1 mg/kg per kg body weight ketamine and xylaxine, respectively). The mice backs were shaved and to induce a thermal injury, a brass bar (10×10×100 mm) was heated in boiling water (95°C ± 2°C) for 15 min. The end of the heated bar was then applied on the shaved back of the mice for 10-20 seconds. Immediately after the creation of the burn, the mice were resuscitated with intraperitoneal injections of 0.5 ml sterile normal saline. P. aeruginosa Xen-5 culture was grown on TSA plate at 37°C overnight. This culture was suspended in normal saline and adjusted to 3 Mc Farland which was then diluted 1:1000 in normal saline. For topical infection, 100 μl of this inoculum containing 1-5 ×104 bacteria was injected s.c. at the sites of the burn. One hour post-infection, mice were treated with ceftriaxone (sc) and levofloxacin (po) 50 mg/kg body weight, twice a day (BID) for 5 days.
Data analysis: In vivo data were transformed to log scale and column statistics was done by graphpad prism 4.1 and statistical significance analysis was done by Dunnett's multiple pair comparison. Image analysis was done by living image softwareR 3.1 (Xenogen, now Caliper Life Science, MA, USA).
Results
In vitro susceptibility assay: Minimum inhibitory concentrations of all the available strains against a panel of commercially available antibiotics are presented in the Table.
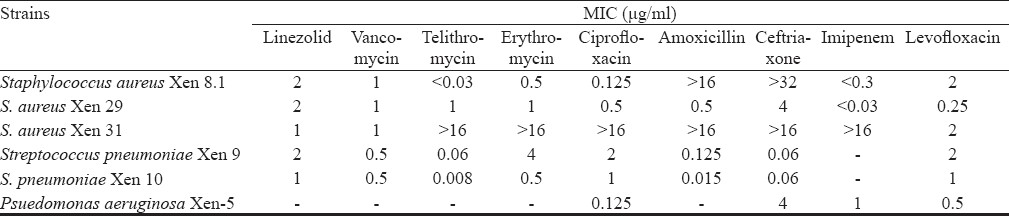
Rapid mouse protection model: This is a monoparametric basic screening model for early hit identification. The infected untreated animals showed a progressive increase in bioluminescent signal from 1 to 6 h, after which the animals started dying. In the treated groups, there was a decline in the signal correlating with the apparently healthy condition and ultimately, survival of the animals. Linezolid and vancomycin showed clearance of infection by 3 h at a dose of 25 mg/kg body weight. However, photon reduction in telithromycin (50 mg/kg) treated group was delayed and was seen at 6 h (Fig. 1).
- Rapid mouse protection model: Real time monitoring of effect of antibiotics on S. aureus Xen 29 intraperitoneally infected mice (n=3 representative mice). Mice were treated 30 min postinfection and all treated and untreated mice were imaged at 3 and 6 h post-infection by using IVIS camera. At 6 h, both linezolid and vancomycin showed less photon signal indicating curing effect. Telithromycin reduced the photon signal partially indicating drug is less effective against S. aureus Xen 29.
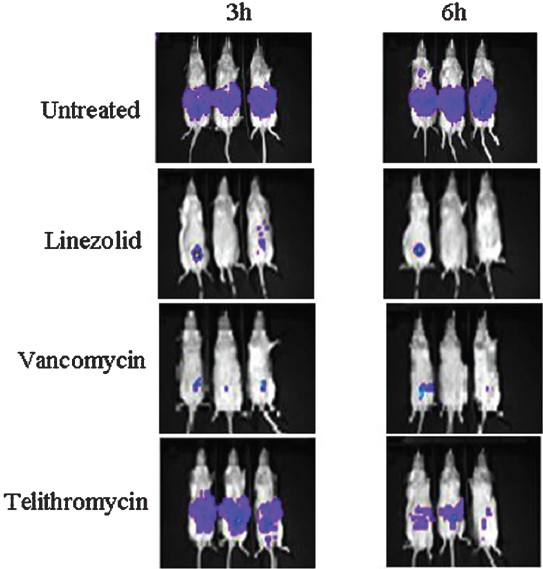
Mouse pulmonary infection model: In murine pulmonary infection, the progression of the bacterial growth assessed by photon intensity in the lung tissue was monitored at 1, 6, 8, 12 and 24 h post infection (Fig. 2) and live bacterial count was assessed at 24 h by the culture method. The difference in photon intensity in terms of RLU in treated group was compared with that of untreated mice. The mean log10 bacterial count (±SEM) in the untreated and telithromycin treated mice was found to be 8.026±0.609 and 3.60 ±0.547, the bacterial load reduction in lungs being 4.426-log10. The photon signal reduction in telithromycin treated mice was approximately 1.74-log as compared to the untreated animals (Fig. 2).
- Murine pulmonary infection model with S. pneumoniae Xen-10 showing the progression of the bacterial growth in untreated control mice as compared to standard drug telithromycin 25 mg/kg body weight treated mice at the beginning of dosing (1 h) and the curing effect of drug at 6, 8, 12 and 24 h.
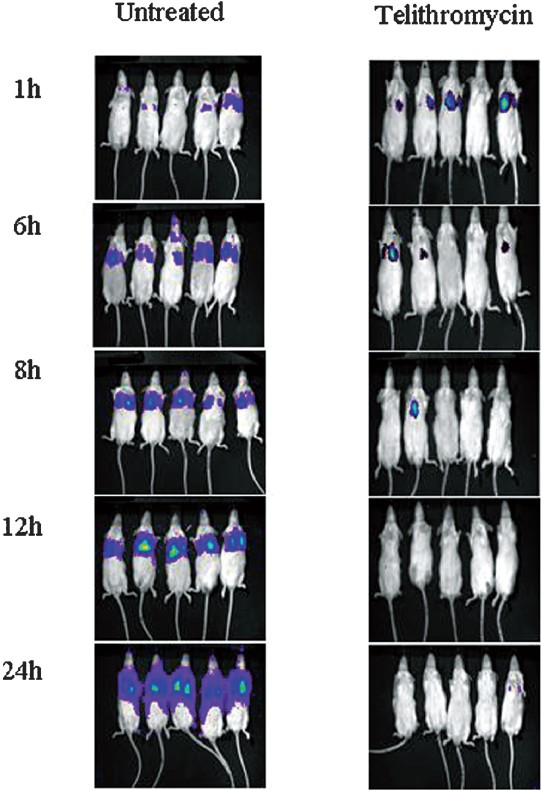
Mouse thigh infection Model: The mean log bacterial count (±SEM) of immunocompromised untreated control mice thigh was 8.884 ± 0.116 and in case of immunocompetent mice it was 6.223 ± 0.247. The log10 bacterial count declined to 7.366 ± 0.323 and 3.513 ± 0.217, respectively, after two doses of linezolid at 100 mg/kg body weight, indicating that bacterial log10 reduction was more in case of immunocompetent (2.71-log10) than that of immunocompromised (1.16-log10) mice. Similarly, reduction in log10 RLU in linezolid treated groups was found to be 1.743-log10 and 0.919-log10 in immunocompetent (Fig. 3A) and immunocompromised (Fig. 3B) mice, respectively, as compared to untreated control.
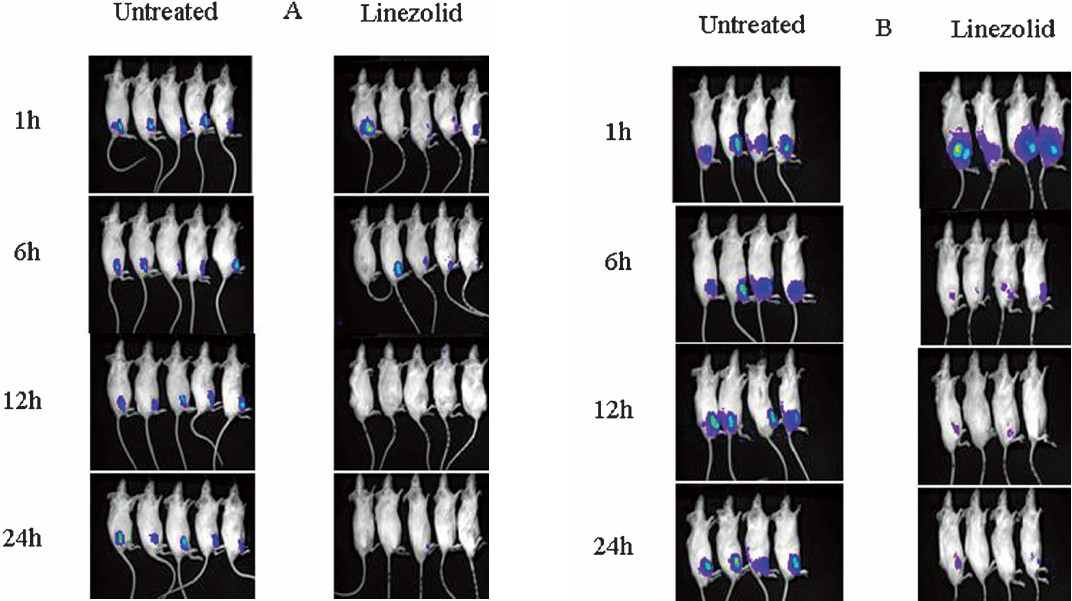
- Thigh infection with S. aureus Xen 8.1 in Immunocompetent (A) and Immunocompromised (B) mice showing the progression of the bacterial growth in untreated control mice as compared to linezolid 100 mg/kg body weight treated mice at the beginning of dosing (1 h) and the curing effect of drug at 6, 12 and 24 h.
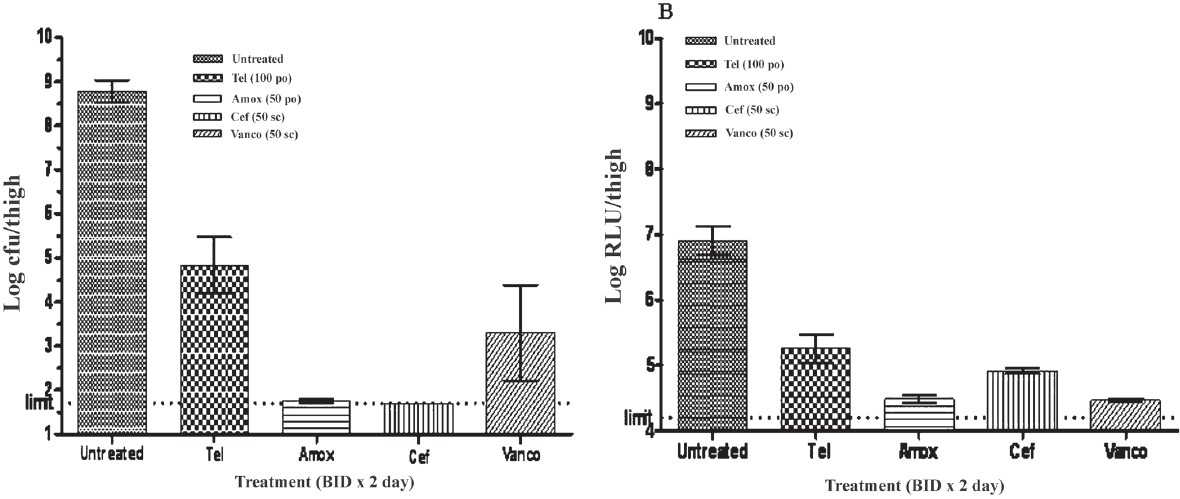
Mouse meningitis model: The mean log10 live bacterial count (±SEM) in untreated control was found to be 8.777 ± 0.253 which declined to 4.826 ± 0.641, 1.749 ± 0.0502, 1.699 ±.000 and 3.299 ± 1.084 in telithromycin, amoxicillin, ceftriaxone, and vancomycin treated mice, respectively after 2 days of treatment regimens. The corresponding RLU values were 6.908 ± 0.215, 5.255 ± 0.224, 4.488 ± 0.055, and 4.918 ± 0.044 and 4.472 ± 0.021. The cfu reduction was found to be the maximum in ceftrixone (7.078-log10) and minimum in telithromycin (3.951-log10) treated group. The reduction in RLU in different treated animals ranged from 1.653-log10 to 2.460-log10 when compared with the RLU of untreated mice (Fig. 4). Bacterial counts of infected brain and meninges and their bioluminescent signals at 48 h are presented in Fig. 5. After 24 h, animals showed strong bioluminescent signals throughout the spinal canal, indicating acute meningitis of the intracranial and intraspinal meninges. A decline in bacterial cell viability, as judged by reduction in the bioluminescent signal, was observed over time in the animals treated with ceftriaxone, epository, telithromycin and vancomycin but not in the untreated groups. Mice treated with the antibiotics survived the infection, whereas all the mice in untreated groups became morbid and finally succumbed to death.
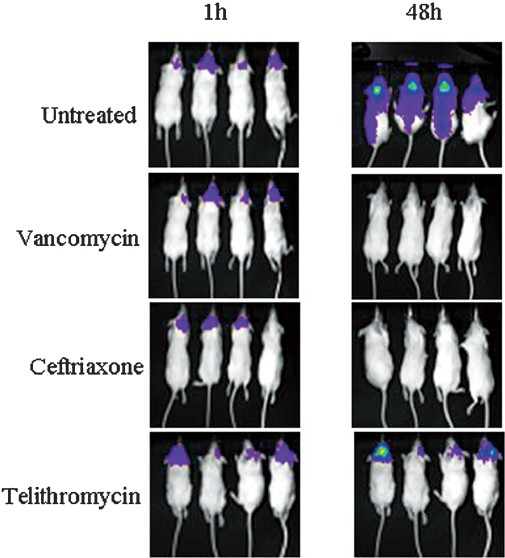
- Murine meningitis model showing photon intensities of infected untreated mice and mice treated with vancomycin, ceftriaxone, and telithromycin at the time of start of treatment (1 h) and curing effect after two days of treatment at 48 h.
-
(A) Mean ± SEM log cfu/ml of various treatment groups in murine meningitis model with S. pneumoniaeXen 9 (B): Mean (± SEM) log RLU/thigh (Photon) of various treatment groups in murine meningitis model with S.pn Xen 9. The treatment groups showing statistically significant reduction in comparison to untreated control group (*P<0.05).
Burn wound infection: In superficial subcutaneous burn wound infection model the mean log10 bacterial count of the untreated control mice was 9.012 ± 0.1758 which declined to 5.382 ± 0.3126 in ceftriaxone and 2.135 ± 0.219 in levofloxacin treated group, indicating that bacterial log10 reduction was more in case of levofloxacin (6.877-log10) than that of ceftriaxone (3.63-log10). The mean log10 RLU in untreated, levofloxacin and ceftriaxone treated group were 7.102 ± 0.185, 4.790 ± 0.093 and 4.486 ± 0.031, respectively (Fig. 6). The dynamic range of log10 RLU reduction was found to be 2.312 to 2.616 which was less as compared to log10 cfu reduction.
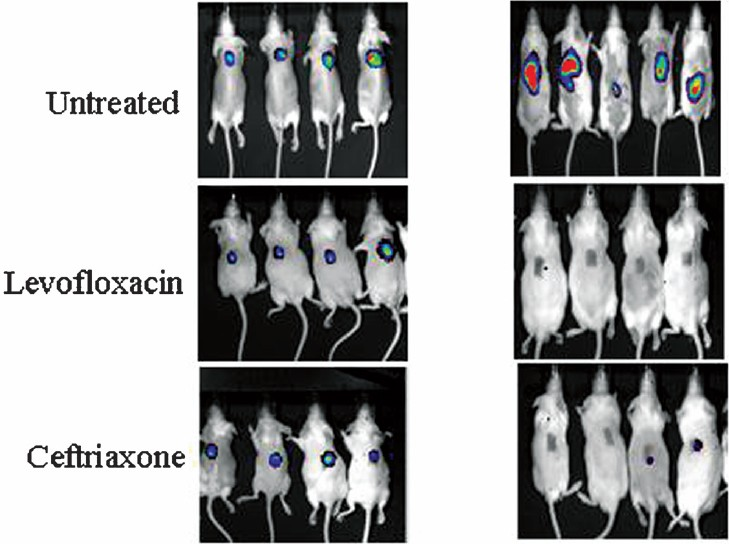
- Mouse superficial burn wound infection model with Pseudomonas aeruginosa Xen-5 treated with levofloxacin (25 mg/ kg) and ceftriaxone (50 mg/kg) at the start of treatment and curing effect on day 5 after completion of treatment protocol as compared to untreated control mice.
Discussion
Rapid, reproducible and non invasive animal models are required for screening new antibiotics1011. It takes lots of time from in vitro hit identification to in vivo leads optimization due to the lengthy process of the animal efficacy studies. Contag et al6 for the first time reported the photonic detection of bacterial pathogens in living animals. We established five rapid, reproducible, and non-invasive murine pulmonary, skin and soft tissue viz. thigh and superficial burn wound; rapid mouse protection assay, and meningitis models using Xenogen bioluminescent strains and specialized IVIS which were validated with commercially available drugs. The protocol followed in the present study was modified from the previous workers to make the model high throughput, reproducible and robust81219–21.
Both quantification of bioluminescent signals in the form of RLU and live bacterial detection as colony forming units per milliliter (cfu/ml) were able to reveal the efficacy of active commercially available drugs. The lower limit of detection in case of cfu was 1.7-log10 whereas the lower limit of RLU was 4.2-log10. These results were in agreement with previous worker1022. The conventional method of cfu determination suffers from many limitations such as requirement of large number of animals, more time, and more labour, difficult extraction process of bacteria from target organs, and contamination of plates due to saprophytes. Most importantly, repeated sampling from same animals is not possible in case of cfu methods as death is the end point in tissue sampling. In case of RLU methods tissue sampling is not required as same animal can be imaged repeatedly. Besides, ethical issues like least invasiveness and less manipulation on animal physiology have added advantages to RLU method.
The rapid mouse protection study using IVIS can be performed and analyzed in one day, and thus can be used as a high throughput screening assay for determining the efficacy of NCEs. Reduction in the number of animals used in this model without reducing the quality of the data obtained will be of tremendous advantage over traditional method.
The reproducibility of intracranial route of infection of meningitis model was much better than that of LP and IC methods reported by Xenogen20. The in vivo imaging of bacterial meningitis permits a more convenient and rapid method to monitor the infection, starting early in the infection and following the disease and efficacy of therapeutic agents at various time points non-invasively, in the same animal without sacrificing the animals. Bioluminescent measurements proved to be an excellent substitute for the traditional methods used to measure pathogen burden.
These animal infection models would help screening of NCEs at an early stage of drug discovery where large numbers of animals are required. Moreover, these models are scientifically logical and convincing as they allow detection of infection in the mice as early as 1 h post inoculation; provide spatial and temporal distribution of bacteria in the target organs throughout the entire disease process and the rapid monitoring of treatment efficacy in a non-invasive manner.
Authors thank the Ranbaxy Research Laboratories for providing the necessary facilities for carrying out this experiment, and acknowledge the support and encouragement of Dr Pradip Kumar Bhatnagar, Senior Vice President, New Drug Discovery Research, Ranbaxy Research Laboratories, Gurgaon, Haryana, India.
References
- The role of animal models in the evaluation of new antibiotics. In: Zak O, Sande M, eds. Handbook of animal models of infection. CA USA: Academic Press; 1999. p. :xxi-xxiv.
- [Google Scholar]
- Evaluation of antimicrobials in experimental animal infection. In: Lorian V, ed. Antibiotics in laboratory medicine. Baltimore, MD: Williams and Wilkins; 1996. p. :604-765.
- [Google Scholar]
- A critique of animal models in antibiotic research. Scand J Infect Dis. 1978;14(Suppl):109-17.
- [Google Scholar]
- Application of therapy in animals to bacterial infection in human diseases. Infect Dis Clin North Am. 1989;3:441-59.
- [Google Scholar]
- Photonic detection of bacterial pathogens in living host. Mol Microbiol. 1995;18:593-603.
- [Google Scholar]
- A non-invasive optical imaging method to evaluate postantibiotic effects on biofilm infection in vivo. Antimicrob Agents Chemother. 2004;48:2283-7.
- [Google Scholar]
- Monitoring bioluminescence Staphylococcus aurues infections in living mice using a novel lux ABCDE construct. Infect Immun. 2000;68:3594-600.
- [Google Scholar]
- Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel Gram-positive lux transposon. Infect Immun. 2001;69:3350-8.
- [Google Scholar]
- Real-time monitoring of bacterial infection in vivo: development of bioluminescent staphylococcal foreign-body and deep-thigh-wound mouse infection models. Antimicrob Agents Chemother. 2003;47:2740-8.
- [Google Scholar]
- Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother. 2005;49:380-7.
- [Google Scholar]
- Direct continuous method for monitoring biofilm infection in a mouse model. Infect Immun. 2003;71:882-90.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved CLSI Standard. M7-A7. Wayne, PA: CLSI; 2006.
- [Google Scholar]
- In vivo pharmacodynamics of a new oxazolidinone (linezolid) Antimicrob Agents Chemother. 2002;46:3484-9.
- [Google Scholar]
- Teicoplanin, vancomycin, rifampicin: in vivo and in vitro studies with Staphylococcus aureus. J Antimicrob Chemother. 1987;19:659-62.
- [Google Scholar]
- Daptomycin in experimental murine pneumococcal meningitis. BMC Infect Dis. 2009;9:50.
- [Google Scholar]
- Pharmacodynamic profile of telithromycin against macrolide- and fluoroquinolone-resistant Streptococcus pneumoniae in a neutropenic mouse thigh model. Antimicrob Agents Chemother. 2005;49:188-94.
- [Google Scholar]
- Rapid direct method for monitoring antibiotics in a mouse model of bacterial biofilm infection. Antimicrob Agents Chemother. 2003;47:3130-7.
- [Google Scholar]
- Therapeutic studies of cefepime (BMY 28142) in murine meningitis and pharmacokinetics in neonatal rats. Antimicrob Agents Chemother. 1990;34:733-8.
- [Google Scholar]
- Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53:393-400.
- [Google Scholar]
- Development and validation of a multi-dose neutropenic rat thigh infection model using real time monitoring of Staphylococcus aureus growth in vivo. In Vivo. 2008;22:667-72.
- [Google Scholar]






