Translate this page into:
Non-invasive prenatal screening & diagnosis of β-thalassaemia in an affected foetus
For correspondence: Dr. Narutchala Suwannakhon, School of Science, University of Phayao, Phayao 56000, Thailand e-mail: narutchala@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Non-invasive prenatal testing (NIPT) of maternally inherited alleles of β-thalassaemia (MIB) remains to be a challenge. Furthermore, current techniques are not available for use as routine tests. NIPT for β-thalassaemia disease was developed by using a specific droplet digital polymerase chain reaction (ddPCR) assay to analyze the cell-free foetal DNA (cffDNA) derived from maternal plasma.
Methods:
Pregnant women and their spouses who are at risk of bearing an offspring with β-thalassaemia disease from common MIB mutations (CD 41/42-TCTT, CD17A>T, IVS1-1G>T and CD26G>A) were enrolled. The ddPCR assay sets were constructed for each of the four mutations. All cell-free DNA samples were first screened for the paternally inherited β-thalassaemia (PIB) mutation. The PIB-negative samples were considered as non-disease and were not further analyzed. For PIB-positive samples, DNA fragments of 50-300 base pairs in size were isolated and purified, and further analyzed for MIB mutation. The allelic ratio between the mutant and the wild-type was used to determine the presence of MIB in cffDNA. All cases underwent a prenatal diagnosis by amniocentesis for a definite diagnosis.
Results:
Forty two couples at risk were enrolled. Twenty two samples were positive for PIBs. Among these 22 samples, there were 10 cases with allelic ratio >1.0 (MIB positive). All foetuses with over-represented mutant alleles were further diagnosed with β-thalassaemia disease; eight with compound heterozygous and two with homozygous mutations. The 20 PIB-negative and 12 MIB-negative foetuses were non-affected.
Interpretation & conclusions:
The results of this study suggest that NIPT utilizing the ddPCR assay can be effectively used for the screening and diagnosis of foetal β-thalassaemia in at risk pregnancies.
Keywords
Droplet digital polymerase chain reaction assay
non-invasive
prenatal diagnosis
β-thalassaemia
β-thalassaemia (MIB) is a common form of severe hereditary chronic anaemia found predominantly in Southeast Asian countries. The disease originates mostly from a combination of two different β-thalassaemia genes (β0β0, β+β0 or β+β+). The clinical presentation of β0β0 and β+β0 is β-thalassaemia major, which manifests as severe chronic anaemia, and these patients are mostly blood transfusion dependent. The optimal treatment of the disease causes an enormous socio-economic burden. The disease exhibits autosomal recessive transmission and is preventable1-3. Prenatal diagnosis using invasive methods (namely, chorionic villus sampling; at 10-12 wk of gestational age, amniocentesis; 15-18 wk and/or cordocentesis; 18-22 wk) is available in most major hospitals. However, such an operation might pose a risk of foetal loss and maternal obstetric complications4.
Non-invasive prenatal testing (NIPT) of maternal blood samples currently allows for the early detection of paternally inherited β-thalassaemia mutation (PIB)5. The absence of PIB implies that the foetus inherited the paternal normal β-globin allele, and was therefore not affected by β-thalassaemia disease. The NIPT decreases the need for invasive procedures by half. However, the detection of maternally inherited alleles by NIPT remains challenging. Presently, there are several methods to detect paternally inherited alleles6-10 whereas there are a only a few reports on the methods to detect of maternally inherited alleles such as relative mutation dosage or relative haplotype dosage using next-generation sequencing (NGS)11-14 and droplet digital (dd) PCR15.
This study was aimed to describe an NIPT utilizing a constructed ddPCR assay for the diagnosis of at risk foetuses with β-thalassaemia disease resulted from four common mutations in Thailand.
Material & Methods
This study was conducted at the department of Pediatrics, University of Phayao Hospital, University of Phayao, Phayao, Thailand, after procuring an ethical approval from the University of Phayao Human Ethics Committee. A written informed consent was obtained from the pregnant women and their spouses before blood collection and prenatal diagnosis.
Subjects: A total of 3000 pregnant women, who attended the Antenatal Clinic, department of Obstetrics and Gynecology, Phayao Provincial Hospital, Phayao, Thailand, from September 2015 to 2017 were included in the study. Forty two at risk couples in which both partners harboured either one or two of the three common β-thalassaemia mutations, namely CD 41/42-TCTT, CD17 A>T and IVS1-1 G>T and haemoglobin E mutation CD 26 G>A, were recruited. Two couples were found to be at risk of having a baby homozygous β-thalassaemia for homozygous CD41/42-TCTT and homozygous IVS1-1 G>T, respectively, while the remaining 40 were compound heterozygous β-thalassaemia. Of the 42 mothers, 22 were CD26 carriers, eight were CD 41/42, eight were CD17 and four were IVS1-1 mutations. Of the fathers, 16 were CD26 carriers, 14 were CD 41/42, seven were CD17 and five were IVS1-1 mutations. The gestation age at the time of the study was 7-16 wk.
Construction of the droplet digital (dd) PCR assay sets: The DNA sequence of the HBB gene was obtained from NCBI GenBank U01317.1 (HBB gene; 62137-63472). Primers and TaqMan probes were designed with Primer Express software (Applied Biosystems, Foster City, CA, USA). Four pairs of primers were designed to amplify the following DNA sequence regions: CD41/42-TCTT, CD26 G>A, CD17 A>T and IVS1-1 G>T (Supplementary Table). All primers and TaqMan MGB probes were purchased from Invitrogen Corporation (Carlsbad, CA, USA).
| Name | Sequence 5′→3′ | Type | Size (bp) | Fluorescent dye |
|---|---|---|---|---|
| CD17-F | GAG GAG AAG TCT GCC GTT ACT G | Primer | 108 | - |
| CD17-R | CTC CTT AAA CCT GTC TTG TAA CCT TGA T | Primer | - | |
| CD17-WT | CAC GTT CAC CTT GCC CCA | Probe | VIC | |
| CD17-MU | ACG TTC ACC TAG CCC CA | Probe | FAM | |
| CD26-F | GAG GAG AAG TCT GCC GTT ACT G | Primer | 110 | - |
| CD26-R | GTC TCC TTA AAC CTG TCT TGT AAC CT | Primer | - | |
| CD26-WT | TTG GTG GTG AGG CCC T | Probe | VIC | |
| CD26-MU | TTG GTG GTA AGG CCC T | Probe | FAM | |
| IVS1-1-F | GGT GAA CGT GGA TGA AGT TGG T | Primer | 90 | - |
| IVS1-1-R | GCC CAG TTT CTA TTG GTC TCC TTA A | Primer | - | |
| IVS1-1-WT | CTG GGC AGG TTG GTA T | Probe | VIC | |
| IVS1-1-MU | CTG GGC AGT TTG GTA T | Probe | FAM | |
| CD41/42-F | TCC CAC CCT TAG GCT GCT GGT G | Primer | 112 | - |
| CD41/42-R | ATG AGC CTT CAC CTT AGG GTT GCC | Primer | - | |
| CD41/42-WT | CCC AGA GGT TCT TTG AGT CCT TTG G | Probe | VIC | |
| CD41/42-MU | CCC AGA GGT TGA GTC CTT TGG G | Probe | FAM |
VIC, an abervation stand forVictoria; FAM, an abervation stand for Fluorescein amidites
Isolation of plasma cell free (cf) DNA: Blood sample (20 ml) collected in EDTA was processed within 2 h after collection. Plasma recovery was performed by conventional two-step centrifugation. Four milliliters of plasma cfDNA was extracted by the QIAamp Circulating Nucleic Acid Kit (QIAGEN, Hilden, Germany) as per the manufacturer’s instructions.
Cell-free foetal DNA (cffDNA) fragment enrichment: The cffDNA enrichment was carried out via automated DNA size selection (Pippin Prep, Beverly, MA, USA) according to the manufacturer’s instructions. To isolate DNA fragments 50-300 base pairs (bp) in size, a two per cent agarose gel cassette was used. The eluate was kept at −20°C until analysis.
Validation of ddPCR mutation detection assays: To validate the effectiveness of the primers and probes used for the ddPCR assay, the plasma cfDNA of CD41/42, CD17, IVS1-1 and CD26 traits was assessed using the Bio-Rad-validated ddPCR mutation detection assays.
Paternally inherited β-thalassaemia mutation (PIB) test using a paternal-specific ddPCR assay: To assess PIB in an at risk pregnancy, a paternal-specific ddPCR assay was carried out according to the standard instructions of the Bio-Rad Q×100 Droplet Digital PCR System. In this study, there were four ddPCR primer and probe sets targeting PIB in maternal plasma (map)-cfDNA.
PIB test using an amplification refractory mutation system (ARMS) real-time PCR: ARMS real-time PCR was carried out to identify the PIB in map-cfDNA, as previously described10.
Maternally inherited β-thalassaemia mutation (MIB) test using maternal-specific ddPCR assay: Quantification of maternal alleles in PIB-positive map-cfDNA was performed using the Bio-Rad QX100 Droplet Digital PCR System. The master mix for the maternal ddPCR assay of each test sample included 1 µl of each FAM probe/primer and VIC probe/primer for the wild-type and mutant alleles, 8 µl of the cfDNA sample and 10 µl of Bio-Rad ddPCR Super Mix. The eight prepared samples, including three PIB-positive maternal-enriched DNA fragments, three PIB-positive map-cfDNA, one PIB-negative map-cfDNA and one normal control map-cfDNA sample, were loaded into a disposable DG8 droplet generator cartridge (2015 Bio-Rad Laboratories, Inc.). The allelic ratio of each sample was determined by analyzing the quantity of the β-thalassaemia mutant and the wild-type alleles.
Prenatal diagnosis by amniocentesis: Prenatal diagnosis by amniocentesis was carried out at the foetal age of 15 to 18 wk. The DNA was extracted from 5 ml of amniotic fluid using the QIA-amp DNA Mini Kit (QIAGEN Inc., Hilden, Germany). The β-thalassaemia mutation was assessed via high-resolution DNA melting analysis and was confirmed by direct DNA sequencing technique16,17.
Postnatal thalassaemia diagnosis: Postnatal blood samples were requested for evaluation of the thalassaemia status. The assessment of β-thalassaemia mutations was carried out using high-resolution DNA melting analysis and direct DNA sequencing. The babies who were unable to undergo thalassaemia blood testing on track received approximately one year of clinical follow up at the Well-Baby Clinic.
Workflow: All map-cfDNA samples were firstly screened for PIB by ddPCR and ARMS real-time PCR. The PIB-negative samples were considered non-disease and were not further analyzed. For PIB-positive samples, cffDNA was enriched and further analyzed for maternally inherited β-thalassaemia mutation (MIB). MIB test positivity indicated the presence of maternally inherited foetal β-thalassaemia mutation. The formulated diagnosis was a compound heterozygous β-thalassaemia. In the case of an at risk pregnancy for homozygous β-thalassaemia, only the MIB test was performed. All cases were undergoing prenatal diagnosis by amniocentesis for a definite diagnosis. The statistical analysis of the allelic ratio of the affected and unaffected foetuses was compared using one-way ANOVA in SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA).
Results
The validated optimal temperature for the ddPCR assay using plasma cfDNA from an individual with a β-thalassaemia trait carrying a CD41/42-TCTT, CD26G>A, CD17 A>T and IVS1-1G>T mutation worked well at 60°C. The ddPCR efficiencies were in the range of 92-98 per cent, whereas the correlation coefficient (R2) of each ddPCR assay set was 0.98-1.0. The allelic ratio between the mutant and the wild-type alleles using ddPCR quantification of the 24 plasma cfDNA of CD26G>A carriers was 1.00±0.13; 0.96±0.08 for 14 of the CD41/42-TCTT carriers; 0.97±0.09 for 10 of the CD17A>T carriers and 0.97±0.13 for seven of the IVS1-1G>T carriers. The cffDNA fragment enrichment via automated DNA size selection exhibited an increase in the concentration of the mutant alleles by 2-5 times. An example of cffDNA enrichment of a compound heterozygous β-thalassaemia/Hb E (CD41/42/CD26) allele is illustrated in Figure 1. The amount of PIB alleles in 17 map-cfDNA samples with a gestational age between seven and 16 wk old using paternal ddPCR assays was 3.38±1.8 per cent, whereas that of ARMS real-time PCR had a threshold cycle of 34.3±0.12.
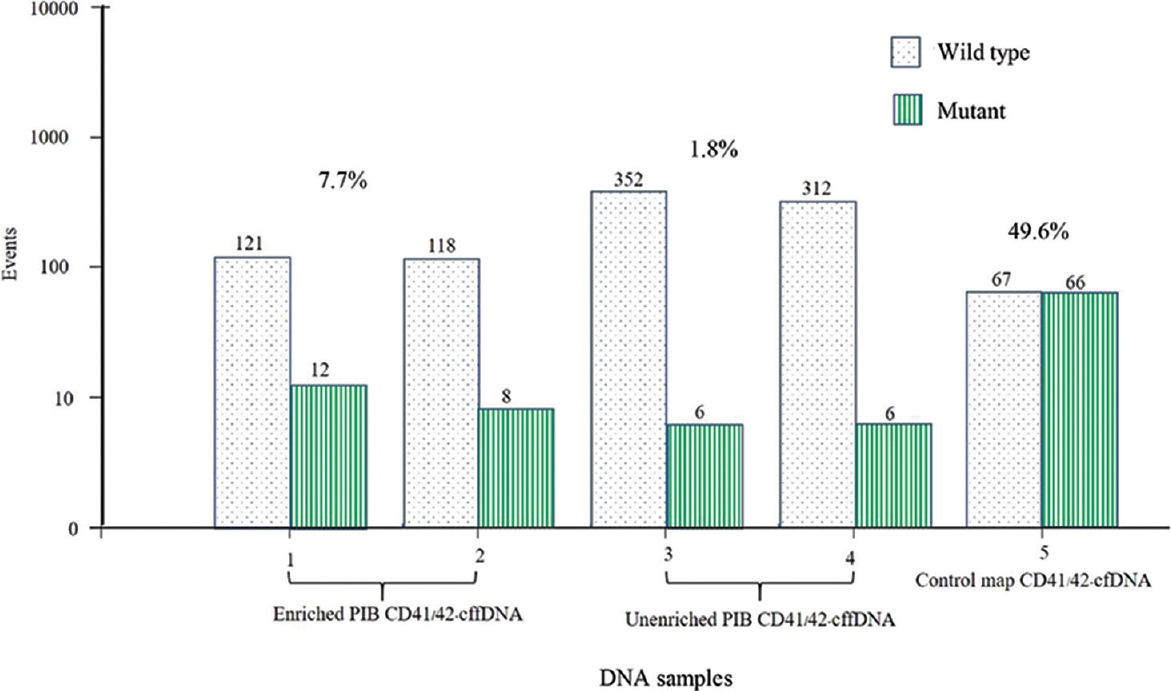
- An image of cffDNA enrichment from a PIB test-positive couple where the mother carried CD 26 and father carried the CD41-42 β-thalassaemia mutation. The cffDNA fragments exhibited an increase in the concentration of the mutant alleles to 4.1 times above the initial value. cffDNA, cell-free foetal DNA; PIB, paternally inherited β-thalassaemia mutation
PIB tests were negative in 20 of the 42 pregnancies (47.6%). The results were in concordance with those of the ARMS real-time PCR tests. Those foetuses which could not undergo thalassaemia blood testing and were on clinical thalassaemia follow up remained unaffected.
Of the remaining 22 PIB-positive samples, 12 had negative MIB tests (28.6%). The allelic ratio (mutant/wild) of the enriched map-cfDNA was 0.87±0.07, while that of unenriched map-cfDNA was 0.71±0.13. After delivery, all negative MIB test foetuses were unaffected. Ten of the remaining were MIB-positive pregnancies. The allelic ratio (mutant/wild) of enriched map-cfDNA was 1.25±0.17, whereas that of unenriched map-cfDNA was 1.10±0.07. The formulated diagnosis was eight foetuses with compound heterozygosity, and two were homozygous for β-thalassaemia. After amniocentesis, five foetuses were found to be affected with CD26 G>A/CD41/42-TCTT, three were affected with CD17 A>T/CD26 G>A and one of each was affected by homozygous IVS1-1 G>T (Figs 2 and 3) and CD41/42-TCTT mutations.
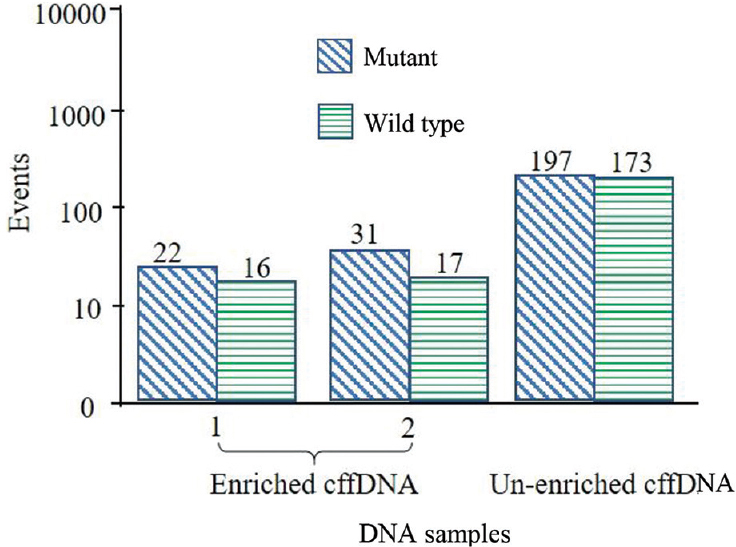
- Box plot of the MIB-positive sample tested with the maternal IVS1-1 G→T ddPCR assay, exhibiting the formulated diagnosis of IVS1-1 G→T homozygosity. ddPCR, droplet digital PCR; MIB, maternally inherited β-thalassaemia mutation
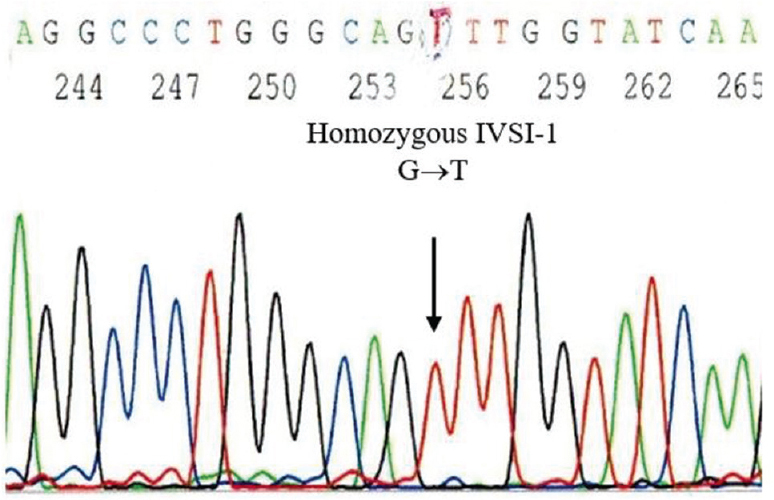
- The results of the direct DNA sequencing technique applied to DNA samples from amniotic cells, which are consistent with the NIPT-formulated diagnosis of an at risk foetus homozygous for beta-thalassaemia IVS1-1 G→T. NIPT, non-invasive prenatal testing
Discussion
NIPT for the detection of pregnant women at-risk of having a baby with β-thalassaemia has been progressively developed. Currently, many techniques are available for the detection of PIBs and can be efficiently be employed for NIPT. However, the characterization and identification of MIBs have been prohibited because the cfDNA is a mixture of both maternal as well as foetal DNA. The detection of MIBs in plasma DNA is harder to infer. Therefore, quantitative methods are required for the same. The detection of cffDNA in maternal plasma is challenging as the proportion of cffDNA in maternal plasma are low, which is difficult to discriminate. Recently, efforts have been made towords developing high-end techniques for foetal diagnosis. For example, the relative haplotype dosage combined with several platform NGS has shown promise as an application for NIPT. However, subjects such as compound heterozygous β-thalassaemia foetuses might be difficult to test with this approach. Moreover, these techniques require extensive sample preparation, computer resources, bioinformatics specialist and parental SNPs haplotype information, are costly and might not be readily available for routine application11-15,18,19. On the other hand, the ddPCR technique is an accurate allele quantification and amplification method to detect allelic imbalances. ddPCR method is able to directly identify genotype of β-thalassaemia mutation and may be easier and less time-consuming to predict the foetal genotype. This assay is convenient for routine screening, but requires individual-specific probe and primer sets to diagnose β-thalassaemia mutation. Furthermore, the ddPCR machine can detect a few fluorochrome dyes to label with specific probes. The multiplex ability application of the ddPCR technique is limited to a few β-mutations.
Through this study, we propose the development of PIB and MIB tests utilizing four common β-globin gene mutations and constructing ddPCR assay sets for the NIPT of pregnancies that are at-risk of having a baby with β-thalassaemia. The method could be used for the screening and testing of an affected foetus with either compound heterozygosity or β-thalassaemia with the same mutation. Principally, cffDNA is foetal DNA. A β-thalassaemia foetus might arise either from the combination of two different or the same β-thalassaemia mutations, one from paternal and the other from maternal origin. Cell-free foetal DNA can be tested by DNA sequence detection and allele quantification using paternal-specific and/or maternal-specific ddPCR assays in map-cfDNA (PIB and MIB tests).
A negative PIB test indicates that the at-risk foetus is unaffected. On the other hand, a positive PIB test could indicate that the at-risk foetus either has a PIB trait or is a β-thalassaemia-affected foetus. To diagnose an at-risk foetus with compound heterozygous β-thalassaemia, firstly the PIB test should be positive. Then, maternally inherited alleles should be further assessed using the MIB test. If the MIB test is negative, then the at-risk foetus is heterozygous. If the MIB test is positive, then there is an over-representation of MIBs, and therefore, the at-risk foetus has compound heterozygous β-thalassaemia. In this study, for couples who share the same β-thalassaemia mutation, the MIB test was positive indicating that there was an over-representation of the parentally inherited mutation meaning the at-risk mother was having a baby with homozygous β-thalassaemia.
These findings suggest that the four constructed ddPCR assay sets could be used for the diagnosis of affected foetuses with inherited β-thalassaemia mutations. The PIB and MIB tests are for the screening and diagnosis of at-risk foetuses with compound heterozygous β-thalassaemia, whereas the MIB test is only for homozygous β-thalassaemia. The MIB test of a foetus affected with β-thalassaemia was always over 1.0. Overall, the proposed NIPT is a simple, precise and accurate method that could reduce the use of invasive procedures among β-thalassaemia at-risk pregnancies.
Financial support and sponsorship
This study was supported by the University of Phayao fund (Gaint no. RD61032).
Conflicts of interest
None.
Acknowledgment
The authors acknowledge Prof Monthon Sanguansermsri and his administrative group for their support of this project
References
- Economic burden of beta-thalassemia/Hb E and beta-thalassemia major in Thai children. BMC Res Notes. 2010;3:29.
- [Google Scholar]
- Progress toward the control and management of the thalassemias. Hematol Oncol Clin North Am. 2016;30:359-71.
- [Google Scholar]
- Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: A systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45:16-26.
- [Google Scholar]
- Non-invasive prenatal diagnosis of beta-thalassemia by detection of the cell-free fetal DNA in maternal circulation:a systematic review and meta-analysis. Ann Hematol. 2016;95:1341-50.
- [Google Scholar]
- Detection of paternally inherited fetal point mutations for β-thalassemia in maternal plasma using simple fetal DNA enrichment protocol with or without whole genome amplification: An accuracy assessment. J Matern Fetal Neonatal Med. 2016;29:2645-9.
- [Google Scholar]
- COLD-PCR and microarray: Two independent highly sensitive approaches allowing the identification of fetal paternally inherited mutations in maternal plasma. J Med Genet. 2016;53:481-7.
- [Google Scholar]
- Postnatal and non-invasive prenatal detection of β-thalassemia mutations based on Taqman genotyping assays. PLoS One. 2017;12:e0172756.
- [Google Scholar]
- Prenatal diagnosis of β-thalassemia with cell-free fetal DNA in maternal plasma. J Coll Physicians Surg Pak. 2019;29:483-5.
- [Google Scholar]
- Noninvasive prenatal screening test for compound heterozygous beta thalassemia using an amplification refractory mutation system real-time polymerase chain reaction technique. Hematol Rep. 2019;11:8124.
- [Google Scholar]
- Non-invasive prenatal diagnosis of beta-thalassemia by semiconductor sequencing: A feasibility study in the Sardinian population. Eur J Hum Genet. 2017;25:600-7.
- [Google Scholar]
- Non-invasive prenatal testing for fetal inheritance of maternal β-thalassaemia mutations using targeted sequencing and relative mutation dosage: A feasibility study. BJOG. 2018;125:461-8.
- [Google Scholar]
- A novel high-throughput molecular counting method with single base-pair resolution enables accurate single-gene NIPT. Sci Rep. 2019;9:14382.
- [Google Scholar]
- Noninvasive prenatal testing for β-thalassemia by targeted nanopore sequencing combined with relative haplotype dosage (RHDO): A feasibility study. Sci Rep. 2021;11:5714.
- [Google Scholar]
- A non-invasive droplet digital PCR (ddPCR) assay to detect paternal CFTR mutations in the cell-free fetal DNA (cffDNA) of three pregnancies at risk of cystic fibrosis via compound heterozygosity. PLoS One. 2015;10:e0142729.
- [Google Scholar]
- High-resolution melting analysis for prenatal diagnosis of beta-thalassemia in northern Thailand. Int J Hematol. 2017;106:757-64.
- [Google Scholar]
- Analysis of beta-thalassemia mutations in northern Thailand using an automated fluorescence DNA sequencing technique. Hemoglobin. 2003;27:89-95.
- [Google Scholar]
- A cell-free DNA barcode-enabled single-molecule test for noninvasive prenatal diagnosis of monogenic disorders: Application to β-thalassemia. Adv Sci (Weinh). 2019;6:1802332.
- [Google Scholar]
- Noninvasive prenatal diagnosis of β-thalassemia by relative haplotype dosage without analyzing proband. Mol Genet Genomic Med. 2019;7:e963.
- [Google Scholar]






