Translate this page into:
NDM-beta-lactamase-1: Where do we stand?
For correspondence: Dr Prabha Desikan, Department of Microbiology, Bhopal Memorial Hospital & Research Centre, Raisen Bypass Road, Karond, Bhopal 462 038, Madhya Pradesh, India e-mail: prabhadesikan@yahoo.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Multidrug-resistant (MDR) Gram-negative bacilli (GNB) have been playing havoc in the field of nosocomial as well as community-acquired infections. Of particular concern are the carbapenem-resistant GNBs, belonging to Enterobacteriaceae and encoding for New Delhi metallo-beta-lactamase-1 (NDM-1) gene. These strains spread rapidly and horizontally in the population, thus exhibiting MDR traits as these can harbour several resistance encoding genes to almost all antimicrobial groups. Several predisposing factors are responsible towards its spread, viz. excessive antibiotic usage, improper aseptic conditions by healthcare workers, lack of awareness, abruptly discontinuing medication course, alternative medications and vector-borne factors contributing to the unchecked harbouring of these super bugs in India. Thus, a bugle call has already been sounded worldwide especially in India, where the country has taken serious cognizance to build up strategy via implementation of several national programs to combat antimicrobial resistance covering human, animal, agriculture and environmental aspects. As there is an exponential rise in variants of NDM-1 harbouring strains, molecular epidemiological investigations of these strains using genotyping techniques are of paramount importance for a better understanding of this rampant spread and curbing resistance thereafter. This review explores the urgent need to develop a cost-effective, rapid molecular assay, viz. the loop-mediated isothermal amplification method for field detection of MBL harbouring bacterial strains, especially NDM-1 and its variants, thus targeting specific carbapenemase genes at a grass root level even to the remote and rural regions of the country.
Keywords
Carbapenemase
enterobacteriaceae
Gram-negative bacilli
NDM-β-1
susceptibility
Carbapenem-resistant Enterobacteriaceae (CRE) isolates have been found to display high resistance to broad-spectrum antimicrobials than non-CRE, implying carbapenem resistance might be linked with resistance to several other antibiotics as well. Since carbapenem are considered to be the last resort drugs of choice, the highly resistant CRE carrying the New Delhi metallo-β-lactamase-1 gene (NDM-1) can limit the therapeutic options12. Excessive antibiotic usage in health care, agriculture and veterinary care, has led to a selection pressure favouring the survival and spread of such resistant organisms, resulting in increased hospital stay, morbidity and mortality34. NDM-1 producers belonging to Enterobacteriaceae are mainly associated with urinary tract infections (UTI), peritonitis, septicaemia, pulmonary infections, tissue and other device–associated infections5. There are reports of the presence of NDM-1 in Cedecea lapagei isolated from the neonatal intensive care unit in Aligarh67. Studies have revealed widespread dissemination of blaNDM variants through horizontal gene transfer among the Gram-negative bacilli (GNB) in developing countries co-existing with other resistance markers8. As observed for other gut-colonizing multidrug-resistant (MDR) bacteria, which precede infections by NDM producers, their transmission mainly takes place via the oro-faecal route9. The most abundant carbapenemase producer globally is the Klebsiella pneumoniae carbapenemase (KPC) (blaKPC gene), followed by the Verona integron-encoded metallo-ß-lactamase (VIM), NDM, Imipenemase (IMP) metallo-β-lactamase, and oxacillinase-48-type (OXA-48). These are all harboured by GNBs, viz. K. pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumanii and Enterobacter cloacae1011. Aggressive acquirement of antimicrobial resistance (AMR) and harbouring of mobile genetic elements by members of Enterobacteriaceae and non-Enterobacteriaceae have greatly affected the community1213.
AMR menace prevails worldwide, especially among the carbapenem group of drugs. The Ministry of Health and Family Welfare, Government of India has been actively addressing AMR issues by launching several programs spanning the length and breadth of the country towards its containment. India is a member of the 10-Global Health Security Agenda (GHSA) steering group and the largest of 17 GHSA phase 1 countries supported by several international organizations to fight against AMR14. The Indian Council of Medical Research (ICMR) and National Centre for Disease Control (NCDC) have been working together on the five-year plans, implementing AMR surveillance, health-care associated infection control and reinforcing various laboratories. With the onset of the COVID-19 pandemic, treating potentially fatal secondary bacterial infections in COVID-19 patients became imperative. In such a situation the increased levels of antimicrobials released in wastewater from hospitals may have affected levels of antimicrobials in the environment at large. Thus, in such challenging times, antibiotic stewardship plays a crucial role15.
The background of blaNDM-1 transmission
NDM-1-producing bacteria was first detected in India from a patient with UTI caused by carbapenem-resistant K. pneumonia16. What was alarming was that, the latter was found to be resistant to most of the antimicrobials16. Subsequently, reports of NDM-1 spread rapidly westward17.
It was found that most of the isolates carrying the blaNDM-1 gene were on plasmids along with several other antibiotic resistance determinants, capable of transferring such massive resistance to the associated bacteria18. These code for resistance to all aminoglycosides, macrolides and sulphamethoxazole, thus converting resident bacterial isolates to multidrug-resistant ones18. Subsequently, blaNDM-1 and blaVIM-2 genes were found together in a strain of P. aeruginosa in West Bengal, displaying co-existence of different carbapenem resistance gene19. However, others have reported different reasons behind the background of NDM-1 dissemination, stating plasmids of different sizes with genetic signatures on either side of blaNDM-1 gene being responsible for its mobility and pathogenesis. Similar gene cassette carrying resistant traits were also found in a tertiary care hospital among northern India among other Gram-negative bacteria from the same patient2021. These genes colonize together, thus spreading extensive resistance which is alarming as these bugs are usually related to causing life-threatening diseases222324.
Acquiring the NDM-1 gene and its proliferation: A multifaceted problem
Although studies related to NDM-1 have mostly been associated to the Indian subcontinent1, the Balkan countries are considered as a secondary reservoir where NDM-producing bacteria have been isolated from patients following return after seeking medical benefits from abroad9. Karthikeyan et al25 have reported detection of NDM-positive bacteria from the Middle East and North or Central Africa including Afghanisthan, Algeria, Cameroon, Egypt, Iraq, Israel, Kuwait, Lebanon, Morocco, the Sultanate of Oman and the United Arab Emirates25. The National Reference Laboratory of the Health Protection Agency, United Kingdom (UK) had investigated the rise in the unusual carbapenem-resistant isolates from UK patients26. These isolates of enterobacteriaceae showed NDM-1, but were negative for other known carbapenemase genes. All these cases had a history of travel to India or Pakistan within a brief span in common26. This calls for a routine detection and accurate reporting of NDM-1 in diagnostic laboratories, especially those from India. With the rampant dissemination of the NDM gene variants (NDM-2 to NDM-8) with varying capacity to cleave carbapenem drugs, molecular epidemiological investigations of these isolates especially from faecal specimen. The hospital sewage is a potential source of blaNDM variants outbreaks which is of major concern27. Genotyping techniques are of paramount importance for a better understanding of their rampant spread and infection control28.
Kumarswamy et al29, have reported the prevalence of NDM-1 in enterobacteriaceae isolates from India, Pakistan and in the United Kingdom. Their study selectively identified NDM-1 isolates from Guwahati, Mumbai, Varanasi, Bengaluru, Pune, Kolkata, Hyderabad, Port Blair, and New Delhi in India, several cities in Pakistan and Dhaka in Bangladesh pointing to its rampant dissemination29. Similar cases of those having travelled to Asian countries and may have acquired infection while undergoing treatment are also available2. Distribution of AMR and colistin resistant pathogenic strains have also been isolated from Tamil Nadu, West Bengal, Punjab303132. In a study conducted in south India, MDR Enterobacteriaceae colonizing the gut of adult rural population was observed indicating faecal carriage of AMR strains with a threat of rapid spread in the community33.
Several human (improper antibiotic stewardship and infection control, human migration) and bacterial factors (nosocomial spread of blaNDM gene) have consequently led to the rapid spread of this gene3435(Figure).
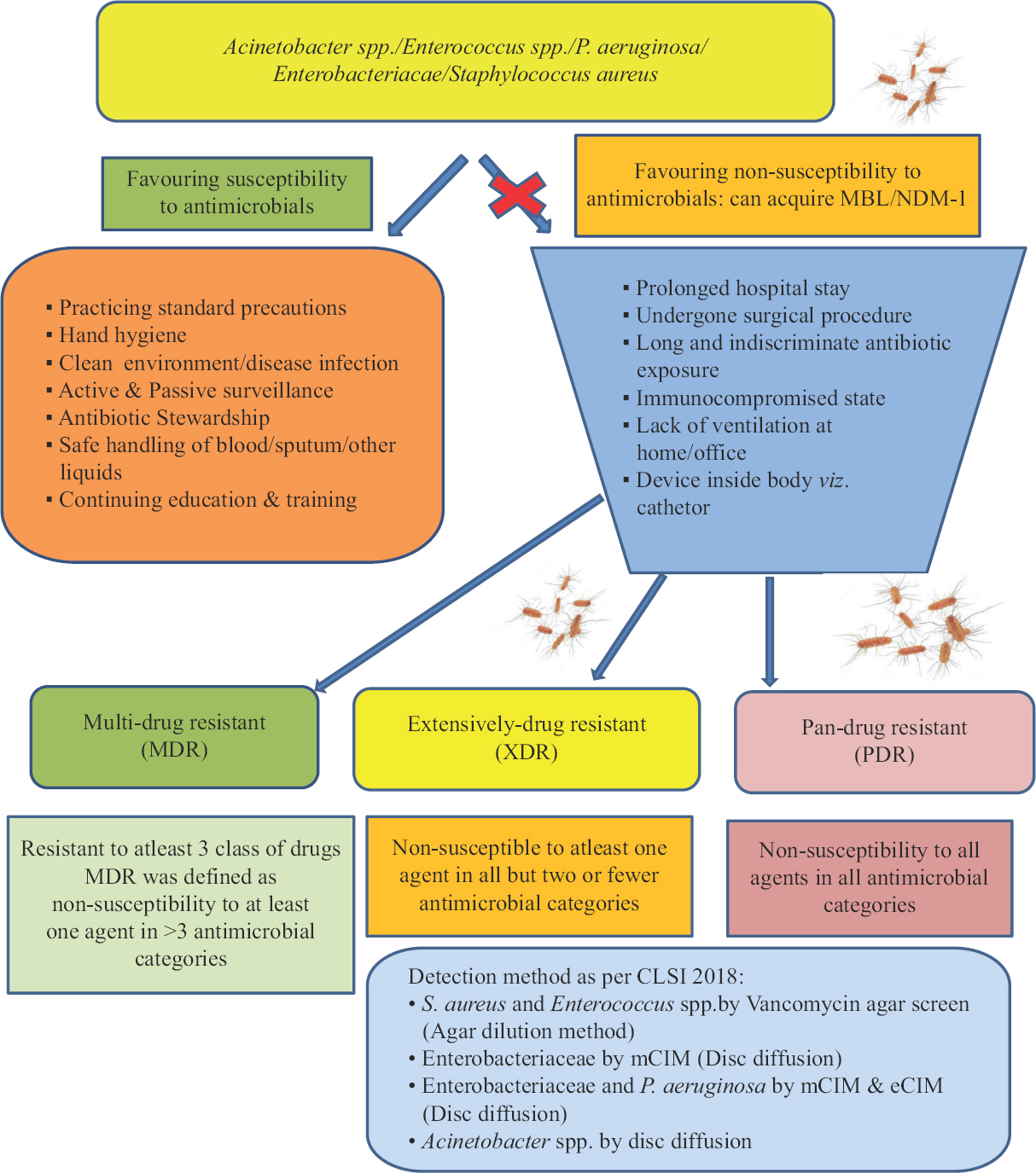
- Flow chart showing screening of Gram-negative bacteria resistant to carbapenem drugs.
Drinking water quality, defective sewage system, vector–mediated viz. house flies, uncontrolled circulation of antibiotics are other major factors promoting the spread of these resistance microbes in a healthy population. Furthermore, there is a tendency to switch to alternative medication particularly the Indian traditional medicinal system such as the Ayurveda, Siddha and Unani having yielded successful results worldwide especially in remote and rural areas36.
Routine laboratory diagnosis of NDM
Currently, the NDM-1 class of carbapenemase is of clinical concern as it shares 32.4 per cent amino acid match with VIM-1/VIM-2, on the other hand minute identity match with other metallo-β-lactamases (MBLs). NDM-1 binds strongly to most cephalosporins compared to VIM-2. NDM-1 effectively hydrolyzes broad range of β-lactams including penicillin, cephalosporin and carbapenem and just sparing monobactams like other MBL9.
Screening of carbapenem-resistant Gram-negative bacilli (GNBs) carrying NDM-1 gene
Currently, detection of NDM producers is based on preliminary screening using the antibiotic susceptibility test (AST)9. Susceptibility to NDM-1 is usually by disc diffusion method in accordance with Clinical and Laboratory Standard Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) or the British Society for Antimicrobial Chemotherapy (BSAC) guidelines as done in practice- and/or determination of minimum inhibitory concentration (MIC) by manual or automated methods373839. It is noteworthy that there might be certain differences in MIC values for isolates depending on the reference used to interpret antibiograms. Susceptibility to carbapenems are observed for some NDM producers and additional tests for carbapenemase detection are needed to identify them accurately9. Different chromogenic plate methods are used for performing AST and identification of carbapenemase producers which are detailed as:
Chromogenic plate method: Carbapenemase producers are presumptively identified using chromogenic plates useful for direct screening of high-risk asymptomatic carriers viz. stool samples. This cost-effective, rapid and simple detection of carbapenemase producers is vital for effective infection management control interventions and also in preventing outbreaks of nosocomial infections by these organisms. Different chromogenic media for detection of NDM-1 producers are available viz. ChromID ESBL and CHROMagar KPC media alongwith acceptable specificity and sensitivities ranging from 53-100 per cent40. However, its disadvantage being chromogenic media is not reliable detection for all types of carbapenemase producing isolates and requires confirmation tests41. Screening stool specimen of patients hospitalized for carbapenemase producers is done using screening culture media, viz. CHROMagar media and ChromID ESBL which is a bit time-consuming before the actual status of the patient is known4243444546. This is of primordial significance as these NDM-1 carrying enterobacteriaceae isolates mainly inhabit the gut region.
Several other routinely and commercially available automated systems which used to identify and detect NDM-1 carbapenemase-producing isolates include the Vitek 2 automated system (France). Studies evaluating the accuracy of these platforms in the detection of carbapenemase activity have shown appropriate sensitivities but inadequate specificities, which leads to the requirement of confirmatory tests4045.
Confirmatory detection of carbapenemase activity among the screened Gram-negative bacilli (GNBs)
Different techniques are available for identification of carbapenemase producers based on several phenotypic and genotypic methods41. The phenotypic methods are based on inhibitors or breaking down of carbapenem drugs or else on spectrophotometric approach, whereas genotypic detection is much more precise based on polymerase chain reaction (PCR)/real-time PCR (RT-PCR)/DNA sequencing/micro array based101842. However, phenotypic methods are much simpler, less time consuming and cheap. Phenotypic assays are designed based on the following principles:
Inhibition of metallo-carbapenemase: These phenotypic tests rely on the combined effect of discs viz. meropenem alongwith ethylenediamine-tetraacetic acid (EDTA), phenylboronic acid (PBA)/ both EDTA and PBA for differentiation of class A and B enzymes or dipicolinic acid (DPA) to specifically inhibit metallo-carbapenemase activity4647. Considering that PBA inhibits other β-lactamases as well, AmpC β-lactamases, cloxacillin (an AmpC inhibitor without activity against KPC and other class A carbapenemases) is also used routinely to differentiate between AmpC and KPC production4849 as it was found that strains harbouring both KPC and MBL could be detected using both EDTA and PBA in a single disk. Thus, ruling out of other common mechanisms for carbapenem resistance can indirectly help the laboratory to diagnose the presence of NDM-1 by virtue of exclusion50.
Combined disc test (CDT): Studies evaluating CDT in comparison to other molecular tests have shown high sensitivities and specificities (90-100%) with reference to blaNDM-1, blaKPC, blaVIM, and blaIMP in carbapenemase-producing isolates from Hyderabad4151.
Double disk synergy test (DDSTs): This method uses Mg-EDTA to detect MBL-producing strains, including NDM-1 producers4152. This method has demonstrated good sensitivities (100%) and specificity of 91.0 per cent. However, this method also reports certain shortfalls, such as test outcome interpretation is subjective requiring a qualified personnel5354.
Gradient diffusion strips: For detecting MBL and KPC various gradient diffusion strips formats have been designed, which separately detect MBL and KPC. The E-test (epsilometer test) MBL using imipenem and imipenem-EDTA was simple for their detection, except in two cases (E. cloacae D and K. pneumoniae), in which interpretation of the results was not possible because the imipenem MICs were too low40555657. The sensitivity of E-test MBL for blaNDM-1 positive isolates was 66.7 per cent and specificity was 100 per cent. However, gradient diffusion strips are quite expensive58.
Detection based on carbapenem hydrolysis
The cloverleaf method [modified Hodge test, (MHT)]: According to Aguirre-Quinonero and coworkers, MHT is still being used for carbapenemase detection following CLSI guidelines4159. However, as a result of the poor performance of the Hodge test displaying ambiguous results, modifications of the assay such as the addition of ZnSO445 or cloxacillin58 to the agar plate was done. Pasteran and co-workers have reported inclusion of Triton X-100 (a non-ionic surfactant) to improve NDM-1 carbapenemases detection, as the compound solubilizes membrane lipoproteins and consequently membrane-bound carbapenemases60. This latter version was known as the Triton Hodge Test (THT)6162. The MHT did not show good results in detecting NDM-producing isolates (merely 20 and 32.5% sensitivity for meropenem and ertapenem, respectively). On the other hand, sensitivities of the THT were 100 per cent with ertapenem and 92.5 per cent with meropenem for the latter MBL producing strains60.
Colorimetric assays
Assays displaying colour change due H+ ion concentration in the media has been exploited that is, pH based detection method or colorimetric assays including pH indicators viz. phenol red for Carba NP41 or bromothymol blue for Blue-CARBA.
Carba NP: This assay was initiated by Nordmann et al63 and named thereafter as Carba NP. This assay has reported specificity of 100 per cent and sensitivity between 90 and 100 per cent in the detection of carbapenemases5860. This assay was thus used for identifying blaNDM-4, blaNDM- 5, blaNDM-7 from rectal and stool swabs from a neonatal intensive care unit in Aligarh, India64.
Blue-CARBA: The Blue-CARBA which is a modification of the Carba NP yields similar results as the Carba NP9606163. The Blue-CARBA is an inexpensive test with 93.3 per cent sensitivity and 100 per cent specific detecting any type of carbapenemase of enterobacteriaceae including NDM-1 producers4965.
Carbapenem inhibition method (CIM): Initiated by Van der Zwaluw et al66, the CIM method display high sensitivity (98-100%) and specificity of 100 per cent. This method has proved to be inexpensive and easy to interpret. Moreover, this assay has been modified and further recommended to be used for carbapenemase detection (mCIM) in enterobacteriaceae and P. aeruginosa in the 28th edition of CLSI67. If mCIM comes positive, then only eCIM (carbapenemase activity is inhibited in the presence of EDTA) is done to differentiate MBL67.
Spectrometry techniques: Mass spectrometry (MS) is more often being used for the detection of carbapenemase-producing isolates.
MS: Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS is reliable for isolate identification of bacteria and fungi and is routinely in use in laboratories. This method is effective for detection of carbapenemase-producing Gram-negative bacteria especially NDM-1. Studies have found sensitivities ranging between 77 to 100 per cent and specificities from 94 to 100 per cent6668. However, MALDI-TOF is time-consuming and costly and its operation requires trained microbiologists. This technique was based on detection of a carbapenem spectrum and of its main derivatives resulting from carbapenem hydrolysis9.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS): The LC-MS/MS methods are analytical, reliable and sensitive. The chromatographic retention time, precursor ion mass, and product combine to impart a good analytical specificity. LC-MS/MS is the gold standard for small-molecule detection and quantitation6970.
Immunochromatographic assay: Detection of NDM-1 by immunochromatographic assay, using rat monoclonal antibodies are also being studied63.
Molecular CP-carbapenem resistant Enterobacteriaceae (CRE) detection methods: Varying susceptibility patterns of isolates towards various carbapenem drug depends on the efficiency with which the drugs are hydrolyzed4243. This variability in hydrolysis spectrum hampers identification. Phenotypic tests are good for screening the carbapenemases, but most of them are incapable of detecting the specific carbapenemase responsible for drug resistance. The World Health Organization (WHO) in an effort towards AMR surveillance and prevention program, through the Global Antimicrobial Resistance Surveillance System (GLASS) has emphasized on the reliability of phenotypic assays whereas, molecular testing could provide additional data regarding resistance profile and mechanisms. Molecular diagnostic assays require costly set-up, rapid, with high specificity and sensitivity for identifying the harboured resistance genes viz. blaNDM-1,blaVIM,blaIMP. Thus, depending on the laboratory capacity and prevalent AMR data, WHO has stratified molecular diagnostic testing to be performed depending on its complexity and cost effectiveness. Thus molecular diagnostics are available for laboratories with low capacity, especially belonging to the low- and middle-income countries the molecular methods recommended are either fully integrated and automated cartridge-operated PCR or the loop-mediated isothermal amplification (LAMP) devices and lateral flow assays requiring visual inspection for interpreting results. Whereas, high-capacity laboratories with good experience in molecular diagnostics might prefer complex diagnostic assays, such as microarrays technology and the whole genome sequencing (WGS) to analyses/interpret the raw data. However, WHO does not endorse nor validate any particular molecular method but relies on the phenotypic detection of resistance in isolates using antimicrobial agents by measuring the MIC which is the gold standard71.
PCR allows for rapid identification of specific carbapenemase genes using primers and probes to conserve regions in the gene target for real-time assays, can be carried out by PCR. PCR being specific to a given gene, and can be further tailored to detect specific subgroups of a gene family72. As blaNDM-1 encoded plasmids are rampantly spreading worldwide especially among members of Enterobacteriaceae faecal screening should be prioritized using PCR-based molecular screening techniques viz. multiplex PCR nucleic acid amplification test (NAAT) for efficient detection of faecal pathogens73. A multilocus sequence typing (MLST) can be performed to identify house-keeping genes viz. on plasmids in pathogens resistant to carbapenem drugs and displaying widespread resistance in the community74. Plasmid sizing is done using S1-nuclease pulsed-field gel electrophoresis (PFGE) and Southern blot is performed for identification of NDM-1 plasmid4473. The specificity and sensitivity using qPCR of blaNDM-1 has been reported as 98.4 and 100 per cent, respectively3542. The primary limitation of PCR is that the only known genes can be targeted while, those encoding novel carbapenemase will be missed with molecular approaches75.
On the other hand, the LAMP method is promising tool. It is simple, rapid, cost-effective, requiring no sophisticated instrument. It is a single tube amplification reaction requiring Bst DNA polymerase for strand displacement and DNA synthesis under isothermal conditions based on auto-cycling. The LAMP technology has been widely used in clinical diagnosis; field detection of MBL harbouring bacterial strains, qualitative and quantitative detection of epidemic bacteria, viruses, and parasites; as well as in fetal sex identification76. It is suggested that time demands requiring further research in the development of the LAMP assay as a molecular tools for the detection and confirmation of NDM-1 producers along with its variants and other MBLs. Thus, a simple and affordable detection assay at a genetic level would certainly help combat MBLs spread, with screening remote and rural areas.
On the other hand, various commercial PCR-based customised assays are also available viz. the Xpert Carba-R test and the hyperplex SuperBug ID475877. These assays are specific and sensitive but costly. The controls used for culture-based tests, phenotypic assays and PCR for NDM-1 detection is usually K. pneumoniae ATCC BAA-1705 as positive control and K. pneumoniae ATCC BAA-1706 as negative control, however, other controls can be selected from in-house confirmed positive /negative strains confirmed by sequencing78.
Microarray technology is another promising tool and can be paired with PCR-based target amplification. It utilizes probes that hybridize to DNA targets, including resistance genes. Microarrays can be used to target and extract DNA from bacterial isolates or patient specimens and can handle hundreds of DNA targets, thus multiplexing with numerous carbapenemase genes, along with distinguishing between closely related variants798081.
The WGS is versatile and comprehensive with a capacity to recognize all resistance mechanisms across the bacterial genome. Apart from targeting various carbapenemases, it can also identify other contributors to resistance, such as porin loss, efflux genes, etc. The data generated in WGS can also help to elucidate the source in outbreak investigations by extracting information regarding the type of plasmid carrying resistance genes, evolutionary lineage of the isolate and its relatedness with other isolates4356. Currently, WGS is expensive and requires qualified personnel for necessary data extraction and interpretation82.
Other promising approaches worth exploring:
Using macrophage: The versatility of macrophages in response to environmental stimuli and their engagement largely in pathogenesis of numerous human diseases makes them a desired target cells to be considered suitable for future therapy. This combined with nano-biotechnology can offer advantage in improving therapy outcome83.
Lytic phage proteins: Using lytic phages or else lytic phage proteins, a concept which originated in the beginning of the 20th century84 for combating AMR holds promise in curbing its spread.
Reactive oxygen therapy (ROS): Targeted use of ROS to the site of infection in various forms has also been suggested as a potential alternative as ROS has antimicrobial activity towards pathogens including biofilm breakdown85.
Conclusion
The extent of threat owing to the spread of pathogens producing NDM-1 belonging to Enterobacteriaceae worldwide is alarming. This is of serious concern in the Indian sub-continent, parts of Asia, Europe and America. The Indian government has taken cognizance of the situation thus creating public awareness against unhygienic practices by initiating cleanliness drive throughout the country.
The lack of a routine standardized phenotypic test for NDM variant detection, expensive molecular tests may have led to under-reporting. The efforts made by various national institutions viz. NCDC, ICMR and GHSA of India and other nations worldwide; along with other international organizations viz. the WHO can steer the world to a safer and securer environment.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019;25:943-50.
- [Google Scholar]
- Global spread of antibiotic resistance:The example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol. 2013;62:499-513.
- [Google Scholar]
- Antimicrobial resistance in the environment:The Indian scenario. Indian J Med Res. 2019;149:119-28.
- [Google Scholar]
- Detection of emerging antibiotic resistance in bacteria isolated from subclinical mastitis in cattle in West Bengal. Vet World. 2017;10:517-20.
- [Google Scholar]
- Antimicrobial treatment challenges in the era of carbapenem resistance. Diagn Microbiol Infect Dis. 2019;94:413-25.
- [Google Scholar]
- First reported New Delhi metallo-β-lactamase-1-producing Cedecea lapagei . Int J Antimicrob Agents. 2017;49:118-9.
- [Google Scholar]
- Occurrence of blaNDM variants among Enterobacteriaceae from a neonatal Intensive Care Unit in a northern India hospital. Front Microbiol. 2018;9:407.
- [Google Scholar]
- Outbreak of efficiently transferred carbapenem-resistant blaNDM-producing gram-negative bacilli isolated from neonatal Intensive Care Unit of an Indian hospital. Microb Drug Resist. 2020;26:284-9.
- [Google Scholar]
- Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int. 2014;2014:249856.
- [Google Scholar]
- Prevalence and mechanisms of carbapenem resistance among Acinetobacter baumannii clinical isolates in Egypt. Microb Drug Resist (25):480-8.
- [Google Scholar]
- Screening for carbapenem-resistant Enterobacteriaceae:Who, when, and how? Virulence. 2017;8:417-26.
- [Google Scholar]
- The evolution and transmission of multi-drug resistant Escherichia coli and Klebsiella pneumoniae:The complexity of clones and plasmids. Curr Opin Microbiol. 2019;51:51-6.
- [Google Scholar]
- Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1340.
- [Google Scholar]
- Global health security agenda: Action packages. Available from: https://www.cdc.gov/globalhealth/healthprotection/ghs/pdf/ghsa-action-packages_24-september-2014.pdf
- SARS-CoV-2, bacterial co-infections, and AMR:The deadly trio in COVID-19? EMBO Mol Med. 2020;12:e12560.
- [Google Scholar]
- Establishing antimicrobial resistance surveillance &research network in India: Journey so far. Indian J Med Res. 2019;149:164-79.
- [Google Scholar]
- The global spread of healthcare-associated multidrug-resistant bacteria:A perspective from Asia. Clin Infect Dis. 2013;56:1310-8.
- [Google Scholar]
- Phenotypic identification &molecular detection of blaNDM-1 gene in multidrug resistant Gram-negative bacilli in a tertiary care centre. Indian J Med Res. 2014;139:625-31.
- [Google Scholar]
- Occurrence of co-existing bla VIM-2 and bla NDM-1 in clinical isolates of Pseudomonas aeruginosa from India. Ann Clin Microbiol Antimicrob. 2016;15:31.
- [Google Scholar]
- Genetic linkage of blaNDM among nosocomial isolates of Acinetobacter baumannii from a tertiary referral hospital in northern India. Int J Antimicrob Agents. 2013;41:452-6.
- [Google Scholar]
- Genetic acquisition of NDM gene offers sustainability among clinical isolates of Pseudomonas aeruginosa in clinical settings. PLoS One. 2015;10:e0116611.
- [Google Scholar]
- Evaluation of co-transfer of plasmid-mediated fluoroquinolone resistance genes and blaNDM gene in Enterobacteriaceae causing neonatal septicaemia. Antimicrob Resist Infect Control. 2019;8:46.
- [Google Scholar]
- A close look onto structural models and primary ligands of metallo-β-lactamases. Drug Resist Updat. 2018;40:1-12.
- [Google Scholar]
- Extremely drug-resistant Citrobacter freundii isolate producing NDM-1 and other Carbapenemases identified in a patient returning from India. Antimicrob Agents Chemother. 2011;55:447-8.
- [Google Scholar]
- Coexistence of bla OXA-23 with blaNDM - 1 and arm –A in clinical isolates of Acinetobacter baumanii from India. J Antimicrob Chemother (65):2253-70.
- [Google Scholar]
- Hospital sewage water:A reservoir for variants of New Delhi metallo-β-lactamase (NDM)- and extended-spectrum β-lactamase (ESBL)- producing Enterobacteriaceae. Int J Antimicrob Agents. 2018;51:82-8.
- [Google Scholar]
- Molecular epidemiology and genome dynamics of New Delhi metallo-β-lactamase producing extra-intestinal pathogenic Escherichia coli strains from India. Antimicrob Agents Chemother. 2016;60:6795-805.
- [Google Scholar]
- Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK:A molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
- [Google Scholar]
- The distribution of carbapenem- and colistin-resistance in Gram-negative bacteria from the Tamil Nadu region in India. J Med Microbiol. 2017;66:874-83.
- [Google Scholar]
- Pattern of colistin resistance in Klebsiella isolates in an Intensive Care Unit of a tertiary care hospital in India. J Infect Public Health. 2020;13:1018-21.
- [Google Scholar]
- Prevalence of colistin-resistant, carbapenem-hydrolyzing proteobacteria in hospital water bodies and out-falls of West Bengal, India. Int J Environ Res Public Health. 2020;17:1007.
- [Google Scholar]
- Multidrug-resistant Enterobacteriaceae colonising the gut of adult rural population in South India. Indian J Med Microbiol. 2018;36:488-93.
- [Google Scholar]
- The spread and acquisition of NDM-1:A multifactorial problem. Expert Rev Anti Infect Ther. 2014;12:91-115.
- [Google Scholar]
- Country-to-country transfer of patients and the risk of multi-resistant bacterial infection. Clin Infect Dis. 2011;53:49-56.
- [Google Scholar]
- Revival, modernization and integration of Indian traditional herbal medicine in clinical practice:Importance, challenges and future. J Tradit Complement Med. 2017;7:234-44.
- [Google Scholar]
- Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria:An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-81.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests, 10th ed. CLSI document M2-A10. Wayne, PA: CLSI; 2009.
- 2010. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 1.1. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_breakpoints_v1.1.pdf
- Non-molecular detection of carbapenemases in Enterobacteriaceae clinical isolates. J Infect Chemother. 2017;23:1-11.
- [Google Scholar]
- Real-time PCR for detection of NDM-1 carbapenemase genes from spiked stool samples. Antimicrob Agents Chemother. 2011;55:4038-43.
- [Google Scholar]
- Phenotypic and molecular characterization of Acinetobacter baumannii isolates causing lower respiratory infections among ICU patients. Microb Pathog. 2019;128:75-81.
- [Google Scholar]
- Fecal carriage of carbapenem-resistant Enterobacteriaceae and risk factor analysis in hospitalised patients:A single centre study from India. Indian J Med Microbiol. 2017;35:555-62.
- [Google Scholar]
- What it the appropriate meropenem MIC for screening of carbapenemase-producing Enterobacteriaceae in low-prevalence settings? Antimicrob Agents Chemother. 2016;60:1556-9.
- [Google Scholar]
- First occurrence of KPC-2-possessing Klebsiellapneumoniae in a Greek hospital and recommendation for detection with boronic acid disc tests. J Antimicrob Chemother (62):1257-60.
- [Google Scholar]
- Sensitive screening tests for suspected class A carbapenemase production in species of Enterobacteriaceae . J Clin Microbiol. 2009;47:1631-9.
- [Google Scholar]
- A sensitive and specific phenotypic assay for detection of metallo-ß-lactamases and KPC in Klebsiella pneumonia with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin Microbiol Infect. 2011;17:552-6.
- [Google Scholar]
- Inhibitor-based methods for the detection of KPC carbapenemase-producing Enterobacteriaceae in clinical practice by using boronic acid compounds. J Antimicrob Chemother. 2010;65:1319-21.
- [Google Scholar]
- Combined disc methods for the detection of KPC- and/or VIM-positive Klebsiella pneumoniae:Improving reliability for the double carbapenemase producers. Clin Microbiol Infect. 2013;19:412-5.
- [Google Scholar]
- Comparative evaluation of multiplex PCR and routine laboratory phenotypic methods for detection of carbapenemases among gram negative bacilli. J Clin Diagn Res. 2014;8:C23-6.
- [Google Scholar]
- Alarming emergence, molecular characterization, and outcome of blaNDM-1 in patients infected with multidrug-resistant Gram-negative bacilli in a tertiary care hospital. J Lab Physicians. 2017;9:170-6.
- [Google Scholar]
- Detection of metallo-betalactamase producing Pseudomonas aeruginosa in Intensive Care Units. Australas Med J. 2013;6:686-93.
- [Google Scholar]
- Evaluation of the double-disk synergy test for New Delhi metallo-β-lactamase-1 and other metallo-β-lactamase producing gram-negative bacteria by using metal-ethylenediaminetetraacetic acid complexes. Microbiol Immunol. 2013;57:346-52.
- [Google Scholar]
- Phenotypic identification &molecular detection of blaNDM-1 gene in multidrug resistant Gram-negative bacilli in a tertiary care centre. Indian J Med Res. 2014;139:625-31.
- [Google Scholar]
- Carbapenem-resistant bacteria and laboratory detection methods. Arch Pedia Infect Dis. 2014;2:188-91.
- [Google Scholar]
- Detection of carbapenemases in Enterobacteriaceae:A challenge for diagnostic microbiological laboratories. Clin Microbiol Infect. 2014;20:839-53.
- [Google Scholar]
- Phenotypic and genotypic characteristics of carbapenem-resistant Enterobacteriaceae in a tertiary-level reference hospital in Turkey. Ann Clin Microbiol Antimicrob. 2016;15:20.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 21st informational supplement. CLSI document M100-S21. Wayne, PA: CLSI; 2011.
- Triton hodge test:Improved protocol for modified hodge test for enhanced detection of NDM and other carbapenemase producers. J Clin Microbiol. 2016;54:640-9.
- [Google Scholar]
- Modified Hodge test using Mueller-Hinton agar supplemented with cloxacillin improves screening for carbapenemase-producing clinical isolates of Enterobacteriaceae . J Med Microbiol. 2015;64:774-7.
- [Google Scholar]
- Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae . J Clin Microbiol (50):477-9.
- [Google Scholar]
- Rapid detection of carbapenemase-producing Enterobacteriaceae . Emerg Infect Dis. 2012;18:1503-7.
- [Google Scholar]
- Detection of New Delhi metallo-β-lactamase variants NDM-4, NDM-5, and NDM-7 in Enterobacter aerogenes isolated from a neonatal intensive care unit of a north India hospital:A first report. Microb Drug Resist. 2018;24:161-5.
- [Google Scholar]
- Evaluation of the Carba NP test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa . Antimicrob Agents Chemother (57):4578-80.
- [Google Scholar]
- The Carbapenemase Inactivation Method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in Gram-negative rods. PLoS One. 2015;10:e0123690.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 28th edition supplement. CLSI document M100. Wayne, PA: CLSI; 2018.
- Detection of carbapenemase activity in Enterobacteriaceae:Comparison of the carbapenem inactivation method versus the Carba NP test. J Antimicrob Chemother. 2016;71:274-6.
- [Google Scholar]
- The real threat of Klebsiella pneumonia carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228-36.
- [Google Scholar]
- Use of imipenem to detect KPC, NDM, OXA, IMP, and VIM carbapenemase activity from gram-negative rods in 75 minutes using liquid chromatography-tandem mass spectrometry. J Clin Microbiol. 2014;52:2500-5.
- [Google Scholar]
- World Health Organization. Molecular methods for antimicrobial resistance (AMR) diagnostics to enhance the global antimicrobial resistance surveillance system. Geneva: WHO; 2019.
- Detection of New Delhi metallo beta lactamase-1 (NDM-1) carbapenemase in Pseudomonas aeruginosa in a single centre in southern India. Indian J Med Res. 2014;140:546-50.
- [Google Scholar]
- A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases:2018 update by the infectious diseases society of America and the American Society for Microbiology. Clin Infectious Dis. 2018;67:e1-94.
- [Google Scholar]
- Molecular characterization of novel sequence type of carbapenem-resistant New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae in the neonatal intensive care unit of an Indian hospital. Int J Antimicrob Agents. 2019;53:525-9.
- [Google Scholar]
- Epidemiology of NDM-1 and its variants in multidrug resistant gram-negative bacilli isolated from infection in cancer patients. Med Chem (Los Angeles). 2017;7:368-70.
- [Google Scholar]
- Sensitive and rapid detection of the New Delhi metallo-beta lactamase gene by loop-mediated isothermal amplification. J Clin Microbiol. 2012;50:1580-5.
- [Google Scholar]
- Simultaneous three Enterobacteriaceae with different blaNDM-1-encoding plasmids in a patient transferred from mainland China to Taiwan. Infect Drug Resist. 2018;11:2555-60.
- [Google Scholar]
- Molecular characterization and antimicrobial susceptibility profile of New Delhi metallo-beta-lactamase-1-producing Escherichia coli among hospitalized patients. J Lab Physicians. 2018;10:149-54.
- [Google Scholar]
- Matrix-assisted laser desorption ionization-time of flight mass spectrometry meropenem hydrolysis assay with NH4HCO3, a reliable tool for direct detection of carbapenemase activity. J Clin Microbiol. 2015;53:1731-5.
- [Google Scholar]
- Comparative evaluation of two chromogenic tests for rapid detection of carbapenemase in Enterobacteriaceae and in Pseudomonas aeruginosa isolates. J Clin Microbiol. 2014;52:3060-3.
- [Google Scholar]
- Molecular detection of blaNDM-1 (New Delhi metallobetalactamase-1) in nosocomial Enterobacteriaceae isolates by nested, multiplex polymerase chain reaction. Med J Armed Forces India. 2018;74:108-15.
- [Google Scholar]
- Rapid screening for carbapenem resistant organisms:Current esults and future approaches. J Clin Diagn Res. 2015;9:DM01-3.
- [Google Scholar]
- Targeting macrophages as a potential therapeutic intervention:Impact on inflammatory diseases and cancer. Int J Mol Sci. 20181953;19
- [Google Scholar]
- Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa . Thorax. 2017;72:666-7.
- [Google Scholar]
- Reactive oxygen species:A novel antimicrobial. Int J Antimicrob Agents. 2018;51:299-303.
- [Google Scholar]






