Translate this page into:
NDM-1 (New Delhi metallo beta lactamase-1) producing Gram-negative bacilli: Emergence & clinical implications
Reprint requests: Dr Bashir Ahmad Fomda, Department of Microbiology, Sher-i-Kashmir Institute of Medical Sciences, Soura, Srinagar 190 011, Jammu & Kashmir, India e-mail: bashirfomda@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Backgound & objectives:
Resistance to carbapenems in Gram-negative bacteria conferred by NDM-1 is a global health problem. We investigated the occurrence of NDM-1 in clinical isolates of Gram-negative bacilli in a tertiary care hospital in Kashmir valley, India.
Methods:
Gram-negative bacilli from different clinical isolates were included in the study. Antimicrobial susceptibility was performed by Kirby Bauer disk diffusion method and interpreted using Clinical Laboratory Standards Institute (CLSI) guidelines. Isolates resistant to carbapenems were subjected to different phenotypic test such as modified Hodge test (MHT), boronic acid and oxacillin based MHT (BA-MHT and OXA-MHT), combined disk test and minimum inhibitory concentration (MIC) with imipenem and imipenem -EDTA for determination of class B metallo enzymes. Presence of blaNDM-1 gene was established by PCR and confirmed by sequencing.
Results:
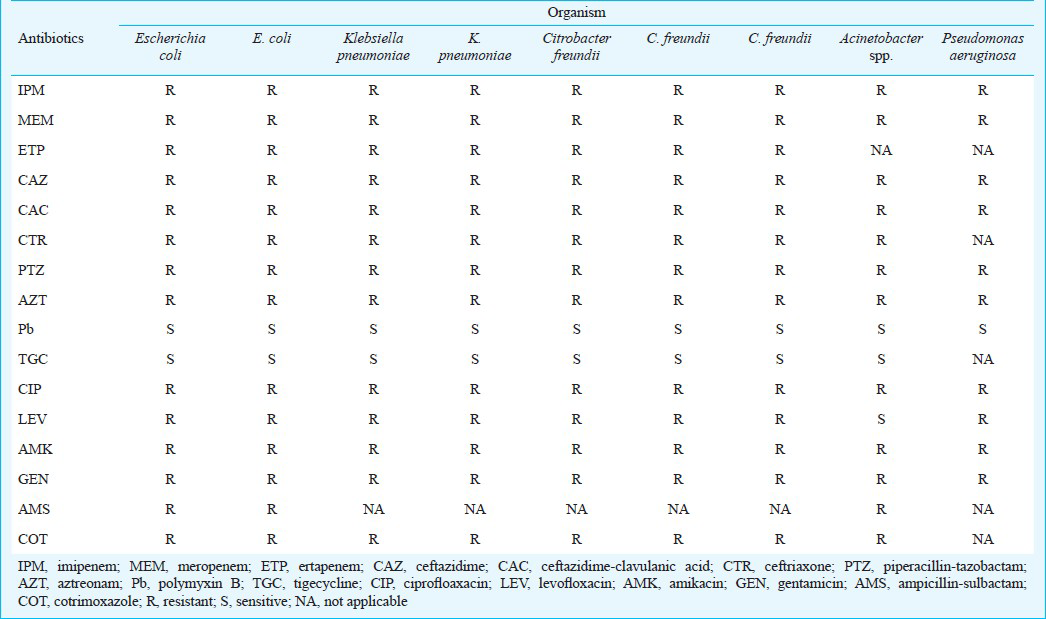
Of the total 1625 Gram-negative isolates received, 100 were resistant to imipenem. Of the 100 isolates, 55 (55%) were positive by modified Hodge test indicating carbapenemase production. Of the 100 isolates tested by MHT, BA-MHT and OXA-MHT, 29 (29%) isolates belonged to Class A and 15 (15%) to Class B, while 56 (56%) isolates were negative. Of the 15 class B metallo beta lactamase producers, nine carried the blaNDM-1 gene. NDM-1 was found among Escherichia coli (2 isolates), Klebsiella pneumoniae (2 isolates), Citrobacter freundii (3 isolates), Acinetobacter spp (1 isolate), and one isolate of Pseudomonas aeruginosa. Isolates were resistant to all antibiotic tested except polymyxin B and tigecycline.
Interpretation & conclusions:
Our study showed the presence of clinical isolates expressing NDM-1 in Srinagar, Jammu & Kashmir, India. These isolates harbour plasmid mediated multiple drug resistant determinants and can disseminate easily across several unrelated genera. To halt their spread, early identification of these isolates is mandatory.
Keywords
BA-MHT
blaNDM-1
combined disk test
MHT
MIC
OXA-MHT
New Delhi metallo beta lactamase-1 (NDM-1) is a novel MBL that confers resistance to all β-lactam antibiotics with the exception of aztreonam12. However, many strains that harbour blaNDM-1 are also aztreonam resistant, presumably by a different resistance mechanism. The blaNDM-1 gene is located on plasmids harbouring multiple resistant determinants, thereby conferring extensive drug resistance, leaving only a few or no therapeutic options3. NDM-1 has been identified mostly in Escherichia coli, Klebsiella pneumoniae and to a lesser extent in Pseudomonas and Acinetobacter4.
The emergence of carbapenem resistance among Gram-negative bacteria is a major cause of concern since carbapenems currently represent the treatment of choice for severe infections caused by multidrug-resistant strains producing extended-spectrum β-lactamases (ESBLs)5. However, the utility of carbapenems is under severe threat with the emergence of cabapenemases including the newly characterized NDM-1. The aim of this study was to determine the occurrence of NDM-1 in clinical isolates of carbapenem resistant Gram-negative bacilli in a tertiary care hospital setting in north India.
Material & Methods
The present study was conducted in the Department of Microbiology, Sher-i-Kashmir Institute of Medical Sciences, Soura, Srinagar, Jammu & Kashmir, India, during a period of six months from December 2011 to May 2012. Ethical clearance for the study was obtained from institute's ethical clearance committee.
Bacterial strains: Non duplicate isolates of Gram-negative bacteria recovered from various specimens such as blood, other body fluids, urine, pus and wound swabs, etc., were identified using conventional techniques. Antimicrobial susceptibility was performed by Kirby Bauer disk diffusion method6 and interpreted using Clinical and Laboratory Standards Institute (CLSI) guidelines7. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as control strains. All isolates resistant to carbapenems were included in the study.
Minimum inhibitory concentration (MIC) of imipenem (Sigma Chemicals, USA) was determined for isolates that were resistant to carbapenems by disk diffusion method6. Breakpoints for imipenem used were as per CLSI guidelines7: susceptible (S), ≤ 1 μg/ml; resistant (R), ≥ 4 μg/ml.
Phenotypic detection of MBLs:
Modified Hodge test (MHT) - Detection of carbapenemases was done by MHT. A clover leaf indentation at the intersection of the test isolate and standard strain was considered to be positive for carbapenemase production89.
Boronic acid based (BA)-MHT and oxacillin based (OXA)-MHT - Modification of MHT, BA-MHT and OXA-MHT as proposed by Pasteran et al10 was also performed. Briefly, a 1:10 dilution of an inoculum of the indicator organism E. coli ATCC 25922, adjusted to a 0.5 McFarland turbidity standard, was used to inoculate the surfaces of plates (diameter, 145 mm; Borosil, India) containing Mueller-Hinton agar (Hi-Media, Mumbai). After the plates were allowed to stand for 10 min at room temperature, disks containing ertapenem (ETP 10 μg) and ETP supplemented with 3-aminophenyl-boronic acid (APB) for the BA-MHT and 10 μl of oxacillin (OXA) for the OXA-MHT were placed onto the agar plates. Concentration of APB (Sigma Chemicals, USA) used in the study was 3,000 μg/disk prepared in dimethyl sulphoxide solution (DMSO; Hi-Media,), while the concentration of OXA (Sigma) used was 1,000 μg/disk. Subsequently, by use of a loop, three to five colonies of the test organisms, grown overnight on an agar plate, were inoculated onto the plate in a straight line from the edge of one disk to that of another containing the same carbapenem. The presence of growth of the indicator strain toward the carbapenem disks was interpreted as positive result for carbapenem hydrolysis (carbapenemase-like pattern). A BA-inhibited or an OXA-inhibited carbapenemase pattern was distinguished due to the absence of growth of the indicator strain toward the carbapenem- plus-APB or OXA disks, respectively compared to the corresponding disk without the inhibitor.
Combined disk test - Phenotypic detection of MBLs was done by combined disk test. Two imipenem disks (10 μg), one containing 10 μl of 0.1 M anhydrous EDTA (292 μg), were placed 25mm apart on Mueller-Hinton plate. Strain producing a zone diameter of >4 mm around the disk with IPM-EDTA when compared to IPM alone was considered positive for MBL11.
MIC for IPM and IPM-EDTA - MIC for IPM (Sigma) alone and IPM-EDTA in combination was determined by microbroth dilution method7. A four-fold reduction of MIC in IPM-EDTA when compared with IPM was taken as positive for MBL production.
Genotypic detection:
PCR assay for blaNDM-1 gene - DNA from bacterial isolates was extracted by alkaline lysis method, presence of blaNDM-1 was established by PCR with specific primers targeting the gene12. The primers (CloniTec, India) used in the study were NDM-Fm (5’-GGTTTGGCGATCTGGTTTTC-3’) and NDM-Rm (5’-CGGAATGGCTCATCACGATC-3’) which amplified an internal fragment of 264 bp of blaNDM-1 gene. PCR conditions were as follows: initial denaturation at 94°C for 5 min; 35 cycles at 95°C for 30 sec, 58°Cfor 30 sec, and 72°C for 30 sec; and final extension for 10 min at 72°C. The 264 bp fragment was amplified complement to the NDM-1 gene. Electrophoresis at 100v for 40 min was performed to separate the products on 1.5 per cent 1xTBE (8.9 M boric acid) and (0.2 M EDTA) agarose gel (Hi-Media). Gel was stained with 5 μg/ml ethidium bromide (Hi-Media) and photograph was taken under gel documentation system (Alpha Innotech Corporation, USA).
Sequencing: PCR products were purified and sequenced. Previously published sequences of NDM-1 isolates retrieved from the National Center for Biotechnology (http://www.ncbi.nlm.nih.gov) were used as the reference sequence. Nucleotide sequence analysis was performed with BLAST sequence algorithms and sequences were aligned using Clustal W13.
Results
Of the 1625 Gram-negative isolates received in the laboratory during the study period, 100 carbapenem resistant isolates were included in the study. Of these, 40 (40%) were Acinetobacter spp., 22 (22%) Klebsiella pneumoniae, 21 (21%) Pseudomonas aeruginosa, 7 (7%) E. coli and 10 (10%) Citrobacter freundii. All isolates resistant to imipenem by disk diffusion method had an MIC ≥ 32 μg/ml. Fifty five isolates were positive by modified Hodge test indicating carbapenemase production. Of the 100 isolates tested by MHT, BA-MHT and OXA-MHT, 29 (29%) isolates belonged to Class A and 15 (15%) to Class B, while 56 (56%) isolates were negative. Phenotypic detection by combined disk test reliably detected all the MBL producers. Also MIC of these isolates for imipenem and imipenem- EDTA showed 8-128 fold reduction (Table I). Of the 15 isolates that were MBL producers, nine showed an amplification band of the expected size (264 base pairs), which was compatible with the blaNDM-1 gene. These were identified as C. freundii (3 isolates), K. pneumoniae (2 isolates), E. coli (2 isolates), P. aeruginosa (1 isolate), and one isolate of Acinetobacter spp. (Table I).
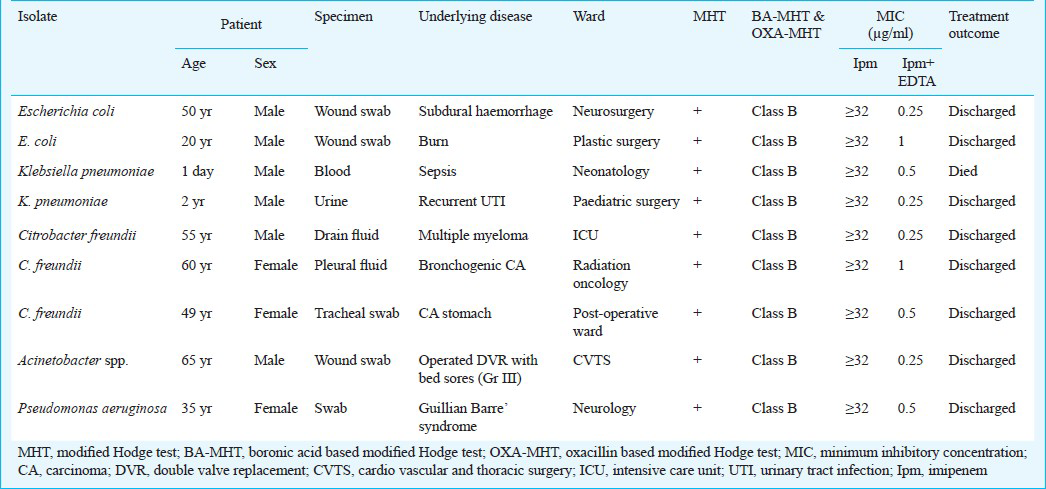
All the NDM-1 enzyme producing isolates were resistant to several antibiotic classes (Table II). Isolates were resistant to all β-lactam antibiotics including aztreonam. In addition, all isolates were resistant to aminoglycosides, tetracyclines, co-trimoxazole and fluoroquinolones, although one isolate was susceptible to levofloxacin. All isolates were susceptible to polymixcine B and tigecycline.
Sequencing of amplified gene was carried out with accession numbers KF468813, KF468814. Nucleotide sequence of the isolates was aligned with reference sequence. The selected isolates showed 100 per cent homology with the reference strain.
Discussion
Current data indicate an increase in the spread of NDM-1 metallo beta-lactamases across several countries14151617181920. Several published studies have reported the presence of NDM-1 in various cities of India124. However, this is perhaps the first report of isolation of NDM-1 from Kashmir valley.
The modified hodge test has been extensively used as a phenotypic assay for detection of carbapenemase activity and has been found to be sensitive in detecting Ambler class A (KPC) and Class D (OXA-48) carbapenemases. However, false positive rates of up to 25 per cent for MHT have been reported9. Also, its sensitivity is low for NDM-1 producers. To circumvent the false positive results, BA-MHT and OXA-MHT were also performed. This has been recommended to improve the performance of MHT and differentiate carbapenemases into different Ambler classes10. Other phenotypic methods like combined disk test and MIC of imipenem and imipenem- EDTA were also used for enhanced detection of NDM-1 producers as has been recommended by other studies10122122.
In the present study, nine of the 15 metallo beta-lactamase carrying organisms were found to be NDM-1 producers. These belonged to different genera: Citrobacter freundii, Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa and Acinetobacter spp. Presence of NDM-1 has not only been reported from Enterobacteriaceae but reports of its presence in Pseudomonas and Acinetobacter have also been published12232425. This highlights the tremendous plasticity and disseminating potential of the blaNDM-1 gene.
Isolates with NDM-1 carbapenemases were highly resistant to many antibiotic classes including β-lactam antibiotics, fluoroquinolone and aminoglycosides. Resistance to aztreonam could be best explained by the presence of other mechanisms of resistance, most importantly AmpC β-lactamase or combination of permeability defects and efflux mechanisms2627. Susceptibility profiling indicated no resistance to polymyxin B and tigecycline in NDM-1 producers and this had been a typical observation among NDM-1 positive isolates227. In contrast to many other resistance mechanisms, NDM-1 is not associated with a single strain but has spread very rapidly to non-clonally related strains. Plasmids carrying the blaNDM-1 gene also carry a number of other genes conferring resistance to aminoglycosides, macrolides and sulphamethoxazole, thus making these isolates multidrug resistant11. Because this carbapenemase is encoded by a genetic element found on different plasmids that may duplicate or jump from bacteria to bacteria easily, rapid dissemination and spread between different bacterial species by lateral gene transfer is favoured20.
In conclusion, our results showed the presence of NDM-1 in clinical isolates for Gram-negative bacteria in Srinagar, Kashmir valley. As these isolates are multi drug resistant, the emergence and spread of NDM-1 producers will limit the therapeutic options and threaten the public health of people. Timely detection, implementation of infection control measures, formulation of antibiotic policy and preventive strategies to control dissemination of such strains are urgently required.
References
- Characterization of a new metallo-β-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046-54.
- [Google Scholar]
- Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
- [Google Scholar]
- Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006-2007. Antimicrob Agents Chemother. 2011;55:1274-8.
- [Google Scholar]
- ESCMID Study Group for Antimicrobial Resistance Surveillance (ESGARS). Metallo-β-lactamases as emerging resistance determinants in Gram-negative pathogens: open issues. Int J Antimicrob Agents. 2007;29:380-8.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493-6.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, Twenty-First informational Supplement. In: CLSI document M100-S21. Wayne, PA: CLSI; 2011.
- [Google Scholar]
- Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo- β-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41:4623-9.
- [Google Scholar]
- Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin Microbiol Infect. 2010;16:112-22.
- [Google Scholar]
- Controlling false-positive results obtained with the Hodge and Masuda assays for detection of class A carbapenemase in species of Enterobacteriaceae by incorporating boronic acid. J Clin Microbiol. 2010;48:1323-32.
- [Google Scholar]
- Phenotypic detection of carbapenem susceptible metallo-β-lactamase producing Gram-negative bacilli in the clinical laboratory. J Clin Microbiol. 2006;44:3139-44.
- [Google Scholar]
- CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673-80.
- [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase-United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:750.
- [Google Scholar]
- NDM-1-producing Klebsiella pneumoniae isolated in the Sultanate of Oman. J Antimicrob Chemother. 2011;66:304-6.
- [Google Scholar]
- Emergence of metallo-beta-lactamase NDM-1-producing multidrug-resistant Escherichia coli in Australia. Antimicrob Agents Chemother. 2010;54:4914-6.
- [Google Scholar]
- Detection of NDM-1-producing Klebsiella pneumoniae in Kenya. Antimicrob Agents Chemother. 2011;55:934-6.
- [Google Scholar]
- Extremely drug-resistant Citrobacter freundii isolate producing NDM-1 and other carbapenemases identified in a patient returning from India. Antimicrob Agents Chemother. 2011;55:447-8.
- [Google Scholar]
- First identification of a patient colonized with Klebsiella pneumoniae carrying blaNDM-1 in Taiwan. J Chin Med Assoc. 2010;73:596-8.
- [Google Scholar]
- New Delhi metallo-beta-lactamase (NDM-1): towards a new pandemia? Clin Microbiol Infect. 2010;16:1699-701.
- [Google Scholar]
- Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol. 2012;50:477-9.
- [Google Scholar]
- New Delhi metallo-β-lactamase (NDM-1)-producing Klebsiella pneumoniae: Case report and laboratory detection strategies. J Clin Microbiol. 2011;49:1667-70.
- [Google Scholar]
- European NDM-1 Survey Participants. New Delhi metallo-beta-lactamase 1-producing Enterobacteriaceae: emergence and response in Europe. Euro Surveill. 2010;15:1-8.
- [Google Scholar]
- Emergence of NDM-1 metallo-β-lactamase in Pseudomonas aeruginosa clinical isolates from Serbia. Antimicrob Agents Chemother. 2011;55:3929-31.
- [Google Scholar]
- Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J Antimicrob Chemother. 2010;65:2253-4.
- [Google Scholar]
- Dissemination of the metallo-β-lactamase gene blaIMP-4 among Gram-negative pathogens in a clinical setting in Australia. Clin Infect Dis. 2005;41:1549-56.
- [Google Scholar]
- Hospital outbreak of carbapenem-resistant Pseudomonas aeruginosa producing VIM-1, a novel transferable metallo-β-lactamase. Clin Infect Dis. 2000;31:1119-25.
- [Google Scholar]






