Translate this page into:
N-acetyltransferase gene polymorphisms & plasma isoniazid concentrations in patients with tuberculosis
Reprint requests: Dr Geetha Ramachandran, Department of Biochemistry & Clinical Pharmacology, ICMR-National Institute for Research in Tuberculosis, Chetpet, Chennai 600 031, Tamil Nadu, India e-mail: geethar@nirt.res.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Variations in the N-acetyltransferase (NAT2) gene among different populations could affect the metabolism and disposition of isoniazid (INH). This study was performed to genotype NAT2 gene polymorphisms in tuberculosis (TB) patients from Chennai, India, and compare plasma INH concentrations among the different genotypes.
Methods:
Adult patients with TB treated in the Revised National TB Control Programme (RNTCP) in Chennai, Tamil Nadu, were genotyped for NAT2 gene polymorphism, and two-hour post-dosing INH concentrations were compared between the different genotypes. Plasma INH was determined by high-performance liquid chromatography. Genotyping of the NAT2 gene polymorphism was performed by real-time polymerase chain reaction method.
Results:
Among the 326 patients genotyped, there were 189 (58%), 114 (35%) and 23 (7%) slow, intermediate and fast acetylators, respectively. The median two-hour INH concentrations in slow, intermediate and fast acetylators were 10.2, 8.1 and 4.1 μg/ml, respectively. The differences in INH concentrations among the three genotypes were significant (P<0.001).
Interpretation & conclusions:
Genotyping of TB patients from south India for NAT2 gene polymorphism revealed that 58 per cent of the study population comprised slow acetylators. Two-hour INH concentrations differed significantly among the three genotypes.
Keywords
Isoniazid
N-acetyltransferase gene
NAT2 polymorphism
tuberculosis
Isoniazid (INH) continues to be the most widely used chemotherapeutic agent for the treatment of tuberculosis (TB). The primary step in the metabolism of INH is acetylation, catalyzed by the enzyme, N-acetyltransferase (NAT2), resulting in the formation of acetyl INH. NAT2 enzyme displays genetic polymorphism, and its activity is expressed at highly variable levels.
Several studies have shown that human subjects show a wide degree of variation in their capacity to acetylate INH to acetyl INH in spite of receiving similar prescribed INH doses1. Individuals can be distinctly characterized phenotypically as being either slow or rapid acetylators (the concentration of the enzyme being higher in rapid acetylators)1. Molecular techniques that are now available permit identification of three genotypes: rapid, intermediate and slow. Slow acetylators are known to be at a risk for most drug-induced toxicities, while rapid acetylators are likely to experience decreased therapeutic efficacy. It has been suggested that NAT2 genotyping before therapy could be useful to predict adverse reactions and make dose adjustments, if necessary23.
The acetylator gene frequency for slow allele differs widely across ethnic groups and countries: 10 per cent in people from the mongoloid race such as the Eskimos, Japanese and Chinese, 90 per cent in the Middle East, 60 per cent in the Negroid and Caucasian populations and 72 per cent in the USA4. Singh et al5 have shown high variation in NAT2 gene frequencies from different regions in India. Zabost et al6 explored the relationship between NAT2 genotypes and serum concentrations of INH in a Polish population and concluded that determining mutations in the NAT2 gene enabled the identification of the INH acetylator type in patients, and the genotyping results were consistent with the phenotype. The present study was conducted to genotype TB patients from Chennai, Tamil Nadu, India, for NAT2 gene polymorphisms and to compare plasma INH concentrations among the different genotypes.
Material & Methods
A prospective study design was followed, in which adult patients with either pulmonary or extrapulmonary TB receiving anti-TB treatment (ATT) in the Revised National TB Control Programme (RNTCP) treatment centres in Chennai, Tamil Nadu, India, during September 2014 to March 2015 were included. Patients were recruited from the TB units in Pulianthope, Chinthadripet, Basin Bridge, Kodambakkam, Saidapet, Tondiarpet, Sembium and Periyar Nagar. Diagnosis and treatment were according to the RNTCP guidelines7. Patients received either Category I or Category II treatment. Category I treatment consisted of a six-month thrice weekly regimen with rifampicin (RMP), INH, pyrazinamide (PZA) and ethambutol (EMB) for two months, followed by RMP and INH for four months7. Category II treatment consisted of a thrice weekly eight-month regimen with streptomycin, INH, RMP, PZA and EMB for two months, followed by INH, RMP, PZA and EMB for one month, and INH, RMP and EMB for the remaining five months. The drug doses were RMP 450 mg (600 mg for those >60 kg body weight), INH 600 mg, EMB 1200 mg, PZA 1500 mg and streptomycin one gram. Patients were eligible to take part in this study if they met the following criteria, (i) aged 18 yr or above, (ii) body weight not less than 30 kg, (iii) received at least two weeks of ATT regularly, (iv) not very sick or moribund, (v) willing to participate and give informed written consent, and (vi) agreeing to come to the same DOT centre until completion of the study. The study commenced after obtaining approval from the Institutional Ethics Committee of the ICMR-National Institute for Research in Tuberculosis, Chennai.
The sample size was calculated based on the study of Singh et al5, which reported mean (95% confidence interval) two-hour plasma INH concentration of 2.4 μg/ml (1.5-3.4 μg/ml) in fast acetylators and 5.6 μg/ml (4.8-6.4 μg/ml) in slow acetylators. With 95 per cent confidence level, 90 per cent power and assuming the expected true difference to be 1 μg/ml, the sample size was calculated as 323.
Patients who were diagnosed with TB were referred by the medical officers at the TB units. The purpose of the study and procedures were explained to those patients who fulfilled the study criteria. Patients who were willing to participate were recruited after obtaining informed written consent. The study was conducted at the respective TB units after patients had received a minimum of seven doses of ATT. On the study day, the anti-TB drugs were administered under direct supervision and three ml blood was collected at two hours post-dosing. The blood was distributed into heparinized and EDTA vacutainer tubes, the former was used for INH estimation and the latter for extraction of DNA and genotyping experiment. Blood collected in the heparinized vacutainer tubes was centrifuged immediately and plasma separated. The plasma samples were stored at -20°C until analysis.
Plasma isoniazid (INH) estimation: This was undertaken within a week of sample collection, by high-performance liquid chromatograph (Shimadzu Corporation, Kyoto, Japan) using validated methods8. INH was extracted from plasma using para-hydrobenzaldehyde and trifluoroacetic acid. Analysis was performed using a C8 column, with ultraviolet detector set at 267 nm. The retention time of INH was 5.5 min. The method was highly specific with no interfering peaks at this retention time. The between and within run variations for all the drugs were below 10 per cent and the lower limit of quantification was 0.25 μg/ml.
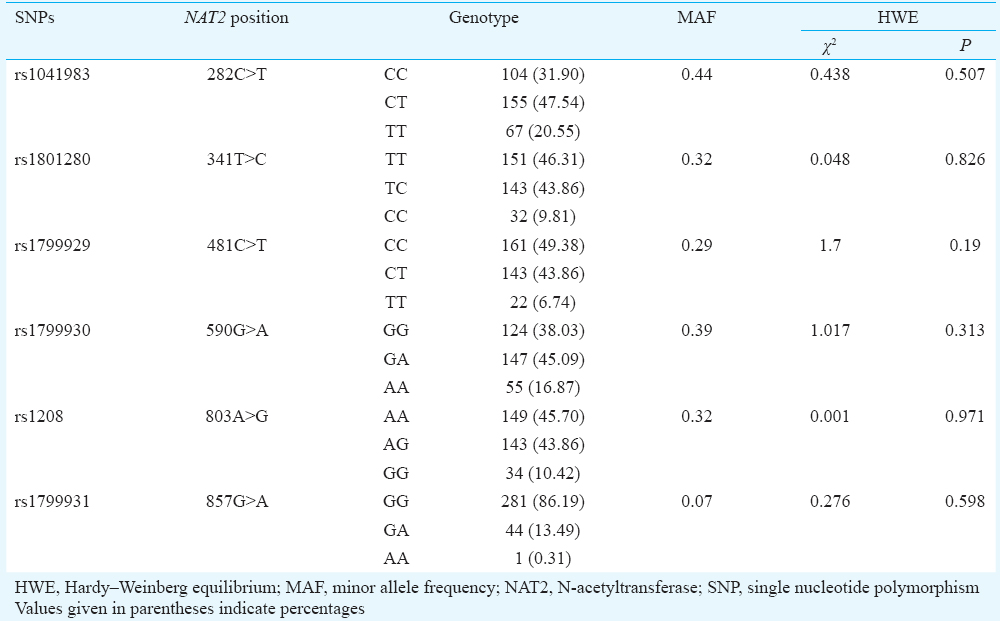
N-acetyltransferase (NAT2) genotyping: Genomic DNA was extracted using QIAamp DNA blood mini kit (Qiagen, Hilden, Germany) and quantitated on Thermo Fisher's NanoDrop 2000 spectrophotometer (NanoDrop Technologies Inc., USA). Six single nucleotide polymorphisms (SNPs, rs1041983, rs1801280, rs1799929, rs1799930, rs1208 and rs1799931) in the NAT2 gene were analyzed using Taqman SNP genotyping assays (Applied Biosystems, USA) in Applied Biosystems 7500 Real-Time PCR System and Sequence Detection Software (SDS) v1.3.1 (Applied Biosystems, USA). The slow, intermediate and rapid NAT2 acetylator phenotypes were determined using NAT2PRED Web server9. This software allows the use of the six polymorphisms in NAT2 to eventually determine the acetylator phenotype.
Statistical analysis: Analysis of data was performed using SPSS, version 20.0 (SPSS Inc., Chicago, Illinois, USA). Data were expressed as median and inter-quartile range (IQR). Marascuillo procedure was carried out to compare proportions. Kruskal–Wallis and Mann–Whitney post hoc tests were performed to study differences in two-hour INH concentrations among the different genotypes; P value was adjusted using the Bonferroni correction method.
Results & Discussion
A total of 326 patients participated in the study. Their demographic and clinical details are given in Table I. Most patients had pulmonary TB and were being treated with Category I regimen. Patients with diabetes mellitus (those with known history of diabetes mellitus and or random glucose >200 mg/dl on the study day) constituted 14 per cent of the study population. The number of slow, intermediate and fast acetylators were 189 (58%), 114 (35%) and 23 (7%), respectively. The genotypes and specific alleles of the NAT2 gene are given in Table II. The distribution followed Hardy-Weinberg equilibrium. Slow acetylators accounted for 55 per cent in southwestern India (Mumbai), 53, 44 and 46 per cent in north India and 74 and 67.4 per cent in south India10111213. We observed 58 per cent of our study population to be slow acetylators, which comprised patients of south Indian origin (Tamil Nadu State).
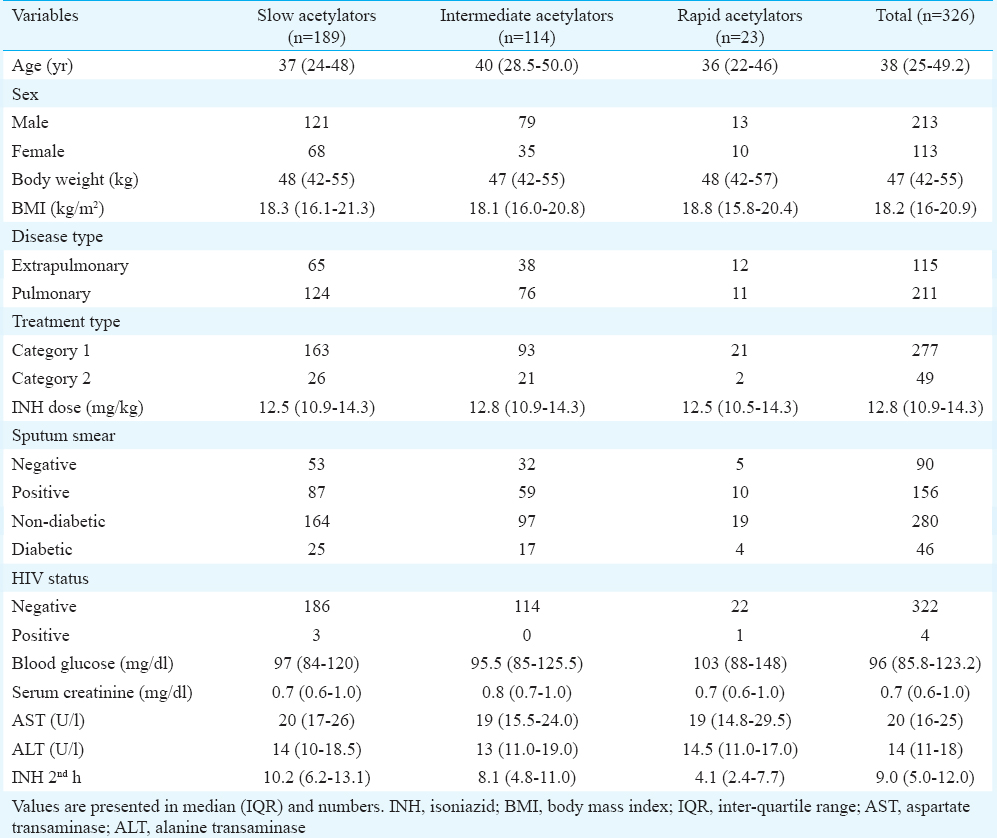
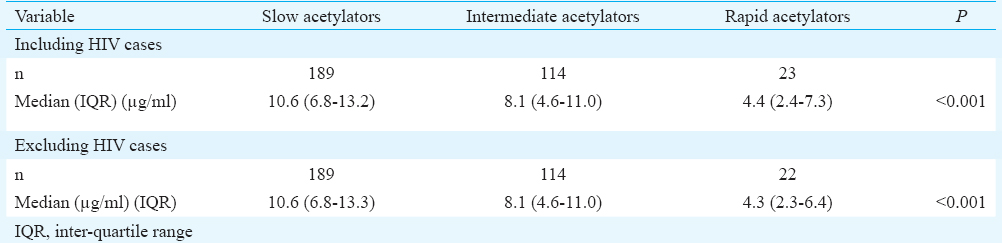
The median two-hour INH concentrations in slow, intermediate and fast acetylators were 10.2, 8.1 and 4.1 μg/ml, respectively. The differences in INH concentrations among the three genotypes were significant (P<0.001). There existed a significant trend in the INH concentrations among the genotypes; the slow acetylators had the highest concentration, followed by the intermediate acetylators and fast acetylators had the lowest INH concentration (Figure). There were four HIV co-infected patients in this study group. Comparison of INH concentrations among the different NAT2 genotypes after excluding the four HIV seropositive patients also yielded similar results (Table III).
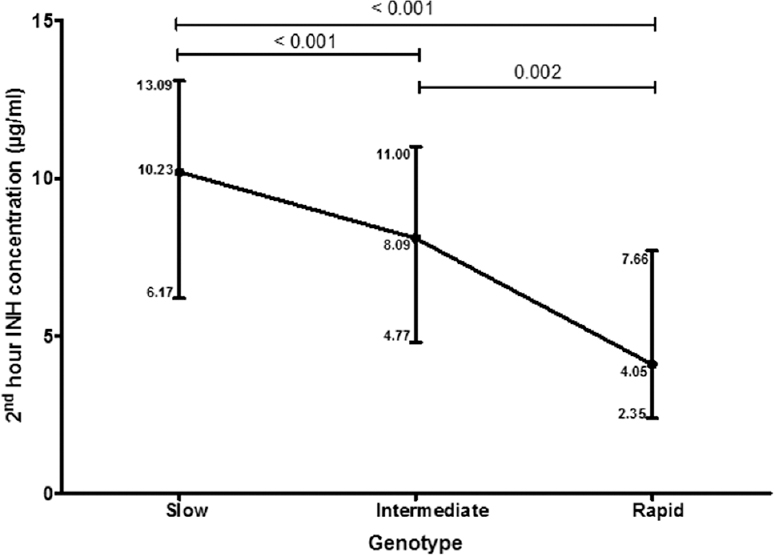
- Median two-hour isoniazid concentrations in the different genotypes. The vertical bars denote inter-quartile range.
Variations in the NAT2 gene among different populations could affect the metabolism and disposition of INH. Several studies to date have documented the influence of NAT2 genotypes on plasma concentrations of INH251415. Drug-induced hepatotoxicity as well as adverse drug events have also been reported16. Viewed in this context, significant variations in INH concentrations among different sets of patients (genotypes) is an important issue, since INH is an important drug in the treatment of TB and is widely used in India. Slow acetylators with higher INH concentrations are more susceptible for drug-induced hepatotoxicity. A meta-analysis by Wang et al17 from 14 studies, comprising 474 cases and 1446 controls showed a significant association between NAT2 slow acetylators and risk of anti-TB drug-induced liver toxicity.
Fast acetylators on the other hand are likely to benefit less from a prescribed drug dose. It has been suggested that rapid acetylators might require INH doses 1.5 times the currently recommended doses18. Comparison of response to TB treatment between slow and rapid acetylators of INH suggested an association between treatment response and rate of inactivation of INH; there was a difference in the rate of conversion to bacteriological negativity between slow and rapid inactivators19.
In this study, we estimated plasma INH concentrations at two hours after a supervised drug administration. It may not always be possible to collect multiple blood samples in the clinical/field setting for logistical and financial reasons; one is typically limited to one or two time points. When only one sample can be obtained, the two-hour post-dose concentrations are usually most informative20 as done in this study. In a separate study, INH peaked at two hours in more than 95 per cent of patients (unpublished findings). It has been reported that the target peak concentration of INH should be in the range of 3-6 μg/ml20. If this range was applied in the present study, 11, 14 and 26 per cent of slow, intermediate and fast acetylators, respectively had their two-hour INH concentrations less than 3 μg/ml. Although there appeared to be a trend in the proportion of patients with sub-therapeutic INH concentrations, the differences were not significant. The drug concentrations observed in our study was higher than that reported from an earlier study from Mumbai, which could be because of differences in the INH dose used in these studies, 600 mg in the present study and 300 mg in the Mumbai study5.
In conclusion, genotyping of TB patients for NAT2 gene polymorphism showed 58 per cent patients as slow acetylators and only seven per cent as rapid acetylators. Two-hour INH levels were significantly different among the different genotypes. Pharmacogenetic testing would help in reducing the occurrence of adverse drug effects and enhancing treatment success, and in the long run, could decrease the cost of health care.
Acknowledgment
The authors thank all the patients for participation in the study, and Shri S. Manoharan Nesakumar for technical assistance in the laboratory. This study was funded by the Model DOTS project through the United States Agency for International Development.
Conflicts of Interest: None.
References
- Pharmacokinetics of isoniazid metabolism in man. J Pharmacokinet Biopharm. 1976;4:83-113.
- [Google Scholar]
- Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses? Antimicrob Agents Chemother. 2005;49:1733-8.
- [Google Scholar]
- NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: A randomized controlled trial for pharmacogenetics-based therapy. Eur J Clin Pharmacol. 2013;69:1091-101.
- [Google Scholar]
- Role of pharmacogenomics in the treatment of tuberculosis: a review. Pharmgenomics Pers Med. 2012;5:89-98.
- [Google Scholar]
- Study of NAT2 gene polymorphisms in an Indian population: Association with plasma isoniazid concentration in a cohort of tuberculosis patients. Mol Diagn Ther. 2009;13:49-58.
- [Google Scholar]
- Correlation of N-acetyltransferase 2 genotype with isoniazid acetylation in Polish tuberculosis patients. Biomed Res Int. 2013;2013:853602.
- [Google Scholar]
- TB India 2011. Revised National TB Control Programme Annual Status Report. New Delhi: Central TB Division, Government of India; 2011.
- Simple and rapid liquid chromatography method for simultaneous determination of isoniazid and pyrazinamide in plasma. SAARC J Tuberc Lung Dis HIV AIDS. 2012;9:13-8.
- [Google Scholar]
- A web server for inferring the human N-acetyltransferase-2 (NAT2) enzymatic phenotype from NAT2 genotype. Bioinformatics. 2009;25:1185-6.
- [Google Scholar]
- Association of genetic polymorphisms of N-acetyltransferase 2 and susceptibility to esophageal cancer in North Indian population. Cancer Invest. 2007;25:340-6.
- [Google Scholar]
- Genetic polymorphism of the N-acetyltransferase 2 gene, and susceptibility to prostate cancer: A pilot study in North Indian population. BMC Urol. 2005;5:12.
- [Google Scholar]
- Association of CYP2E1 and NAT2 gene polymorphisms with chronic obstructive pulmonary disease. Clin Chim Acta. 2007;382:37-42.
- [Google Scholar]
- Arylamine N-acetyltransferase 2 polymorphism in the ethnic populations of South India. Int J Mol Med. 2003;11:125-31.
- [Google Scholar]
- The influence of NAT2 genotypes on the plasma concentration of isoniazid and acetylisoniazid in Chinese pulmonary tuberculosis patients. Clin Chim Acta. 2006;365:104-8.
- [Google Scholar]
- Variations between individuals and populations in the acetylation of isoniazid and its significance for the treatment of pulmonary tuberculosis. Clin Pharmacol Ther. 1976;19(5 Pt 2):610-25.
- [Google Scholar]
- Genotyping of the N-acetyltransferase2 polymorphism in the prediction of adverse drug reactions to isoniazid in Japanese patients. Drug Metab Pharmacokinet. 2002;17:357-62.
- [Google Scholar]
- NAT2 polymorphisms and susceptibility to anti-tuberculosis drug-induced liver injury: A meta-analysis. Int J Tuberc Lung Dis. 2012;16:589-95.
- [Google Scholar]
- Dose-escalation study of isoniazid in healthy volunteers with the rapid acetylator genotype of arylamine N-acetyltransferase 2. Eur J Clin Pharmacol. 2007;63:927-33.
- [Google Scholar]
- Rate of inactivation of isoniazid in South Indian patients with pulmonary tuberculosis 2. Clinical implications in the treatment of pulmonary tuberculosis with isoniazid either alone or in combination with PAS. Bull World Health Organ. 1961;25:779-92.
- [Google Scholar]
- Therapeutic drug monitoring in the treatment of tuberculosis: An update. Drugs. 2014;74:839-54.
- [Google Scholar]






