Translate this page into:
Multi-centric evaluation of a stage-specific reverse transcriptase-polymerase chain reaction assay as a xenomonitoring tool for the detection of infective (L3) stage Wuchereria bancrofti in vectors
For correspondence: Dr Sugeerappa Laxmanappa Hoti, ICMR-National Institute of Traditional Medicine, Nehru Nagar, Belagavi 590 010, Karnataka, India e-mail: hotigood@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
An infective stage specific reverse transcriptase-polymerase chain reaction (RT-PCR) assay utilizing the abundant larval transcript-3 (Alt-3) gene of Wuchereria bancrofti was developed at ICMR-VCRC, Puducherry and found to be stage specific, and sensitive upon validation in the laboratory. This study was aimed at independently evaluating this assay for its utility as a monitoring/surveillance tool in the operational programme for elimination of lymphatic filariasis (LF) by four national research laboratories.
Methods:
Evaluation of the assay was carried out in a multi-centric mode in three phases. In phase I, a workshop was conducted to impart hands-on training to the scientists from the collaborating centres on the RT-PCR assay and in Phase II the assay was evaluated for specificity and sensitivity in detecting the infective (L3) stage larvae of W. bancrofti in its vector, Culex quinquefasciatus, using 50 coded pooled samples. Phase III evaluation was done on wild-caught mosquito vectors from selected endemic areas of Assam and Bhubaneswar States and Andaman Nicobar islands.
Results:
Phase I data indicated that the assay was able to detect all the pools of mosquito samples contaning L3 stage larvae of W. bancrofti as positive, even in the presence of other vector stages of the parasite indicating its stage specificity (100%). The assay was found highly sensitive (100%), detecting all the infected pools as positive and specific detecting all uninfected pools as negative. The results of phase II showed inter-laboratory variation. Phase III evaluation from all the centres suggested that the infectivity rate determined for pooled mosquitoes by the RT-PCR assay (0.5%) was comparable to that by dissection method (1.2%) (95% confidence interval overlaps).
Interpretation & conclusions:
Overall, the results from three of the four participating centres indicated that the assay is at least as sensitive and stage specific as the conventional mosquito dissection technique, and hence, may be useful as a xenomonitoring tool for Transmission Assessment Survey in Mass Drug Administration programmes for LF.
Keywords
Infective (L3) stage
L3 specific reverse transcriptase-polymerase chain reaction assay
multi-centric evaluation
Wuchereria bancrofti
Human lymphatic filariasis (LF) is endemic in 73 countries and territories of the tropical and subtropical regions of the globe, putting 1.39 billion people at risk of developing this debilitating disease1. LF continues to be an important public health problem in India, contributing about 44.3 per cent to the global burden. In an effort to eliminate LF, combined drug regimens of antifilarial drugs are being mass administered in >48 endemic countries2. The Global Programme to Eliminate Lymphatic Filariasis (GPELF) has two principal aims: (i) to interrupt LF transmission, and (ii) to manage morbidity and prevent disability1. Transmission of LF has two components: transmission from man to mosquito vector and transmission from the mosquito vector to the human host3. The success of the GPELF has led to a significant decrease in microfilaria and antigenemia levels in several countries that have accomplished several rounds of Mass Drug Administration (MDA)4. However, the current challenge is to decide on an appropriate time for cessation of MDA. Success of the global programme to eliminate LF through MDA depends on the availability of tools to monitor the outcome of the elimination efforts. The decision for cessation of MDA is complex and multiple decision support tools have been reported so far for arriving at the decision5. Transmission is a key parameter in this regard and can therefore be monitored and evaluated by measuring changes in infection status of either vectors or humans. Direct detection of microfilariae (Mf) of the parasite in the vector is indicative of both the presence of patent circulating Mf infections in humans and transmission of the infection from humans to the vector. Transmission of LF is a function of both the prevalence of mosquitoes with infective-stage larvae and the man-biting rate6. Detection as well as differentiation of the parasites and their stages in vectors is therefore essential to determine the infection (proportion of vectors harbouring any stage of the filarial parasite) and infectivity (proportion of vectors harbouring L3 stage of the filarial parasite) rates for assessment of the transmission of filarial infection in endemic areas.
Interruption of transmission of infection is largely being monitored using human infection measures such as antigenaemia7, microfilarial loads6 and antifilarial antibodies89. Detection of L3 stage larvae of filarial parasites by dissection and microscopic examination is the gold standard method to estimate the transmission levels in endemic settings10. However, this method is cumbersome, subjective, has low through-put and not applicable in areas with ultra-low parasite prevalence in vectors. These attributes make this method unsuitable for use in assessing large scale programmes such as GPELF.
PCR-based methods for the detection of infective (L3) stage larvae of Wuchereria bancrofti and Brugia malayi have previously shown to be useful detection tools1011. Molecular xenomonitoring (MX) using PCR based detection of filarial DNA in mosquito vectors, is a sensitive and less invasive tool which can help in indirect detection of filarial infection in communities121314. Infective stage-specific reverse transcriptase-PCR (RT-PCR) assays, targeting two individual L3 specific genes [Cuticular collagen 2 (CoL2, U370160) and Abundant larval transcript-3 (Alt-3, U370163) respectively] of W. bancrofti, were developed at ICMR-Vector Control Research Centre (VCRC), Puducherry and validated in the laboratory for stage specificity and sensitivity11. These assays could detect only a minimum number (1-2) of L3 among all other stages in a pool of 25 mosquitoes. The intensity of the positive signal obtained with pools of 25 mosquitoes with single L3 parasite was stronger in Alt-3-based than in Col 2-based assay. Therefore, RT-PCR assay targeting Alt-3 gene was taken forward for further validation.
The sensitivity and specificity of the Alt-3 assay was found to be 98 and 100 per cent [95% confidence interval (CI): 88-100% and 91-100%] respectively. Consequently, the process of detection of the infective stage parasite of W. bancrofti in vector mosquitoes by Alt-3-based RT-PCR assay was patented15 (Indian patent no.257150). However, this assay needed an independent evaluation for its utility as a monitoring/surveillance tool in the operational programme. Therefore, the objective of this study was to evaluate the Alt-3 gene-based RT-PCR assay as a xenomonitoring tool from the perspective of end points for the programme.
Material & Methods
Evaluation of the infective stage-specific RT-PCR assay was carried out in a multi-centric mode involving four national research laboratories located in four different regions of India namely ICMR-Regional Medical Research Centre (RMRC), Dibrugarh, Assam; ICMR-RMRC, Bhubaneswar, Odisha; ICMR-RMRC, Port Blair, Andaman & Nicobar Islands; Centre for Research in Medical Entomology (CRME), Madurai, Tamil Nadu from January 2012 to August 2013.
Uninfected Culex quinquefasciatus mosquitoes were obtained from the laboratory colony maintained at the rearing and colonization facility at ICMR-Vector Control Research Centre (VCRC), Puducherry. Mf of W. bancrofti (nocturnally periodic strain) were purified from blood collected from Mf positive patients residing in and around Puducherry town, which is a known endemic area for bancroftian filariasis16 after procuring clearance from the Institutional Ethics Committee at CRME, Puducherry. Blood smears from microfilaraemic individuals were collected from >100 individuals during 2100-2300 hr by finger prick method and examined for Mf after Giemsa’s staining. About 5 ml of blood was drawn from five high-count (>20-100 Mf/20 µl) Mf carriers after obtaining a written informed consent from the study participants. Mf separation from blood was done by Percoll-gradient technique17.
Female Cx. quinquefasciatus mosquitoes were fed on high-count microfilaraemic blood (about 50 Mf/cc blood) by employing the artificial membrane feeding technique18. After dissecting infected mosquitoes on day four, L1 (short and sausage-shaped, length: 125-150 µm diameter 10-17 µm) stage and day seven, L2 (length: 200-300 µm; diameter 15-30 µm) stage post-infection respectively, were picked up using micro-needle and stored individually in sterile microfuge tubes. Infective stage larvae, L3, (active filiform, length: 1400 µm; diameter 20 µm) were harvested using Bearman’s funnel technique on day 14 post-infection18. Each parasite (L1 or L2 or L3 larva) was stored individually in 20 µl Trizol® (Invitrogen, USA) in a microfuge tube at −80°C.
The evaluation of the assay was carried out in three phases (Fig. 1) and the experimental design of the study was as follows:
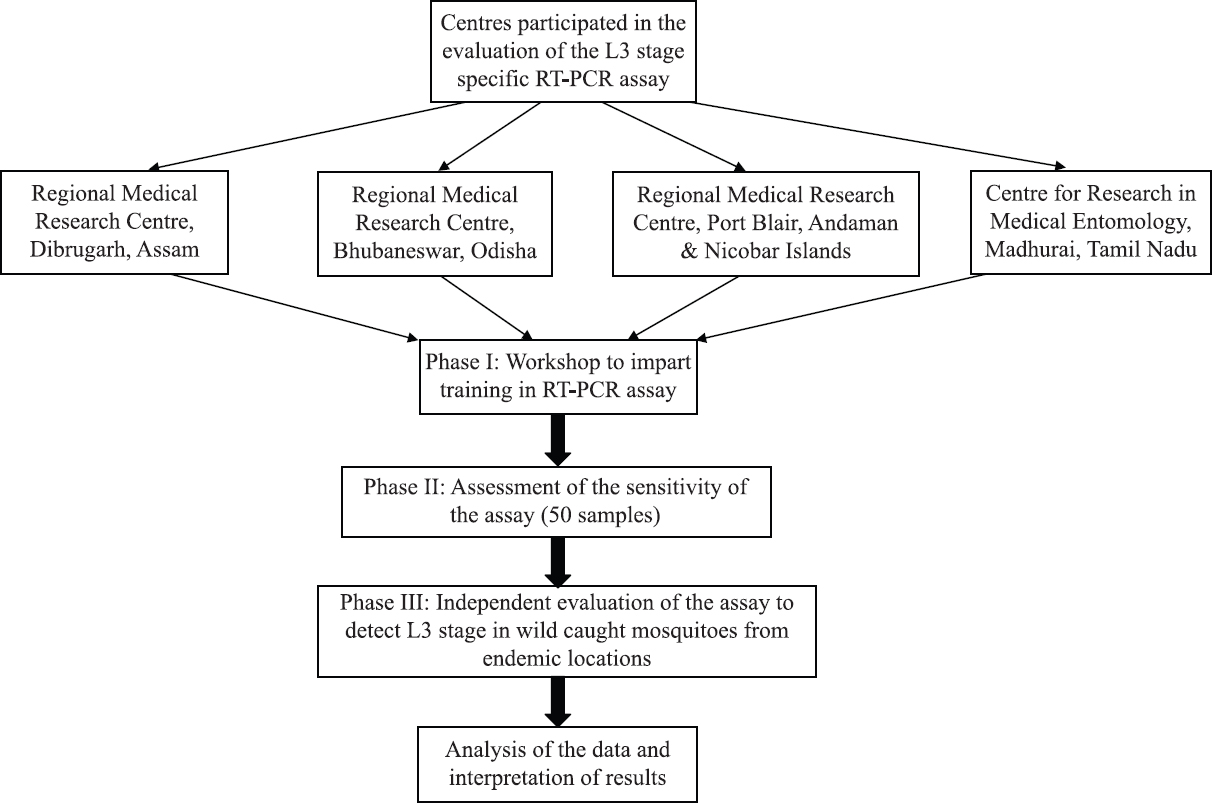
- Design of the multi-centric evaluation of a stage-specific RT-PCR assay for the detection of infective (L3) stage Wuchereria bancrofti in vectors.
-
In phase I, a workshop was conducted to impart hands-on training to the scientists from the collaborating laboratories and their technical staff on the RT-PCR assay for detecting the infective (L3) stage larvae of W. bancrofti in its vector, Cx. quinquefasciatus. For assessing the stage specificity of the assay, pools of mosquitoes (25 numbers per pool) each containing a mix of different stages of the parasite were prepared. The positive pools contained (in the following order): (a) L3 + Mf, (b) L3 + L1, (c) L3 + L2, (d) L3 alone (+ve control) and the negative pools contained (x) Mf + L1, (y) L1 + L2, and (z) only mosquitoes (−ve control, in duplicate, coded by an external expert and subjected to L3 specific RT-PCR assay by each participant. Thus, 8 positive pools and 6 negative pools were distributed to each Centre. Further, the participants also assessed the sensitivity of the assay on coded samples of five infected (spiked with L3 stage larva) and five uninfected mosquitoes (laboratory reared) pools, four pools each with Mf, L1, L2 and L3, along with a positive control (L3 only) and negative control (mosquitoes only), forming a total of 7 positive and 9 negative pools and these pools were not in duplicate (unlike the previous ones).
Coding of the samples was done by an external expert to avoid bias or subjectivity by the participant in assessing the outcome of the experiment. Decoding means the results given by the participant for the coded (unknown) samples matched with the known original sample numbers given by the external expert.
Before analyzing the coded samples in phase II, the participating centres pre-tested the known (12) samples; five each of positive and negative pools, a positive and negative control, were provided by the VCRC to each centre.
-
In phase II, 50 mosquito pools comprising 25 infected (spiked with single W. bancrofti L3) and 25 uninfected (without L3) pools of 25 mosquitoes each were coded by the external expert and sent to each of the participating laboratories for processing further by RT-PCR assay.
-
In phase III, field surveys were conducted in the LF endemic areas (Mf rate ≥1.0) of Assam and Odisha States and Andaman & Nicobar Islands. Cx. quinquefasciatus mosquitoes, the vector of W. bancrofti were collected from different locations which included LF endemic tea gardens in Dibrugarh district of Assam State, endemic areas of Cuttack, Khurdha and Nayagarh districts in Odisha State, endemic areas of Thirukoyilur district in Tamil Nadu and two endemic locations viz. Haddo and Dairy Farm in South Andaman, Andaman & Nicobar Islands. From the selected endemic areas, indoor resting collections in households were made in the morning hours, following standard entomological procedures19 for a period of one hour using mechanical aspirator. Collected mosquitoes were knocked down using diethyl ether, identified to species level and 25 gravid Cx. quinquefasciatus were pooled into a microfuge tube (1.5 ml) to which 350 µl Trizol® (Invitrogen, USA) was added. The tube was labelled, sealed with parafilm and stored at −20°C until further use. Pools of 25 mosquitoes each were processed by Alt-3-based RT-PCR assay and an equal numbers of mosquitoes were subjected to dissection and microscopy for detection of infective (L3) stage W. bancrofti for assessing the transmission.
RNA extraction: Mosquito samples in 1.5 ml microfuge tubes were thoroughly homogenized with the pestle fitted to a hand-driven motor. After completing homogenization, the pestle was washed with 250 µl of Trizol® (Invitrogen, USA) and the homogenate was processed for extraction and purification of total RNA using AxyPrepR Multi-source total RNA miniprep Kit (Axygen, USA) at room temperature following the protocol prescribed by the manufacturer with minor modifications. The extracted RNA was subsequently utilized as the template for reverse transcription for cDNA synthesis or stored at −80°C for future use.
Reverse transcription-polymerase chain reaction (RT-PCR) for cDNA synthesis: First-strand cDNA was synthesized using Sensiscript Kit (Qiagen, Germany) following the manufacturer’s instructions. An aliquot of the finished reverse-transcription reaction was taken as template to the PCR mix for the amplification of the second strand.
The PCR reaction mixture (total volume, 25 μl) included 5.5 μl of sterile MilliQ water, 12.5 μl of Go Taq green mastermix (Promega, USA), and 2.0 µl of 25 pmol of each primer based on Alt-3 gene (forward: WbL33F and reverse: WbL33R):
WbL33F: 5’- GAGTCGTTTGGTTGGGGATA-3’ and WbL33R: 5’- TCTTCTTGCCCAGTACAGCA-3’. The amplification was carried out in a Thermal Cycler GeneAmp PCR system 2720 (Applied Biosystem, USA) using the following conditions: at 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1 min and 72°C for 10 min.
A sample of 10 μl of the PCR product was electrophoresed in 2 per cent (w/v) agarose gel along with the molecular weight marker (100 bp), stained with ethidium bromide, to check for an amplicon size of 111 bp (GenBank: EU370163) on a UV transilluminator and the results documented in a gel documentation system (UVP gelDoc-It, UK).
Data analysis: The Kappa statistic was used to measure the degree of agreement between actual and test results based on pool screen method. The Kappa coefficients were interpreted as the agreement between actual and test is 0.01-0.20 slight; 0.21-0.40 fair; 00.41-0.60 moderate; 0.61-0.80 substantial; 0.81-1.0 almost perfect. Poolscreen V2.0 software developed by Katholi et al20 was used to obtain the maximum likelihood estimate of the vector infectivity rate and its 95 per cent CI from the data generated from RT-PCR assay. For the mosquitoes dissected, the infectivity rate was calculated as the percentage of mosquitoes positive for L3 larvae. The 95 per cent CI for the infectivity rate by dissection was calculated based on the normal approximates to binomial data. The difference in estimated infectivity rates between the two methods (Poolscreen and dissection) was evaluated by comparing 95 per cent CI’s for the estimates.
Results
During the workshop (Phase I), stage specificity of the assay was tested by each participant in pools of mosquitoes containing mixed stages of W. bancrofti. This was done to simulate the infection of mosquitoes in field condition. The results were decoded by the same expert who coded them and it was observed that all the samples that contained L3 stage larvae, irrespective of the presence of other stages, were found positive, while all those samples that did not contain L3 larvae were negative (Fig. 2). Thus, the assay was able to detect L3 stage larva with specificity in pools of mosquitoes even in the presence of other vector stages of the parasite indicating its stage specificity (Table IA). Further, the participants also assessed the sensitivity of the assay on coded samples and the results indicated that the assay was sensitive in detecting all the L3 infected pools as positive and specific in detecting all the negative pools as negative (Fig. 3 and Table IB).
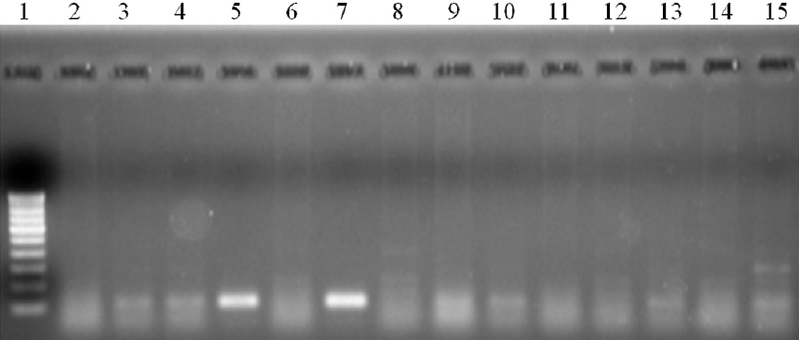
- Stage specificity of L3-specific RT-PCR in detecting the infective stage W. bancrofti in pools of mosquitoes containing mixed stages of W. bancrofti in Cx. quinquefasciatus . Lane 1- Molecular weight marker (100 bp); lanes 2, 6, 8, 11, 12, 14 – mosquito pools with mixed stages without L3; lanes 3, 4, 5, 7, 9, 10, 13, 15 – Mosquito pools with mixed stages including L3.
| Name of the centre | Number of positive pools | Number detected as positive (sensitivity %) | Number of negative pools | Number detected as negative (specificity %) |
|---|---|---|---|---|
| CRME, Madurai | 8 | 8 (100) | 6 | 6 (100) |
| ICMR-RMRC, Dibrugarh | 8 | 8 (100) | 6 | 6 (100) |
| ICMR-RMRC, Port Blair | 8 | 8 (100) | 6 | 6 (100) |
| ICMR-RMRC, Bhubaneswar | 8 | 8 (100) | 6 | 6 (100) |
| Overall | 32 | 32 (100) | 24 | 24 (100) |
CRME, Centre for Research in Medical Entomology; RMRC, Regional Medical Research Centre
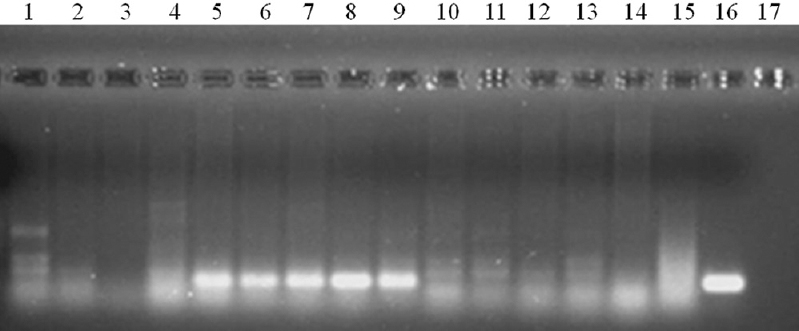
- Specificity and sensitivity of L3-specific RT-PCR in detecting the infective stage W. bancrofti in Cx. quinquefasciatus by the participant. Lane 1: Molecular weight marker (100 bp); lanes 2, 3, 4; pools containing Mf, L1, L2; lanes 5-10: pools containing L3 larvae; lanes 11-15: pools without L3; lane 16: positive control (L3 larvae; lanes 17: negative control (mosquito).
| Name of the centre | Number of positive pools | Number detected as positive (sensitivity %) | Number of negative pools | Number detected as negative (specificity %) |
|---|---|---|---|---|
| CRME, Madurai | 7 | 7 (100) | 9 | 9 (100) |
| ICMR-RMRC, Dibrugarh | 7 | 7 (100) | 9 | 9 (100) |
| ICMR-RMRC, Port Blair | 7 | 7 (100) | 9 | 9 (100) |
| ICMR-RMRC, Bhubaneswar | 7 | 7 (100) | 9 | 9 (100) |
| Overall | 28 | 28 (100) | 36 | 36 (100) |
Results of Phase II evaluation by the participating Centres after decoding showed that the results of the two laboratories were 90-92 per cent concordant, but that of the other two laboratories were less concordant (42-60%), showing inter-laboratory variation. The Kappa statistic indicated that the agreement between actual and test detected were almost ideal for two laboratories (CRME and RMRC, Dibrugarh), and moderate for all the laboratories combined. However, for two laboratories, the results were not in agreement (Table II).
| Name of the centre | Number of positive pools | Number detected as positive (sensitivity %) | Number of negative pools | Number detected as negative (specificity %) | Concordant results (%) | κ statistic, K (95% CI) |
|---|---|---|---|---|---|---|
| CRME, Madurai | 25 | 21 (84) | 25 | 24 (96) | 45 (90) | 0.80 (0.63-0.97) |
| ICMR-RMRC, Dibrugarh | 25 | 23 (92) | 25 | 23 (92) | 46 (92) | 0.84 (0.69-0.99) |
| ICMR-RMRC, Port Blair | 25 | 10 (40) | 25 | 11 (44) | 21 (42) | 0.00 (0.00-0.11) |
| ICMR-RMRC, Bhubaneswar | 25 | 15 (60) | 25 | 15 (60) | 30 (60) | 0.00 (0.00-0.47) |
| Overall | 100 | 69 (69) | 100 | 73 (73) | 142 (71) | 0.42 (0.29-0.55) |
CI, confidence interval
Phase III evaluation was done on wild-caught mosquito vectors from selected endemic areas. Forty to 100 pools of 25 mosquitoes each (coded by a third party) were subjected to W. bancrofti Alt-3 RT-PCR assay by each participating centre. Simultaneously, female mosquitoes were subjected to dissection and microscopy for the detection of L3 stage larvae of W. bancrofti (Table III). The results from all the centres showed that the infectivity rate estimated by PCR assay (0.5%) was comparable to that by the dissection method (1.2%; 95% CI overlaps). Centre-wise analysis showed that the infectivity rates by PCR assay did not differ significantly from that by dissection method for two of the centres (RMRC, Dibrugarh and RMRC, Port Blair) but for RMRC, Bhubaneswar it differed significantly (no overlap of 95% CI).
| Evaluating centre | Number of locations | Number of Cx. quinquefasciatus collected | Number of pools | Number of pools detected as positive (%) | Number of Cx. quinquefasciatus dissected | Number positive for L3 | Infectivity rate (%, 95% CI) by | |
|---|---|---|---|---|---|---|---|---|
| PCR assay | Dissection | |||||||
| ICMR-RMRC, Dibrugarh | 10 | 2500 | 100 | 19 (19) | 938 | 13 | 0.84 (0.48-1.39) | 1.39 (0.64-2.13) |
| ICMR-RMRC, Bhubaneswar | 3 | 1000 | 40 | 2 (5) | 1550 | 29 | 0.20 (0.002-0.7) | 1.87 (1.2-2.5) |
| ICMR-RMRC, Port Blair | 2 | 1025 | 41 | 0 | 1000 | 0 | 0.0 | 0.0 |
| ICMR-CRME, Madurai* | - | - | - | - | - | - | - | - |
| Overall | 15 | 4525 | 181 | 21 | 3488 | 42 | 0.50 (0.32-0.82) | 1.20 (0.80-1.57) |
*Not completed
Discussion
MX tools are being increasingly used in the recent years e.g., for filarioid helminths2122, multidrug malaria parasites23 and Trypanosoma brucei24. Species specific conventional PCR assays for W. bancrofti and B. malayi were developed earlier2526 and subsequently, a real-time PCR assay for W. bancrofti was developed27. The latter assay has been used for assessing the post-MDA scenario in American Samao28, Sri Lanka29 and Togo30. These studies have amply elucidated the use of MX tools in the end game of GPELF. However, the species-specific PCR assay used in these studies are useful only for determining the infection rates in vectors thus leaving an iota of doubt regarding the assessment of ongoing transmission of infection in an endemic area. A tool that can detect the infective stage of the parasite and hence can measure the infectivity rate in vectors would be much rather useful. In view of this, a conventional RT-PCR based assay utilizing the infective stage-specific gene, Alt-3, for detecting infective (L3) stage larvae of the lymphatic filarial parasite, W. bancrofti, in vector mosquito Cx. quinquefasciatus was developed11. This assay is based on the stage-specific primers designed based on the gene, Alt 3, upregulated in infective stage larvae of the filarial parasite, W. bancrofti. Molecular detection of L3 stage parasites primarily included preservation and efficient extraction of RNA from parasites in pools of mosquitoes. The assay developed by us was able to detect a single L3 parasite spiked in a pool of 25 whole mosquitoes (Table IA). However, this assay required independent multi-centric validation using wild-caught mosquitoes before being employed in the LF elimination programme. The validation was thus conducted in LF endemic areas with the participation of four laboratories, in three phases.
In phase I, assay conducted on a limited number of pooled mosquito samples showed that it was sensitive and specific. Further, the assay conducted on coded pooled mosquitoes containing mixed stages of the parasites also showed it to be stage specific, detecting the L3 stage even in the presence of other parasite stages. In the phase II evaluation, the results of RT-PCR assays performed in the collaborating laboratories on non-coded samples (pre-test) also showed that the assay is highly sensitive and specific, detecting all positive samples as positive and negative samples as negative (Tables IA and B). Followed by this, the sensitivity and specificity of the RT-PCR assay validated on coded samples in their respective laboratory settings indicated inter-laboratory variation, which could be due to issues pertaining to storage and transportation of samples and reagents and handling of RNA extraction protocol (Table II).
The purpose of phase III evaluation was mainly to see whether the assay can be employed for detecting infective stage parasite in wild-caught vectors, while also enabling the establishment of the assay in the collaborating centres. At RMRC, Dibrugarh, the assay was evaluated on 100 pools (each containing 25 mosquitoes) from 10 different LF endemic locations and comparison with staging by dissection and microscopy. The infectivity rates obtained by both methods were statistically comparable (Table III). The investigators also tested pools of mosquitoes from non-endemic areas and those reared in the laboratory and found that both the lots were negative by RT-PCR assay. Thus, the results of the evaluation at this centre suggested that the RT-PCR is specific, sensitive and can be employed for field studies.
Similarly, this assay was conducted by RMRC, Bhubaneswar on mosquitoes collected from three endemic locations (Cuttack, Khurdha and Nayagarh), and the location-wise analysis of the data indicated that in two of the locations the infectivity rates were comparable (95% CI overlap) with that obtained by the dissection method (Supplementary Table). However, in the vectors collected from Nayagarh, infectivity rates obtained by the two methods were not comparable (Supplementary Table). This may be due to the large difference in the number of mosquitoes assessed by the two methods. While 1550 mosquitoes were subjected to dissection and microscopy which yielded infectivity rate of 2.1 per cent (95% CI: 1.0-3.2), only 5 pools (125 mosquitoes) were subjected to RT-PCR assay (infectivity rate: 0%). Thus, the difference in infectivity rate determined by combining data from all the three locations could be due to small numbers of pools (5 pools of 25 mosquitoes each i.e.125 mosquitoes) from Nayagarh subjected to PCR assay compared to that subjected to dissection.
| Study site | RT-PCR assay results | Dissection results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of pools | Total No. of mosquitoes | No. of pools positive for L3 | Infectivity rate (%) | LCL | UCL | No. of dissected | L1/L2 % | No. of positive for L3 | Infectivity rate % | LCL | UCL | |
| Cuttack | 20 | 500 | 1 | 0.20 | 0.006 | 1.05 | 578 | ND | 8 | 1.38 | 0.43 | 2.34 |
| Khurdha | 15 | 375 | 1 | 0.28 | 0.009 | 1.41 | 308 | ND | 7 | 2.27 | 0.61 | 3.94 |
| Nayagarh | 5 | 125 | 0 | 0.00 | 0.000 | 0.00 | 664 | ND | 14 | 2.11 | 1.02 | 3.20 |
| Total | 40 | 1000 | 2 | 0.20 | 0.002 | 0.71 | 1550 | ND | 29 | 1.87 | 1.20 | 2.55 |
At RMRC, Port Blair, 1025 mosquitoes (41 pools) from two locations were collected and none were found positive by RT-PCR, while dissection of 1000 mosquitoes from the same locations also indicated the absence of L3 stage parasite in any of the mosquitoes, thus indicating that the results of the two methods agree with each other. It may be noted that these areas have undergone several rounds of MDA and the Mf rate as reported by the National Vector Borne Disease Control Programme (NVBDCP) is extremely low31. At CRME, Madurai, collection of mosquitoes from the endemic locations and detection of L3 stage by RT-PCR assay could not be completed. In all the above-mentioned laboratories, the personnel were trained and the central laboratory in each centre is well established and the assay conditions standardized.
The impact of MDA in reducing the filarial infection in humans is assessed by Transmission Assessment Survey (TAS) following the protocol recommended by WHO. However, TAS cannot detect low-level infection in certain endemic areas. To achieve elimination of LF, robust surveillance and monitoring tools are needed. MX, a process of screening wild mosquitoes for parasite infection/infectivity is a useful tool to assess whether the parasite transmission is present in an endemic community, during and/or following control/elimination programmes. The MX programme results reported from recent studies19202122 indicate the potential of MX as an alternate or supplementary tool for the enhanced disease surveillance system.
PCR based approach i.e., MX to detect infection/infectivity in mosquitoes has a particular advantage of a real-time assessment of the active transmission which would be useful for evaluating the success of GPELF by monitoring the decline of transmission risk following MDA. The potential of this RT-PCR based assay to rapidly screen pools of 25 mosquitoes/tube and 40 tubes per day (about 1000 mosquitoes per day) will prove particularly valuable when the occurrence of infection in the mosquitoes falls below one per cent, whereas a technician can dissect and detect infection only about 40-50 mosquitoes per day. Thus, the ability to rapidly screen such large numbers of mosquitoes is advantageous in determining the presence or absence of infectivity (L3) in mosquitoes in a defined location or region of the country following the completion of an LF-elimination programme. It would also provide important information regarding possible endpoints for MDA and for detecting resurgent infection following cessation of MDA as an effective surveillance tool for post MDA monitoring. The limitation of possible degradation/contamination of RNA or cDNA due to two steps involved in this assay may be overcome by converting it as a quantitative RT-PCR in real-time format with higher sensitivity. In the present study the multi-centric evaluation of stage-specific RT-PCR assay developed for detecting infective (L3) stage larvae of the lymphatic filarial parasite, W. bancrofti, in vector mosquito Cx. quinquefasciatus showed that it is stage-specific and sensitive, and has potential for application in the assessment of transmission of LF in the national elimination programmes.
Financial support & sponsorship: This study received funding from department of Biotechnology (DBT), Government of India and Indian Council of Medical Research -Task Force, New Delhi.
Conflicts of Interest: None.
References
- Global Programme to Eliminate Lymphatic Filariasis:Progress report on mass drug administration in 2011. WHO Wkly Epidemiol Rec. 2012;87:345-56.
- [Google Scholar]
- The Global Programme to Eliminate Lymphatic Filariasis:Health impact after 8 years. PLoS Negl Trop Dis. 2008;2:e317.
- [Google Scholar]
- A review of the complexity of biology of lymphatic filarial parasites. J Parasit Dis. 2009;33:3-12.
- [Google Scholar]
- Global Programme to Eliminate Lymphatic Filariasis:Progress report on mass drug administration in 2007. WHO Wkly Epidemiol Rec. 2008;83:333-48.
- [Google Scholar]
- Transmission intensity index to monitor filariasis infectionpressure in vectors for the evaluation of filariasis elimination programmes. Trop Med Int Health. 2003;8:812-9.
- [Google Scholar]
- Diagnostic tools for filariasis elimination programs. Trends Parasitol. 2007;23:78-82.
- [Google Scholar]
- The ICT filariasis test:A rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol Today. 1997;13:401-4.
- [Google Scholar]
- Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis –A multi-center trial. Filaria J. 2004;3:9.
- [Google Scholar]
- Pan LF-ELISA using BmR1 and BmSXP recombinant antigens for detection of lymphatic filariasis. Filaria J. 2007;6:10.
- [Google Scholar]
- Rapid detection of Wuchereria bancrofti and Brugia malayi in mosquito vectors (Diptera:Culicidae) using a real-time fluorescence resonance energy transfer multiplex PCR and melting curve analysis. J Med Entomol. 2009;46:158-64.
- [Google Scholar]
- RT-PCR assay for the detection of infective (L3) larvae of lymphatic filarial parasite, Wuchereria bancrofti, in vector mosquito Culex quinquefasciatus. J Vector Borne Dis. 2008;45:207-16.
- [Google Scholar]
- Reverse transcriptase-PCR assay for detecting filarial infective larvae in mosquitoes. PLoS Negl Trop Dis. 2008;2:e251.
- [Google Scholar]
- Molecular xenomonitoring using mosquitoes to map lymphatic filariasis after mass drug administration in American Samoa. PLoS Negl Trop Dis. 2014;8:e3087.
- [Google Scholar]
- Application of a household-based molecular xenomonitoring strategy to evaluate the lymphatic filariasis elimination program in Tamil Nadu, India. PLoS Negl Trop Dis. 2017;11:e0005519.
- [Google Scholar]
- A process for diagnosis of infective (L3) larvae of Wuchereria bancrofti in vector mosquito, Culex quinquefasciatus. Indian Patent Number (257150) Granted On September 6 2013
- [Google Scholar]
- Lymphatic filariasis in India:Epidemiology and control measures. Postgrad Med J. 2010;56:232-3.
- [Google Scholar]
- Isolation of microfilariae from blood on iso-osmotic percoll gradient. Indian J Med Res. 1984;79:497-501.
- [Google Scholar]
- Development of Wuchereria bancrofti (Nematoda:Onchocercidae) in Culex quinquefasciatus (Diptera:Culicidae) after repeated feeding on microfilaraemic blood. Trop Biomed. 1995;12:73-6.
- [Google Scholar]
- Lymphatic Filariasis:The Disease and Its Control. Fifth Report of the WHO Expert Committee on Filariasis. WHO Tech Rep Ser, 702. Geneva: World Health Organization; 1979. p. :1-71.
- Determining the prevalence of Onchocerca volvulus infection in vector populations by PCR screening of blackflies. J Inf Dis. 1995;172:1414-7.
- [Google Scholar]
- Xenomonitoring of mosquitoes (Diptera:Culicidae) for the presence of filarioid helminthes in eastern Austria. Can J Infect Dis Med Microbiol 2018 doi:10.1155/2018/9754695
- [Google Scholar]
- Screening of mosquitoes for filarioid helminthes in urban areas in south western Poland-common pattern in European setaria tundra xenomonitoring studies. Parasitol Res. 2019;118:127-38.
- [Google Scholar]
- Ecotope-based entomological surveillance and molecular xenomonitoring of multidrug resistant malaria parasites in Anopheles vectors. Interdiscip Perspect Infect Dis 2014 doi:10.1155/2014/969531
- [Google Scholar]
- Illuminating the prevalence of Trypanosoma brucei s.l. in Glossina using LAMP as a tool for xenomonitoring. PLoS Negl Trop Dis. 2016;10:e0004441.
- [Google Scholar]
- Detection of Wuchereria bancrofti larvae in pooled mosquitoes by the polymerase chain reaction. Trans R Soc Trop Med Hyg. 1994;89:665-6.
- [Google Scholar]
- A polymerase chain reaction assay for the detection of Brugia malayi in blood. Am J Trop Med Hyg. 1994;51:314-21.
- [Google Scholar]
- A real time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. Am J Trop Med Hyg. 2006;74:826-32.
- [Google Scholar]
- Lymphatic filariasis elimination in American Samoa:Evaluation of molecular xenomonitoring as a surveillance tool in the endgame. PLoS Negl Trop Dis. 2016;10:e0004722.
- [Google Scholar]
- Programmatic use of molecular xenomonitoring at the level of evaluation units to assess persistence of lymphatic filariasis in Sri Lanka. PLoS Negl Trop Dis. 2016;10:e0004722.
- [Google Scholar]
- Molecular xenomonitoring for post-validation surveillance of lymphatic filariasis in Togo:No evidence for active transmission. PLoS Negl Trop Dis. 2018;11:52.
- [Google Scholar]
- Elimination of lymphatic filariasis India-2013-14. Available from: https://nvbdcp.gov.in/WriteReadData/l892s/TAS-National-Guidelines-2013-14.pdf






