Translate this page into:
Mosquitocidal Bacillus amyloliquefaciens: Dynamics of growth & production of novel pupicidal biosurfactant
Reprint requests: Dr A.M. Manonmani, Scientist F, Head, Unit of Microbiology & Immunology, Vector Control Research Centre (ICMR), Indira Nagar, Puducherry 605 006, India e-mail: ammanonmani@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
A strain of Bacillus amyloliquefaciens (VCRC B483) producing mosquito larvicidal and pupicidal biosurfactant was isolated from mangrove forest soil. The present study was aimed at studying the kinetics of growth and production of the mosquitocidal biosurfactant by this bacterium.
Methods:
Dynamics of growth, sporulation and production of mosquitocidal biosurfactant were studied by standard microbiological methods. The mosquitocidal biosurfactant was precipitated from the culture supernatant and bioassayed against immature stages of mosquito vectors to determine lethal dose and lethal time. The activity, biological and biochemical properties of the biosurfactant have also been studied.
Results:
The pupal stages of mosquitoes were found to be more vulnerable to the biosurfactant produced by this bacterium with Anopheles stephensi being the most vulnerable species. The median lethal time (LT50) was found to be 1.23 h when the pupal stages of the above species were exposed to lethal concentration LC90 (9 µg/ml) dosage of the biosurfactant. Production of biosurfactant was found to increase with incubation time and maximum biomass, maximum quantity of biosurfactant (7.9 mg/ml), maximum biosurfactant activity (6 kBS unit/mg) and maximum mosquitocidal activity (5 µg/ml) were attained by 72 h of growth. The lipopeptide nature of the biosurfactant was confirmed by β-haemolysis, lipase activity, biofilm forming capacity, thermostability and biochemical analysis.
Interpretation & conclusions:
The mosquitocidal biosurfactant produced by B. amyloliquefaciens (VCRC B483) may be a prospective alternative molecule for use in mosquito control programmes involving bacterial biopesticides.
Keywords
Bacillus amyloliquefaciens
biosurfactant
lipopeptide
mosquitocidal
pupicidal
surface tension
The mosquito-borne diseases including malaria, filariasis, dengue and viral encephalitis remain the important diseases of humans, with an estimated two billion people worldwide living in areas where these are endemic1. Potential strategies to control vector borne diseases include vaccines, new drugs, transgenic mosquitoes refractory to the causative disease agents and vector control. Among these, vector control methods are given more importance because research on the development of vaccines for many of the mosquito borne diseases has not yet resulted in a product. The concept of mosquito control using their natural enemies is conceived as a novel, non-polluting, cost-effective and environmentally sustainable method by which vector mosquitoes can be kept at bay. The first bacterial agent to challenge the larval mosquitoes was isolated from a mosquito breeding habitat in Negev Desert in Israel and named as Bacillus thuringiensis subsp. israelensis2. Since then a number of bacterial and fungal agents have been isolated for use against mosquitoes. A strain of B. subtilis subsp. subtilis isolated from Andaman & Nicobar islands has been found to kill not only the immature stages but also the adult mosquitoes34.
Recently a Bacillus amyloliquefaciens (VCRC B483) strain producing a mosquito larvicidal and pupicidal biosurfactant was isolated from the same ecosystem5. The earlier biosurfactant reported to be mosquitocidal was surfactin produced by B. subtilis subsp. subtilis. Because of their biodegradable nature, biosurfactants gain a lot of importance. In the present study, the kinetics of growth and production of biosurfactant by the bacterium VCRC B483 was investigated.
Material & Methods
Source of bacterium: The study was conducted in the Microbiology and Immunology laboratory, Vector Control Research Centre, Puducherry, India. The B. amyloliquefaciens (VCRC B483) strain isolated from soil samples collected from the mangrove forests of Andaman and Nicobar islands5 was used for the production of the mosquitocidal biosurfactant. Stock cultures were stored in 10 per cent glycerol (Sigma, USA) at -80°C and maintained on nutrient agar slants.
Source of mosquito samples: For laboratory bioassay, third instar larvae and freshly moulted pupae (2-3 h old) of Culex quinquefasciatus, Anopheles stephensi, Aedes aegypti [Rearing condition: temperature 30 ± 2°C; relative humidity: 70-80 per cent; larval food: dog biscuits and yeast powder (6:4 ratio)] were obtained from a cyclic colony of mosquitoes maintained at the Rearing and Colonization Unit of the Vector Control Research Centre.
Growth conditions of the bacterium, B. amyloliquefaciens (VCRC B483): The bacterium was grown aerobically on nutrient yeast salt mineral medium (NYSM)6. Tubes containing 10 ml NYSM broth were inoculated with a loopful of bacterial cells from the slant culture. The tubes were incubated overnight on a rotary shaker (New Brunswick Scientific Co. Inc., USA) at 28 ± 2°C. After incubation, culture was inoculated to fresh 50 ml of NYSM broth and incubated again for a further period of 7 h to synchronize the growth. From this young culture, 5 per cent inoculum was added to 2 l Erlenmeyer flasks containing 600 ml of the medium and incubated with shaking for 72 h as mentioned above.
Separation of mosquitocidal biosurfactant: Bacterial cells were removed from the medium by centrifugation at 9000 × g for 25 min in a Sorvall Evolution RC superspeed centrifuge (Kendro Lab. Products, Asheville, NC, USA) using SLA-1500 rotor. The culture supernatant (CS) obtained was precipitated with 6 N HCl for isolation of the crude mosquitocidal biosurfactant (CMB) and the precipitate was collected by centrifugation at 9,000 × g for 25 min and lyophilized.
Growth and sporulation of B. amyloliquefaciens (VCRC B483): To study the growth pattern, 1 ml of culture samples was removed aseptically at 6 h interval for up to 72 h. The determination of generation time of VCRC B483 was carried out as per the methodology described by Stephenson7. Growth was determined by viable cell count assay by plating. For spore count determination, culture samples collected as above were heat treated at 80°C for 15 min. prior to plating and presence of spores in the treated samples was confirmed by Schaeffer-Fulton stain (Hi Media, India).
Assessment of mosquitocidal activity of CMB: CMB obtained from the culture supernatant was bioassayed against larvae and pupae of An. stephensi, Cx. quinquefasciatus and Ae. aegypti following WHO standard protocols1. For experimental treatment, 100 mg of CMB was dissolved in 10 ml of distilled water and used as the stock solution. To 150 ml capacity, disposable wax-coated paper cups, 100 ml of chlorine-free tap water was added and either 25 larvae or pupae were transferred to each cup. Each experiment was performed using four replicates and an equal number of controls were set up simultaneously. The treated and control cups were held at 27±2°C, 80-90 per cent relative humidity and a photoperiod of 12 h of light followed by 12 h of dark. All the bioassay cups were covered with mosquito net cloth to prevent the escape of emerging adults, if any. The mortality of the larvae/pupae was scored after 24 h of exposure by counting the number of live ones present in the bioassay cup. The moribund and dead pupae in four replicates were combined and expressed as a percentage of pupal mortality of each concentration. Dead pupae included those found at the bottom of the bioassay cups as straightened pupae by losing their typical comma shape as well as the dead late stage pupae which were moulted to adult but were unable to come out of the pupal exuvia. The experiments were repeated twice. In cases where the control mortality was between 5 and 10 per cent, the observed percentage mortality was corrected using Abbott's formula8. Data from all replicates were pooled for analysis.
Determination of median lethal time: Median lethal time for An. stephensi pupae, the most vulnerable mosquito species was determined using LC90 (9 μg/ml) dose determined from the above experiment in quadriplicate with appropriate controls. The number of live pupae present in the bioassay cups was counted after every 30 min until the death of all the pupae and median lethal time (LT50) and LT90 was determined9.
Dynamics of production of mosquitocidal biosurfactant: Whole culture was drawn at hourly intervals from 4 to 24 h of incubation. Bioassays were conducted using a fixed dose of 1 ml of whole culture in wax coated paper cups containing 25 freshly molted pupae of An. stephensi in 100 ml chlorine-free tap water. Appropriate controls without the addition of the bacterial culture, but containing 1 ml of un-inoculated NYSM broth were maintained.
Determination of biomass and mosquitocidal biosurfactant: Whole culture (100 ml) was drawn at 12, 24, 48, 72, 96, 120, 144 of incubation and used for the determination of biomass and mosquitocidal biosurfactant production. Biomass was determined by gravimetry10 and biosurfactant production was determined by turbidometric estimation method11. The calibration curves for biosurfactant were prepared as follows: aqueous solutions of the lyophilized CMB (pH 7.0) were prepared containing various concentrations viz, 1, 2, 4, 6, 8 and 10 mg/ml of the crude biosurfactant. These solutions were acidified using 6 N HCl and then allowed to stand for 30 min at 4°C for proper precipitation of biosurfactants and vortexed to get a homogeneous suspension of the insoluble bio-surfactants. Optical density of these suspensions was measured at 600 nm using a UV-visible spectrophotometer. Water was taken as the reference in all these turbidity measurements. The optical density values obtained were plotted against crude biosurfactant concentration to get a linear calibration curve11. For biosurfactant estimation in culture samples collected at different hours, 1 ml of culture supernatant was taken in a sterile tube, acidified for precipitation of the mosquitocidal biosurfactant followed by optical density mesurement at 600 nm in UV-visible spectrophotometer. The quantity of mosquitocidal biosurfactant was calculated using the calibration curve.
Determination of biosurfactant activity: Biosurfactant activity of the mosquito larvicidal and pupicidal metabolite was estimated by oil displacement activity12. Seven ml of distilled water was taken in a Petri dish of 100 mm diameter and 8 µl of mineral oil (Sigma, USA) was placed on its surface. Then, 5 µl of the culture supernatant was gently placed on to the centre of the thin membrane of the oil formed on the surface of the water. A clear halo was visible under light and the area of the circle was measured. The same test was performed with un-inoculated plain culture medium for comparison.
Biological and biochemical properties of the mosquitocidal biosurfactant: Biological properties such as haemolytic activity, lipase activity13 were performed on blood agar plates and Tributyrin agar plates, respectively using samples such as whole culture, cell free supernatant and CMB. Thermostability was tested by subjecting the CMB at 121°C for 15 min. Biofilm forming capacity of the culture was analyzed on solid surface using glass tubes and plastic plates14. CMB was used at 10 mg/ml for the estimation of carbohydrates, proteins and lipids151617.
Statistical analysis: LC50/LT50 and LC90/LT90 and their 95 per cent confidence intervals (CI) were calculated from a log dosage-probit mortality regression line using SPSS version 19.0 for windows (IBM Corp, USA). Simple linear regression analysis was used to quantify the relationships of optical density versus concentration of biosurfactant, biomass versus biosurfactant production and biosurfactant activity versus log incubation time.
Results
Growth and sporulation of B. amyloliquefaciens (VCRC B483): Growth pattern of the mosquitocidal bacterium B. amyloliquefaciens (VCRC B483) was studied and its generation time was calculated as 32 min. As this is a spore forming bacterium, the growth curve was drawn based on the vegetative cells as well as the spores (Fig. 1a & 1b). In the given conditions, the lag phase was from 0-3 h. The exponential phase, which started from 4th h, extended up to 48 h, after which the bacterium entered into stationary phase of growth. The production of pupicidal metabolite was initiated after the lag phase, i.e., by 4th h and the maximum mosquitocidal activity was obtained by 24 h but sporulation was initiated by 30th h and complete sporulation was seen by the end of 48 h (22 × 108).
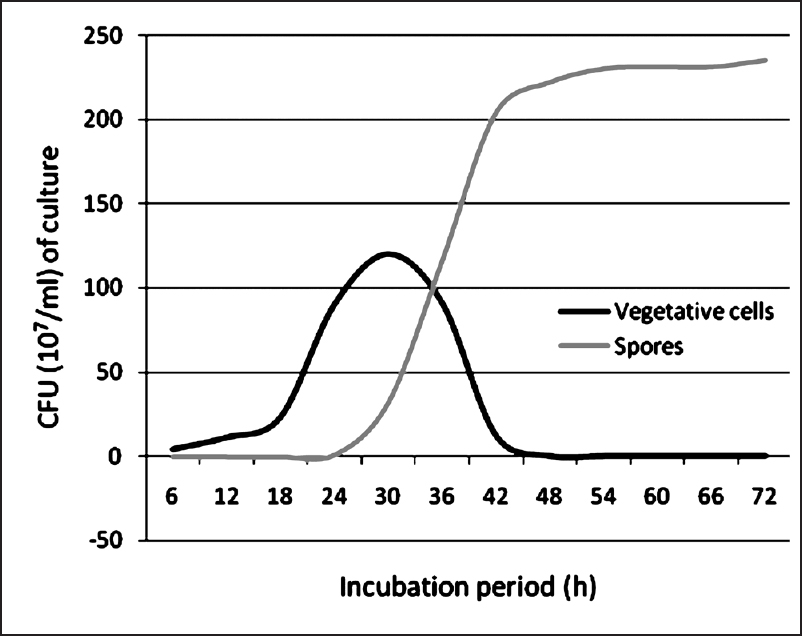
- Growth curve of the mosquitocidal bacterium B. amyloliquefaciens.
Assessment of mosquitocidal activity of CMB: Bioassay guided determination of biosurfactant production was assessed using the 24, 48 and 72 h sample. The LC50 dose reported earlier by us3 was used as a diagnostic dose. Since the CMB obtained after 24 h and 48 h of incubation was not very effective, 72 h CMB was bioassayed against the larval and pupal stages of An. stephensi, Cx. quinquefasciatus and Ae. aegypti (Table). Among the mosquito immatures tested, pupal stages were found to be the most vulnerable and among the three mosquito genera tested, An. stephensi were found to be the most vulnerable with LC50 of 5 μg/ ml. To elicit the same response Cx. quinquefasciatus and Ae. aegypti pupae required 8.1 and & 13.2 μg/ml which were 1.62 and 2.64 times higher than that required for An. stephensi. Similar vulnerablity profile was observed with the larval stages but the dose required was 2- 4 fold higher than that required to kill the pupal stages of mosquitoes.
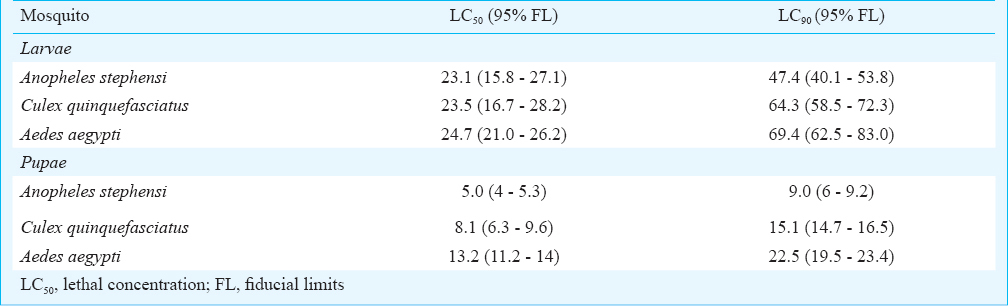
When pupal stages of An. stephensi were exposed to LC90 dosage of the CMB to determine the median lethal time, the LT50 and LT90 were found to be 1.23 and 2.18 h, respectively.
Dynamics of production of mosquitocidal biosurfactant: The results showed that the production of the mosquitocidal biosurfactant was initiated at 4th h of incubation and 50 per cent of mortality was observed at 6th h of incubation. However, 100 per cent mortality was observed only with 24 h old culture. The quantity of biomass was found to increase with the incubation period. At 72 h it was found to be 26.9 g/l and thereafter the quantity of biomass was maintained till 144 h (25.8 g/l) of incubation. The calibration curve for biosurfactant concentration was prepared using CMB (Fig. 2). The relation between biosurfactant concentration and OD600nm was linear. The dry weight values and the estimated biosurfactant yields (by turbidometric measurements) showed a significant (P<0.001) linear correlation with R2 0.99. The relationship showed that concentration of CMB explained 99 per cent of the variation in OD. Using the calibration curve, concentration of biosurfactant produced at different hours of incubation was determined (Fig. 3). Production of biosurfactant was found to increase with incubation time and maximum quantity (7.9 mg/ml) was obtained at 72 h of growth. Thereafter the quantity of biosurfactant was maintained till 144 h. Production of biosurfactant with respect to biomass is plotted in Fig. 4. Biomass and production of biosurfactant were found to be significantly (P<0.001) linearly related [intercept (95% CI): -32.2 (-56.1, -8.3); slope (95% CI): 7.59 (4.3, 10.8)]. The coefficient of determination (R2 = 0.88) indicated that the increase in biosurfactant quantity was in proportion to the quantity of biomass and about 90 per cent of variability in the production of biosurfactant could be explained by biomass.
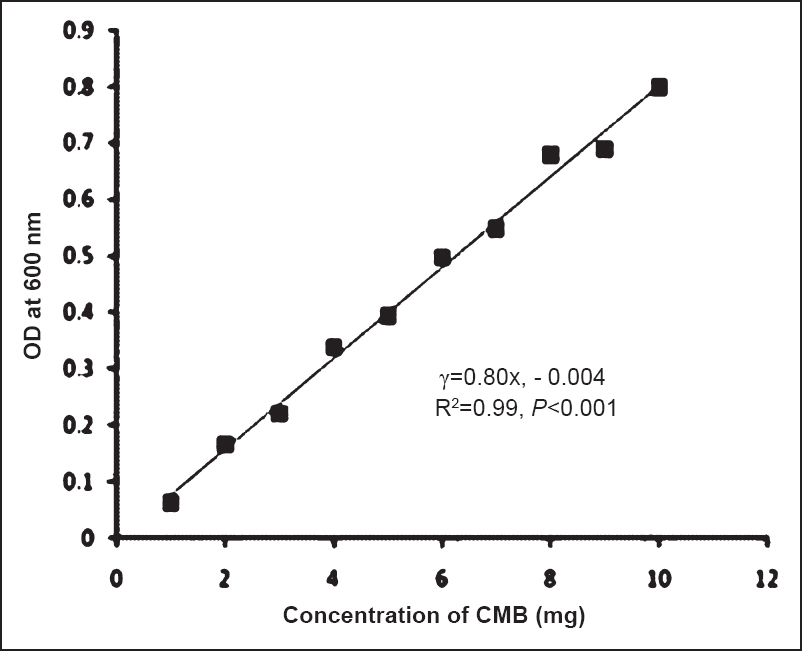
- Calibration curve for crude mosquitocidal biosurfactant (CMB) produced by B.amyloliquefaciens.
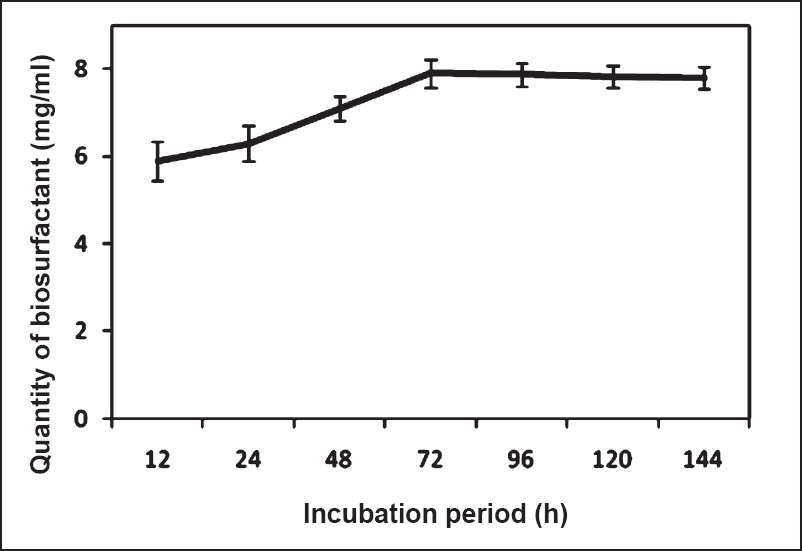
- Dynamics of production of mosquitocidal biosurfactant (mean ± SD) by B. amyloliquefaciens.
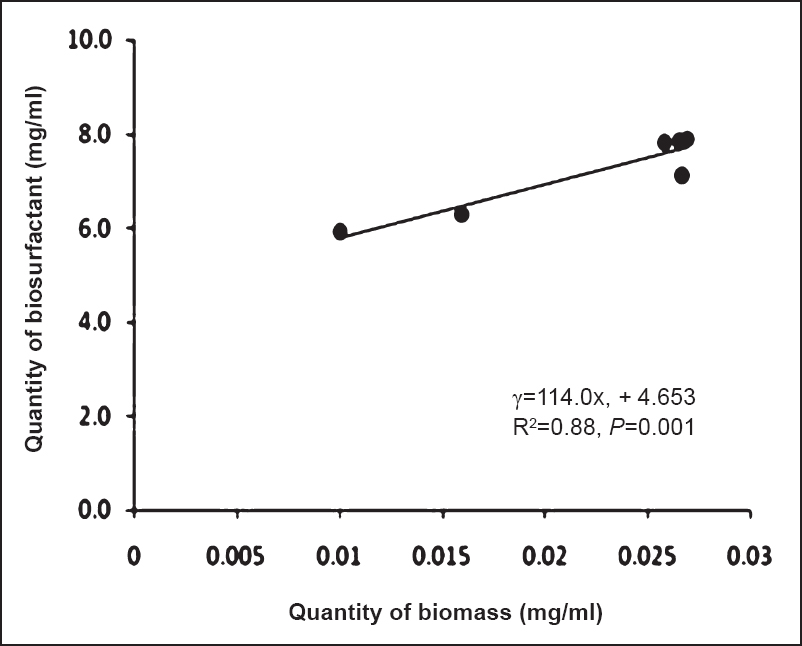
- Relationship between biomass and biosurfactant production in whole culture by B. amyloliquefaciens.
Determination of biosurfactant activity: Biosurfactant activity was studied at different hours by oil displacement test (Fig. 5). The production of biosurfactant was found to increase with the growth of the organism and maximum activity was observed with 72 h culture, i.e. 6000 BS (BioSurfactant) or 6 kBS unit/mg and this value was almost maintained till 144 h of incubation. The activity of biosurfactant coincided with the time of maximum production. There was a significant (P<0.002) correlation between logarithm of time and oil displacement activity and it was logarithmically linearly related with log incubation time [R2 = 0.87; Intercept (95% CI): -3.15 (-6.5, 0.24); Slope (95% CI): 1.89 (1.07, 2.7)].
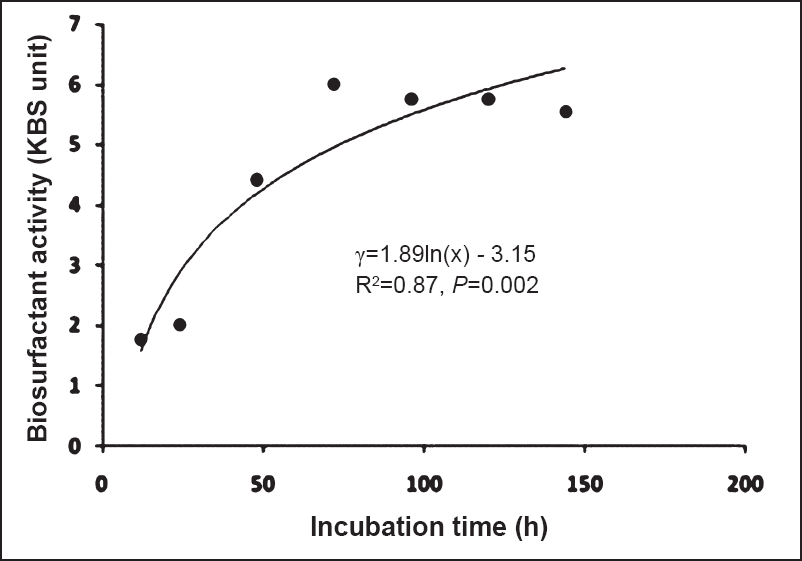
- Determination of biosurfactant activity of CMB produced by B. amyloliquefaciens by oil displacement method.
Biological and biochemical properties of the mosquitocidal biosurfactant: β-haemolysis, i.e., complete haemolysis and also lipase activity were seen with whole culture, CS and CMB. Biofilm forming capacity was analyzed on solid surface using glass tubes and polystyrene immune plates (Nunc, Denmark). Maximum binding capacity was observed on both surfaces i.e., glass and polystyrene. The heat treated CMB (121°C, 15 min.) showed 54 per cent pupal mortality at LC50 dose indicating its thermostable nature. Results of biochemical analysis showed that the CMB contained carbohydrate (0.2 mg), protein (3.6 mg) and lipid (6.2 mg).
Discussion
Generation time for the mosquitocidal bacterium B. amyloliquefaciens (VCRC B483) was calculated as 32 min as against 30 min reported by Wilson and Young18 for a wild strain of the same bacterium. The difference in the generation time may be because the strain we have used was obtained from a different environment (mangrove forest). Experiments on growth and sporulation in B483 showed that the mosquitocidal biosurfactant was produced during the vegetative phase of growth unlike in the case of B. thuringiensis and B. sphaericus where mosquito larvicidal toxin production accompanied sporulation19. It is well known that biosurfactants are produced by non ribosomal peptide synthetases (NRPS) which are not regulated by sporulation20.
The biosurfactant produced by B483 was found to possess larvicidal and pupicidal activity. The vulnerablity profile of the mosquito species to CMB of B. amyloliquefaciens was in the following order: An. stephensi > Cx. quinquefasciatus > Ae. aegypti. The higher vulnerability of pupal stages to the CMB might be because of the high levels of physiological activity exhibited by this stage when it is in the process of transformation from immature to adult21. The median lethal time (LT50) for the mosquitocidal biosurfactant against pupae of An. stephensi was found to be 1.23 h which was higher than the median lethal time of surfactin produced by B. subtilis subsp. subtilis, another mosquito pupicidal bacterium22.
This study showed that relationship between biomass and production of biosurfactant was significant and biomass quantity determined the quantity of biosurfactant. In VCRC B483, 72 h of growth was necessary for maximum production of biomass and biosurfactant. In the case of B. subtilis subsp. subtilis maximum biomass production was achieved at 48 h and maximum production of mosquitocidal metabolite was observed at 24 h22. Though, both the bacterial species produced mosquitocidal biosurfactants, the dynamics of growth/production of the mosquitocidal metabolite was not similar.
It is well known that surfactin is a powerful biosurfactant reported to show 5.78 - 6.83 kBS unit/mg and our results confirm that the pupicidal metabolite is a biosurfactant23. The biosurfactant production remained same until 144 h indicating the stability of the compound and the self resistance of the strain to the biosurfactant. β-haemolysis exhibited by B483, is an indication that the mosquitocidal surfactant may be surfactin24. Also, the strain possessed the biofilm forming capacity on glass and polystyrene. In general, the biofilm producer primarily initiates the formation of the biofilm at the gap between air and liquid interfaces, and finally matures into biofilm. Aerobic colonies are reported to form aggregation onto the interphase air and liquid medium meeting junction25 as this system provides nutrients as well as maintains aerobic state. This leads to microcolony formation which matures into biofilm structure with the production of high amounts of extracellular polysaccharides. Considering the action of biosurfactants in air-liquid interfaces, the lipopeptide biosurfactant produced by the strain B. amyloliquefaciens might have a functional role on its biofilm formation and stability. The mosquitocidal biosurfactant was thermostable, as it was effective even after exposure to 121°C for 15 min. Biochemical analysis showed that the biosurfactant contained lipid as major component (6.2 mg/10 mg). The properties like haemolysis, biofilm formation, thermostability and biochemical composition of the surfactant indicate that the mosquitocidal biosurfactant may be a lipopeptide.
The biosurfactant produced by the bacterium VCRC B483 was found to lower the surface tension of water from 72 to 27.7 mN/m3. Surfactin, one of the most powerful biosurfactants is reported to reduce the surface tension of water from 72 to 27 mN/m3 and reduction to levels between 41-31 mN/m has resulted in total pupal mortality of mosquitoes262728. Piper and Maxwell29 determined the relationship between surface tension reducing properties of non-ionic surfactants and mortality of larval and pupal stages of Cx. quinquefasciatus. As pupal stages of mosquitoes are solely dependent on their trumpets for respiration, reduction in surface tension of water caused by surfactants prevented the trumpets to retain their position at the water's surface. As a result, the pupae loose contact with the air and ultimately die because of respiratory arrest. The larval stages of all the three mosquito species required greater concentration of CMB to produce 50 and 90 per cent kill. This might be due to the greater ability of larvae to overcome suffocation via cuticular respiration29. In addition to reducing the surface tension, other properties of the mosquitocidal factor may also be responsible for the activity.
Biosurfactants are surface active agents being used in environmental applications such as bioremediation and dispersion of oil spills, enhanced oil recovery and transfer of crude oil30. However, the results of the present study on B. amyloliquefaciens have revealed that biosurfactants also have potential for controlling mosquitoes vectoring diseases like malaria, filariasis, dengue, etc.
Acknowledgment
Authors acknowledge Dr P. Jambulingam, Director, Vector Control Research Centre and Dr S.L. Hoti, Scientist ‘F’, for their support provided throughout the study and critically reviewing the manuscript and thank Dr S. Subramaniam, Scientist ‘D’ for statistical analysis.
References
- Report of the WHO informal consultation on the evaluation and testing of insecticides. Geneva. Geneva: World Health Organization (document CTD/WHOPES/IC/96.1); 1996.
- [Google Scholar]
- A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata, Culex univitattus, Aedes aegypti and Culex pipiens. Mosq News. 1977;37:355-8.
- [Google Scholar]
- Surfactin: a novel mosquitocidal biosurfactant produced by Bacillus subtilis ssp. subtilis (VCRC B471) and influence of abiotic factors on its pupicidal efficacy. Lett Appl Microbiol. 2010;51:406-12.
- [Google Scholar]
- Mosquito adulticidal activity of a biosurfactant produced by Bacillus subtilis subsp. subtilis. Pest Manag Sci. 2012;68:1447-50.
- [Google Scholar]
- Bacillus amyloliquefaciens: a mosquitocidal bacterium from mangrove forests of Andaman & Nicobar islands, India. Acta Trop. 2011;120:155-9.
- [Google Scholar]
- Comparison between bacteriophage typing and serotyping for the differentiation of Bacillus sphaericus strains. Ann Microbiol (Paris). 1980;131:297-308.
- [Google Scholar]
- Cell growth. In: Calculations for molecular biology and biotechnology - a guide to mathematics in the laboratory. USA: Academic Press; 1994. p. :42-75.
- [Google Scholar]
- A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265-7.
- [Google Scholar]
- Determination of minimum lethal time of commonly used mosquito larvicides. J Commun Dis. 1984;16:162-4.
- [Google Scholar]
- Substrate dependent production of extracellular biosurfactant by a marine bacterium. Bioresour Technol. 2009;100:1015-9.
- [Google Scholar]
- Rapid quantification of a microbial surfactant by a simple turbidometric method. J Microbiol Methods. 2009;76:38-42.
- [Google Scholar]
- A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J Bacteriol. 1993;175:6459-66.
- [Google Scholar]
- Comparison of methods and screening of biosurfactant producing marine Actinobacteria isolated from Nicobar marine sediment. IIOAB J. 2010;2:34-8.
- [Google Scholar]
- Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295-304.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-54.
- [Google Scholar]
- Intergenetic transformation of the Bacillus subtilis genospecies. J Bacteriol. 1972;111:705-16.
- [Google Scholar]
- Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science. 2004;303:1805-10.
- [Google Scholar]
- Mosquito pupicidal toxin production by Bacillus subtilis subsp. subtilis. Biol Control. 2008;44:242-7.
- [Google Scholar]
- Production and characterization of biosurfactants from Bacillus licheniformis F2.2. Biosci Biotechnol Biochem. 2003;67:1239-44.
- [Google Scholar]
- Characterization of biochemical properties and biological activities of biosurfactants produced by Pseudomonas aeruginosa mucoid and non-mucoid strains isolated from hydrocarbon-contaminated soil samples. Appl Microbiol Biotechnol. 2005;69:192-9.
- [Google Scholar]
- Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour Technol. 2010;101:2389-96.
- [Google Scholar]
- Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl Environ Microbiol. 1981;42:408-12.
- [Google Scholar]
- Ovicidal activity of aliphatic amines and petroleum oil against two species of mosquitoes. J Econ Entomol. 1968;61:510-5.
- [Google Scholar]
- Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol. 2000;53:495-508.
- [Google Scholar]






