Translate this page into:
Molecular & serological study of dengue virus-infected patients attending a tertiary hospital of Dhaka city, Bangladesh (2013 to 2016)
*For correspondence: saifmunshi@yahoo.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Dengue fever is a mosquito-borne disease caused by serotypes of dengue virus (DENV-1, -2, -3 and -4). Infection by one serotype confers long-term immunity to that serotype but subsequent infection by another serotype causes severe disease including dengue haemorrhagic fever and dengue shock syndrome1. Several molecular markers such as dengue viral load, serotype and serological markers such as dengue NS1 (non-structural protein 1) antigen and immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies against DENV (anti-dengue IgM and IgG) are used for diagnosis of dengue. Dengue serotype(s) and viral load are the two critical factors that modulate the outcome of dengue infections12. Dengue NS1 antigenemia is detectable within 24 h and may persist for nine days following the onset of illness. Simultaneous testing for dengue NS1 along with anti-dengue IgM/IgG provides the diagnostic potential for both early and late dengue disease3. These serological and molecular markers are used not only to confirm the diagnosis but also to categorize the disease severity or increase in risk of progression to more severe forms of dengue infection. These are also essential to gather epidemiological data regarding the evolution of dengue epidemics. In several studies, these markers have been studied either singly or combined in different geographical locations45, but there are limited studies from Southeast Asia. Therefore, with a view to determine the circulating dengue serotype(s) and to study the inter-relationship of all the above markers, this observational study was initiated in a tertiary care hospital of Dhaka city, the capital of Bangladesh from 2013 to 2016. During the study period, patients with fever for 1-7 days attending the outpatient care facilities of Bangabandhu Sheikh Mujib Medical University (BSMMU) from different parts of Dhaka city were referred to the department of Virology, BSMMU, for diagnosis of dengue virus infection. The study was approved by the Institutional Review Board of BSMMU (IRB No. BSMMU 2016/3467).
After obtaining written informed consent, 5 ml of venous blood was collected from the patients and serum was separated to perform immunochromatographic tests for dengue NS1 antigen and anti-dengue IgM/IgG using qDetect™ dengue NS1 antigen and qDetect™ dengue IgM/IgG test kit (OMCH, Dhaka, Bangladesh), respectively. The serum samples positive for either dengue NS1 or anti-dengue IgM±IgG were aliquoted (~1 ml), preserved at −70°C and included in this study. Viral RNA was extracted from preserved serum using Virus DNA/RNA Extraction Kit III VI100 (Geneaid Biotech, New Taipei City, Taiwan) for detection of DENV serotypes and quantitation by Real-time PCR (StepOnePlus, Applied Biosystem, Foster City, CA, USA) using the commercially available kit (genesig® Advance Kit; Primer Design™ Ltd., Camberley, UK).
The statistical analysis i.e. Student's t test or analysis of variance was performed using SPSS v 16.0 (SPSS Inc., Chicago, IL, USA) to assess the differences between means where appropriate. Continuous and categorical variables were displayed as means±standard error (SE) and percentages, respectively.
During the study period, 571 febrile patients were screened for acute dengue infection, and of them, 160 (age: 18-57 yr; males: 93, females: 67) were found positive for either dengue NS1 or dengue IgM antibody (±IgG) (Table). Among the serum samples collected in acute phase (n=118), 25 (21.2%), 22 (18.6%), 33 (28%), and 38 (32.2%) were dengue RNA positive in 2013, 2014, 2015 and 2016, respectively. When serotyping was performed, 57.6 per cent (68/118) samples were found positive for DEN-1 and 42.4 per cent (50/118) for DEN-2. In 2013 and 2014, DEN-2 was detected in 84 (21/25) and 77.3 per cent (17/22) serum samples and DEN-1 was detected in 16 (4/25) and 22.7 per cent (5/22) samples, respectively. In 2015 and 2016, 87.9 (29/33) and 79 per cent (30/38) samples were positive for DEN-1 and 12.1 (4/33) and 21 per cent (8/38) were positive for DEN-2, respectively (Fig. 1A). No case of DEN-3 and DEN-4 was detected throughout the study period. The mean difference in viral load was significantly (P<0.01) higher for DEN-2 (6.23±1.9) than the viral load (mean±SE log10 RNA copies/ml) of DEN-1 (4.2±0.22) (data not shown). Dengue RNA detection rate was higher i.e. 92 (45/49) in patients presented on the third day of the fever and was decreased to 87 (27/31), 83.3 (20/24), 61.9 (13/21) and 37.1 per cent (13/35) on 4th, 5th, 6th to 7th days of fever, respectively. Viral load was highest on the third day (6.65±0.20) and reduced to 5.2±1.3, 5.1±1.0, 4.3±0.9, 3.6±0.8, 2.9±0.9 on 4th, 5th, 6th to 7th days of fever, respectively (data not shown). This decreasing trend was also observed when each serotype was considered individually in each day of fever (Fig. 1B). All the dengue RNA-positive serum samples were categorized into five groups according to the presence of dengue NS1, IgM, IgG and serotypes (Table & Figure C, D). Dengue RNA was detected in all dengue NS1-positive serum samples (100%) with high mean viral load (6.6±0.7l), while the detection rate reduced to 69.2 in the samples positive for both dengue NS1 and anti-dengue IgM, and viral load significantly (P<0.01) reduced to 5.0±0.7 (Table & Figure C). Comparing with dengue NS1- and IgM-positive serum, dengue RNA was detected in 64.7 per cent samples which were only positive for anti-dengue IgM, and viral load was found significantly reduced to 3.5±0.5 (P<0.001). The detection rate was increased to 88.9 per cent in the samples which were positive for all three serological markers of dengue i.e. dengue NS1, anti-dengue IgM and IgG, and viral load was found significantly higher (6.8±1.1) than the patients who were positive for both dengue NS1 and IgM (P<0.01). Among the samples positive only for anti-dengue IgM and IgG, 52.6 per cent was positive for dengue RNA, and viral load was detected significantly (P<0.001) reduced to 5.4±1.2 than those positive for only anti-dengue IgM. Viral load when considered separately for individual serotype also showed the similar trend (Figure D).
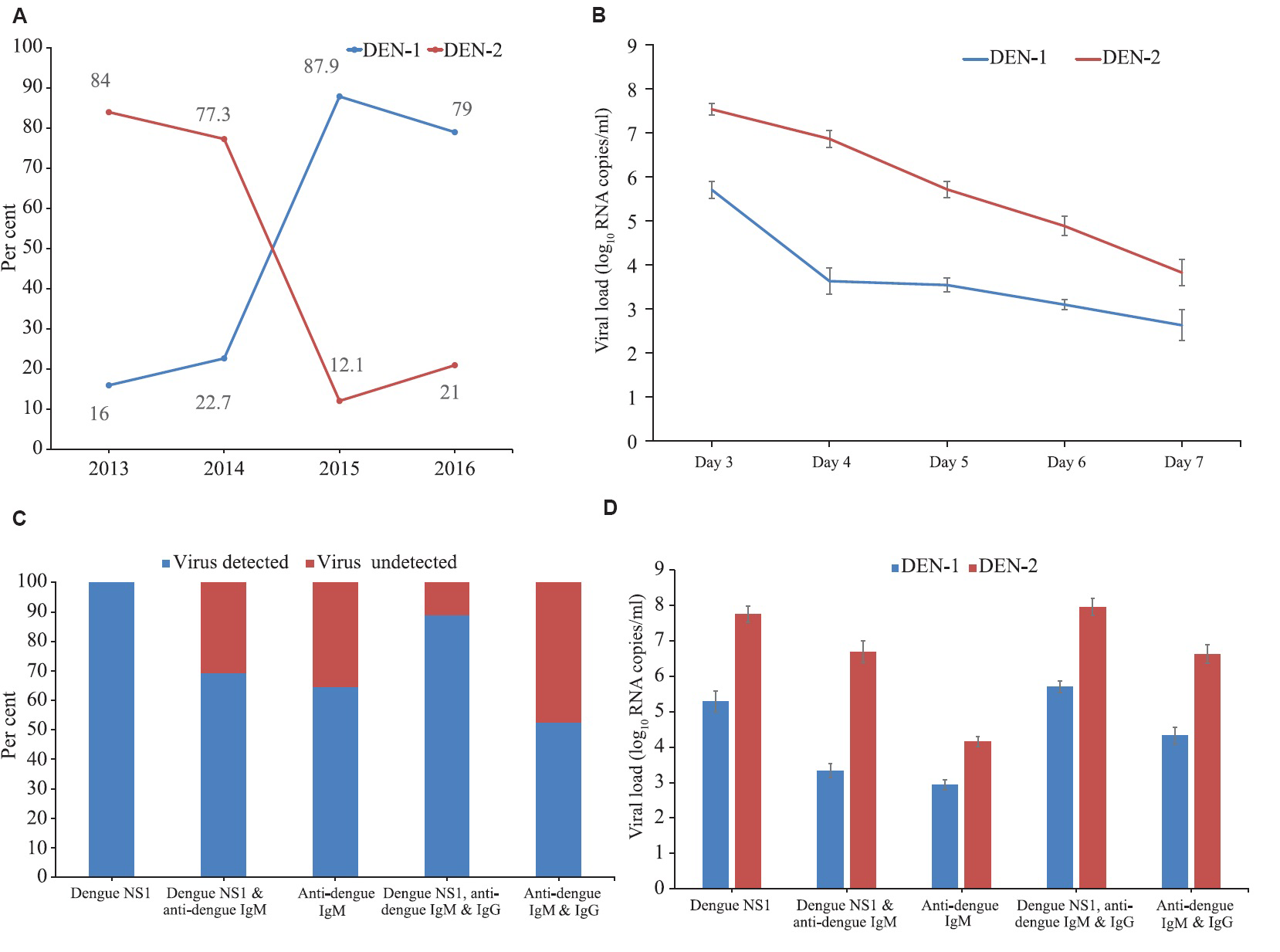
- Serotypes, viral loads and serological markers of dengue infection during 2013-2016 in Dhaka city, Bangladesh. (A) Year-wise distribution of dengue serotypes, (B) relation of days of fever with dengue viral loads and virus detection rate (mean±SE), (C) detection rate of virus in different serostatus, and (D) viral load in different samples with dengue virus-1 and 2 infections according to serostatus.
| Category (total number of serum samples tested) | Total number of serum samples positive for dengue RNA (%) | Serotype | Viral load (mean±SE log10 RNA copies/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DEN-1 | DEN-2 | |||||||||
| Number of DEN RNA positive serum samples in each year | ||||||||||
| 2013 | 2014 | 2015 | 2016 | 2013 | 2014 | 2015 | 2016 | |||
| Dengue NS1 (24) | 24 (100.0) | 2 | 2 | 4 | 5 | 4 | 4 | 1 | 2 | 6.6±0.7l |
| Dengue NS1 and anti-dengue IgM (39) | 27 (69.2) | 1 | 1 | 7 | 9 | 4 | 3 | 0 | 2 | 5.0±0.7 |
| Anti-dengue IgM (51) | 33 (64.7) | 1 | 1 | 9 | 9 | 7 | 3 | 1 | 2 | 3.5±0.5 |
| Dengue NS1, anti-dengue IgM and IgG (27) | 24 (88.9) | 0 | 1 | 5 | 4 | 5 | 6 | 2 | 1 | 6.8±1.1 |
| Anti-dengue IgM and IgG (19) | 10 (52.6) | 0 | 0 | 4 | 3 | 1 | 1 | 0 | 1 | 5.4±1.2 |
| Total (160) | 118 (73.7) | 4 | 5 | 29 | 30 | 21 | 17 | 4 | 8 | 5.22±0.20 |
IgM, immunoglobulin M; IgG, immunoglobulin G; NSI, non-structural protein 1
In the present study, DEN-1 and DEN-2 were detected as two of the prevailing serotypes during the years 2013-2016 in Dhaka city where DEN-1 gained predominance at the end of 2016. The presence of DEN-3 and DEN-1 was reported first from Bangladesh in 19646 and in 19807, respectively, while in 2000, all four serotypes were found to be co-circulating, with the predominance of DEN-389. A recent study in three metropolitan cities of Bangladesh also showed that DEN1 and DEN2 were in the circulation10. Serotype DEN-3 and DEN-4 were not found during 2013-2016, indicating the displacement of the previously prevailing serotypes. Dengue virus infection with DEN-1 has been reported from China, Malaysia and India in 2005, 2010 and 2014, respectively11121314. Therefore, circulation of DEN-1 in Dhaka city may have a link with any of these geographical areas.
It was observed that DEN-2-infected patients had a higher viral load than DEN-1 which was contrary to the studies performed in Vietnam1516. Therefore, the variations in viral load of each serotype may differ from geographical area to area in which dengue genotype may have a role. It has been shown earlier that different genotypes of the same dengue serotype may have different replication rates17. This study also showed that viral load was higher in the presence of dengue NS1 irrespective of the presence or absence of anti-dengue IgM and IgG antibodies indicating that dengue NS1 antigenemia was associated with viral load and independent of immune status4. Samples positive for anti-dengue IgM had the lowest dengue RNA detection rate and the lowest level of virus, suggesting DENV-specific IgM as a strong negative modulator of viraemia. Inverse correlation between IgM seropositivity and dengue viral load has also been observed previously1819. This may happen due to the clearance of virus by IgM antibodies5. The viral load and detection rate in the serum samples positive for all three serological markers were higher than all other groups of samples. The RNA detection rate and viral loads of the samples positive for anti-dengue IgM and IgG and those positive for dengue NS1 and anti-dengue IgM group were almost similar, but the rate and viral load were lower than samples positive for dengue NS1, anti-dengue IgM and IgG. This indicates that anti-dengue IgG which is supposed to neutralize the DENV increases the viral load. This phenomenon of antibody-mediated enhanced viral replication and higher viraemia has been reported in the animal model also20.
In this study, age- and sex-matched blood samples could not be collected and genotyping of the circulating DENV could not be done. These were the limitations of the study. The findings of this study provided information about circulating dengue serotypes in Dhaka city, Bangladesh, and relation of DENV serotypes, viral load and other available dengue serological markers in the same group of patients.
Financial support & sponsorship: The study was partially supported by grants from Center for Disease Control Unit, Directorate General of Health Services, Bangladesh.
Conflicts of Interest: None.
References
- Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445-59.
- [Google Scholar]
- Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2-9.
- [Google Scholar]
- Dengue symptoms significance in anti-dengue drug development: Road less travelled. Bioinformation. 2017;13:131-5.
- [Google Scholar]
- Relationship between nonstructural protein 1 detection and plasma virus load in dengue patients. Am J Trop Med Hyg. 2010;83:696-9.
- [Google Scholar]
- Viral load in patients infected with dengue is modulated by the presence of anti-dengue IgM antibodies. J Clin Virol. 2013;58:258-61.
- [Google Scholar]
- Recovery of dengue viruses from patients during epidemics in Puerto Rico and East Pakistan. Am J Trop Med Hyg. 1966;15:573-9.
- [Google Scholar]
- Serological evidence of dengue fever in the Bangladesh republic. Acta Virol. 1980;24:153.
- [Google Scholar]
- Isolation and serotyping of dengue viruses by mosquito inoculation technique from clinically suspected cases of dengue fever. Bangladesh Med Res Counc Bull. 2002;28:104-11.
- [Google Scholar]
- Predominance of the DEN-3 genotype during the recent dengue outbreak in Bangladesh. Southeast Asian J Trop Med Public Health. 2002;33:42-8.
- [Google Scholar]
- Circulating dengue virus serotypes in Bangladesh from 2013 to 2016. Virusdisease. 2018;29:303-7.
- [Google Scholar]
- The prevalence and endemic nature of dengue infections in Guangdong, South China: An epidemiological, serological, and etiological study from 2005-2011. PLoS One. 2014;9:e85596.
- [Google Scholar]
- Characterizing a large outbreak of dengue fever in Guangdong province, China. Infect Dis Poverty. 2016;5:44.
- [Google Scholar]
- The Telegraph. 2016. Dengue Stings, Excuses Bite. Available from: https://www.telegraphindia.com/1160809/jsp/calcutta/story_101382.jsp#.WItRFkV96b8
- [Google Scholar]
- Dengue infections and circulating serotypes in Negeri Sembilan, Malaysia. Malaysian J Public Health Med. 2012;12:21-30.
- [Google Scholar]
- Kinetics of viremia and NS1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl Trop Dis. 2011;5:e1309.
- [Google Scholar]
- Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: Differential effects according to serotype and immune status. J Infect Dis. 2011;203:1292-300.
- [Google Scholar]
- Detection of immune-complex-dissociated nonstructural-1 antigen in patients with acute dengue virus infections. J Clin Microbiol. 2003;41:4154-9.
- [Google Scholar]
- Selection for virulent dengue viruses occurs in humans and mosquitoes. J Virol. 2005;79:853-9.
- [Google Scholar]
- Low sensitivity of NS1 protein tests evidenced during a dengue type 2 virus outbreak in Santos, Brazil, in 2010. Clin Vaccine Immunol. 2012;19:1972-6.
- [Google Scholar]
- First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS Pathog. 2014;10:e1004031.
- [Google Scholar]





