Translate this page into:
Molecular characterization of locus of enterocyte effacement pathogenicity island in shigatoxic Escherichia coli isolated from human & cattle in West Bengal, India
Reprint requests: Dr. Suresh Chandra Das, ICAR-Indian Veterinary Research Institute, Eastern Regional Station, 37, Belgachia Road, Kolkata 700 037, West Bengal, India e-mail: dasivriers@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Shigatoxic Escherichia coli (STEC) recovered from dairy animals of Kolkata, India, harboured the putative virulence genes; however, the animals did not exhibit clinical symptoms. Similarly, human isolates in this locality also showed variations in degree of symptoms. Hence, this study was designed to know the presence of recognized gene(s) in the locus of enterocyte effacement (LEE) pathogenicity island in these STEC isolates and functional status of the cardinal gene (eae) related to pathogenicity.
Methods:
Genes were characterized using polymerase chain reaction (PCR) assays, and functional status of cardinal gene (eae) was evaluated by fluorescent actin staining (FAS) assay. Variation in eae gene was determined by intimin PCR.
Results:
Cattle STEC isolates carried 22 genes in LEE pathogenicity island in different frequencies ranging from 5.63 to 47.88 per cent of the isolates. In human isolates, the genes namely ler, escRSTU, orf2, escC, escV, orf3 and tir that are associated with secretory function, were found to be absent and rest of the genes were present in lower frequency. Further, the cardinal gene (eae) responsible for initiation of pathogenesis was in a very low frequency in human (n=2; 10.5%) and cattle (n=11; 15.5%) isolates. None of these eae+ STEC isolates from human and cattle revealed positivity in FAS assay.
Interpretation & conclusions:
Majority of human STEC isolates lacked the cardinal virulence gene (eae), and genes for secretory function that are essential for facilitating pathogenesis. This may partially be attributed to low occurrence of STEC in human clinical diarrhoea in this area. Although a few isolates (11 of 71) from cattle had eae gene, they did not express phenotypically. This could be one of the reasons for not appearing of clinical symptoms in the hosts.
Keywords
Fluorescent actin staining assay
locus of enterocyte effacement
pathogenicity island
shigatoxic Escherichia coli
Shigatoxic Escherichia coli (STEC) has been recognized globally as an emerging human diarrhoeal pathogen that can cause a spectrum of illness ranging from watery diarrhoea to haemorrhagic colitis, or even fatal complications such as haemolytic uremic syndrome. Ruminants, particularly cattle, have been found to be the principal reservoir of this organism1.
Production of one or more shigatoxins (Stx1, Stx2 or variants) has been identified to be the cardinal virulence factor for their pathogenesis, and in addition, the large plasmid (pO157)-borne virulence factors are also presumably associated12. On binding of STEC to the brush border of host mucosa, pathogenesis facilitates cytoskeletal reorganization and formation of a characteristic histopathological feature, termed the ‘attaching and effacement’ (A/E) lesion, by subverting the intestinal epithelial cell function3. The ability to cause A/E lesions is encoded on a large pathogenicity island termed as locus of enterocyte effacement (LEE) where the eae gene (encodes intimin) mediates intimate attachment to the host cell through the intimin receptor (tir) which is chaperoned by CesT and translocated into the host cell plasma membrane by the type III system34. Except enteropathogenic E. coli (EPEC) and STEC isolates, LEE is not present in the normal flora of E. coli or in any other pathogroups of E. coli. In majority of the clinical cases and outbreak incidences, the associated STEC strains carried the LEE and the eae5; however, a few reports revealed the cases devoid of eae6. The presence of eae in cattle STEC isolates has been reported to vary in different geographical set-up such as France, Spain and Japan78910. Detailed sequence analysis of this gene in STEC isolates from human and different animals revealed that N (5’) terminal region is highly conserved whereas C (3’) terminal is variable and is responsible for binding to the host enterocytes and intimin receptor (tir)1112. It has been suggested that different intimins may be responsible for different host tissue cell tropism13.
In our previous studies1415, it was observed that 87.3 per cent of the STEC isolates (stx+) obtained from Kolkata from human and animals did not generate the amplicon for eae, the indicator gene for LEE and A/E phenotype in STEC pathogenesis. A pilot polymerase chain reaction (PCR) assay was conducted with these STEC isolates to understand the sequences of genes mainly associated for type III secretory function (left side, left junction, right junction of LEE and espB, espD and tir) flanking to eae in the LEE, and the results showed that these genes were not present in most of the eae+ STEC isolates14. On the contrary, these genes were common in the eae− STEC isolates. Further, the findings with eae+ STEC isolates showed that these gene sequences were not constantly present suggesting the presence of incomplete LEE. With this background, the present investigation was designed to know the structural gene sequences of the LEE of the STEC isolates obtained from Kolkata, India, and functional status of the eae gene as well as to determine their variants. The findings were compared with the reference strain to relate the importance of LEE genes in pathogenicity of the STEC strains.
Material & Methods
In the present study, a total of 19 and 71 STEC isolates from human diarrhoea stool and cattle faecal samples, respectively, were included. Repository of STEC isolates recovered from human and cattle faecal samples during our earlier study14 was used in this study. Subsequently, the isolates obtained during 2010-2013 were also included. Human isolates were recovered from stool specimens of diarrhoea patients admitted to the Infectious Diseases Hospital and B. C. Roy Memorial Hospital for children, Kolkata, India, from June 2001 to July 2003 and February 2011 to May 2013. The study was approved by the institutional ethics committee of the National Institute of Cholera and Enteric Diseases (NICED) (ICMR), Kolkata.
Briefly, stool specimens from the diarrhoea patients were collected using a sterile catheter in sterile McCartney bottles; however, rectal swabs were taken from the patients from whom stool could not be obtained and finally introduced in Cary-Blair transport medium. Faecal sample from cattle was collected aseptically by digital rectal retrieval. All the collected samples were transported to the laboratory within four hours of collection and processed immediately following the method adopted in earlier studies1415 for isolation and characterization of STEC.
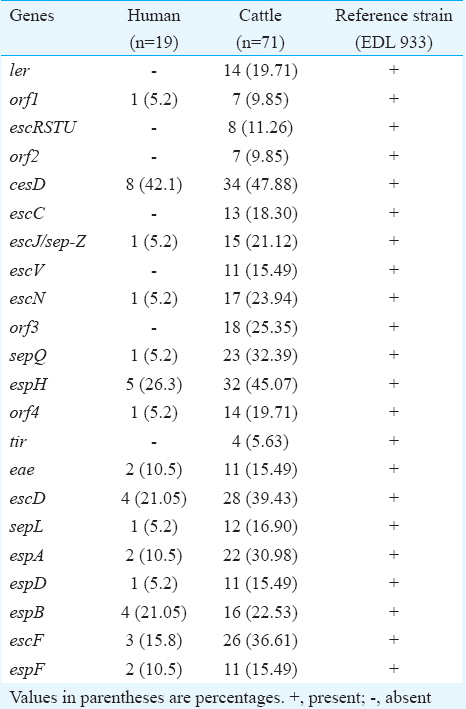
Screening of genes in LEE pathogenicity island: The reference strain (EDL933) was used in the study. The primers for target LEE genes were designed using the software and published EDL 933 sequence (Accession. No. AF071034). The primer sequence for stx1 and stx2 gene (EVT1/EVT2 and EVS1/EVC2) was used as mentioned in the earlier study16. The LEE genes (ler, orf1, escRSTU, orf2, cesD, escC, escJ/sep-Z, escV, escN, orf3, sepQ, espH, orf-4, sepQ, espH, orf4, tir, eae, escD, sepL, espA, espD, espB, escF and espF) (Table I) were screened by standardizing the PCR protocol using the published16 and designed primer sets (Table II) with reference strain (EDL 933) DNA. The obtained PCR amplicons were visualized in agarose gel electrophoresis using the 1 Kb and 100 bp DNA marker.
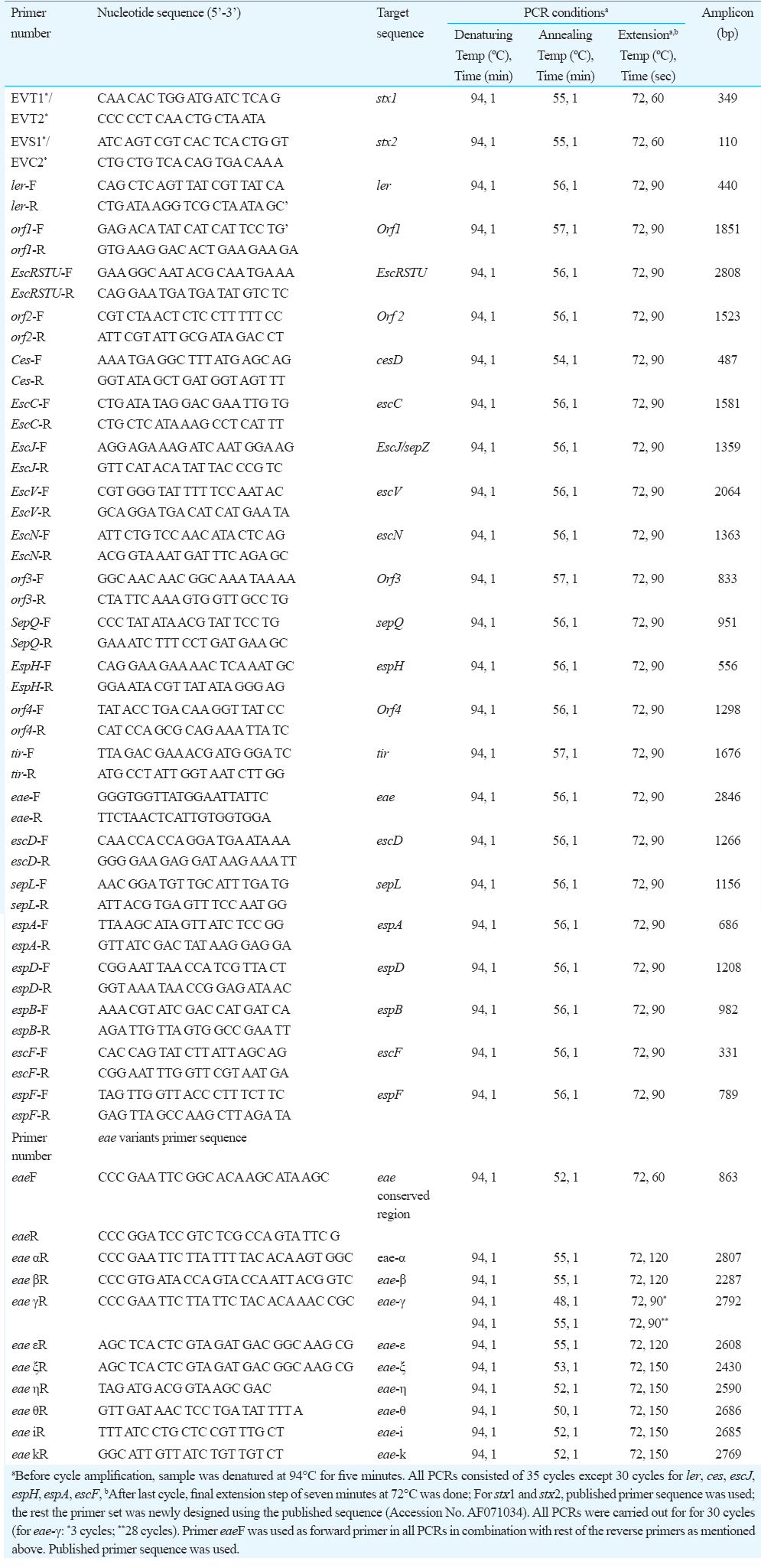
Detection of intimin (eae) variants by PCR assay: The PCR assay was carried out using the published primer sequences (Table II) and methods17.
Determination of functional status of intimin by fluorescent actin staining (FAS): Functional status of eae (intimin gene) in STEC isolates i.e. capability for A/E to the susceptible host epithelia was evaluated by assaying the fluorescent actin staining (FAS). The assay was performed adopting the standard published method18 using the phalloidin dye FITC conjugate and Hep-2 cell line. The EDL933 and E2348/69 were used as positive control and K12 E. coli (DH5α) was used as negative control.
Results
A total of 19 human and 71 cattle STEC isolates were screened for the presence of 22 genes in their LEE pathogenicity island. Among human isolates, cesD was found positive in eight (42.1%) isolates followed by espH [5 (26.3%)], escD and espB [4 (21.05%) each], escF [3 (15.8%)], eae, espA and espF [2 (10.5%) each] and orf1, escJ/sep-Z, escN, sepQ, orf4, sepL and espD [1 (10.5%) each]; however, the isolates did not produce amplicon for other seven genes viz. ler, escRSTU, orf2, escC, escV, orf3 and tir (Table I).
In cattle isolates, cesD gene was detected most frequently (n=34, 47.88%) followed by espH [32 (45.07%)], escD [28 (39.43)], escF [26 (36.61%)], sepQ [23 (32.39%)], espA [22 (30.98%)], orf3 [18 (25.35%)], escN [17 (23.94%)], espB [16 (22.53%)], escJ/sepZ [15 (21.12%)], ler and orf4 [14 (19.71) each], escC [13 (18.30%)], sepL [12 (16.90%)], escV, espD & espF [11 (15.49%) each], eae [11 (15.49%)], escRSTU [8 (11.26%)], orf1 and orf2 [7 (9.85%) each] and tir [4 (5.63%)] (Table I).
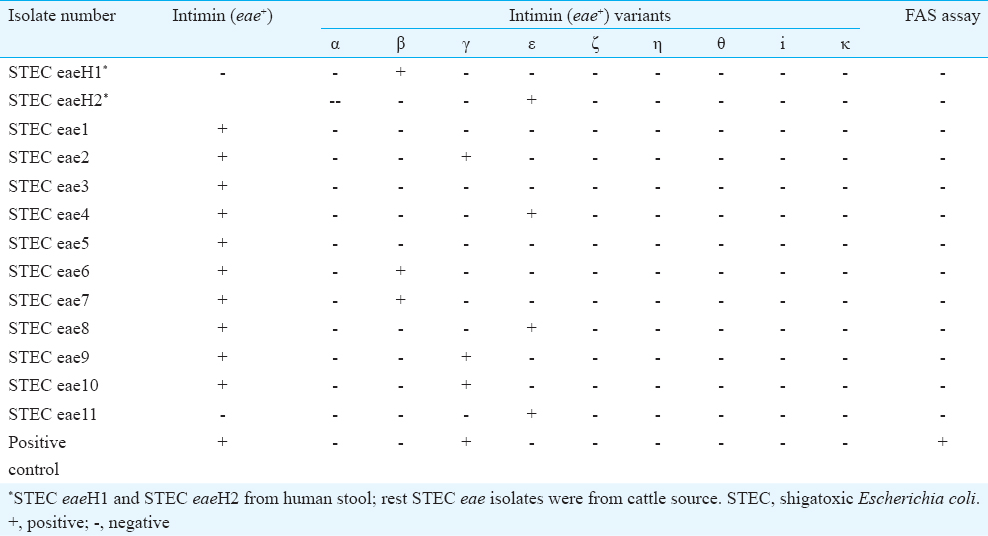
A total of 13 STEC isolates [human (n=2) and animal (n=11)] were found to carry the eae gene and were screened by nine different PCR assays for the detection of variants of eae (α, β, γ, ε, ξ, η, θ, I and k) (Table III). It was observed that three isolates each were detected to be positive for β and γ variants and four isolates for ε variants. Rest three isolates [13−10 (3+3+4)] remained untypable in the PCR assay. All the eae+ STEC isolates (n=13) were assessed by FAS for functional expression of their intimin (eae); however, none of the isolates was found to be positive for intimin (eae) as evident in negative cell adherence.
Discussion
In the present investigation, the data for the presence of genes in LEE of human STEC isolates revealed that seven out of 22 genes in LEE were not detected; moreover, the frequency of the genes present among the STEC human isolates was low. The eae gene which is principally responsible for attaching the host epithelium for initiation of pathogenesis was detected in a very limited number of isolates (2 of 19 isolates). Further, FAS assay findings suggested that their functional status was not expressed. The present study findings suggested that presence of eae gene, principally responsible for A/E function, was in a very low frequency and there was an absence of seven genes viz. ler, escRSTU, orf2, escC, escV, orf3 and tir linked to secretory function in STEC isolates. The present study highlights the frequency of genes in LEE pathogenicity island of STEC isolates; however, screening for the presence and role of other concerned gene(s) and pathogenicity island as carried out in other published studies1819 would be contributory to ascertain the detailed reasons for low incidence STEC in clinical diarrhoea in this area.
STEC isolates from cattle possessed the LEE genes in a comparatively high frequency than human isolates including the presence of eae gene; however, the hosts (cattle) were apparently healthy and did not show any clinical symptoms. The FAS assay was conducted for these eae+ STEC isolates (n=11) from cattle to understand the expression and functional ability (A/E to the host epithelium) of their eae gene; however, none of them showed positivity for the cell adherence in this assay. It appeared that the cattle harboured this organism having the important virulence gene-like eae for establishing pathogenesis but did not express.
The eae+ STEC isolates from human and animal were tallied with history of samples and found that the concerned two human cases had history of loose stool; however, the cattle were apparently healthy. This observation generated two ideas. First, these eae+ isolates might have variation in their eae sequence and as a result could not bind to the host enterocyte. Alternatively, their eae gene is not expressed for functional ability. In earlier study20, heterogeneity in C’(3) terminal of eae sequence in STEC isolates from different sources was reported. This idea prompted us to explore the possibility of eae variants in these STEC isolates, for eae (intimin) variants (α, β, γ, ε, ξ, η, θ, I and k), and to evaluate the status of expression of this gene by FAS. It was observed that three isolates each were detected to be positive for β and γ variants and four isolates for ε variants. Rest three isolates [13−10 (3+3+4)] remained untypable with the used primer sets. It suggests that these three isolates may possess eae variants that differ from the, so far, recognized eae variants. To understand the functional expression of their intimin (eae), all the eae+ STEC isolates were assessed by FAS; however, all were found negative as evaluated by cell adherence in FAS.
The activation of LEE pathogenicity island is regulated at various levels, including transcriptional and post-transcriptional regulators located both inside and outside of the pathogenicity island212223. Several molecules are involved in a complex network of regulation, including mechanisms such as quorum sensing and temporal control of LEE genes transcription and translation23, by the chemical signalling system depending on the factors available in the environment of host epithelium and bacteria (e.g. stressed epithelia and fucose sugar)24. LEE island carries the regulator gene (ler) for its own expression and type III secretory system that infuses the effector molecules to the host cell leading to A/E lesions enterocytes24. Promotion of LEE expression is associated with signal inducer (QseC and QseE) produced by bacteria. On passing through the steps of activation and phosphorylation, these components end at the FusKR complex (fucose sugar repressor) that determines the LEE expression25. Further study on this aspect may supplement information on the pathogenesis with STEC of this area.
In conclusion, the study findings suggested that all the genes in LEE island in STEC isolates of human and animal origin in this study area were not constantly present; particularly, human isolates were carrying 15 of these genes in low frequency and seven genes were lacking. Besides, frequency of cardinal gene (eae) was very low in human and in moderate frequency among cattle isolates; however, the phenotypic expression of the gene was not evident. These findings may relate to the low incidence of this organism in clinical diarrhoea in this area as well as the apparently healthy carrier in cattle. Further study on the screening of other gene(s) and pathogenicity island including the FusKR complex that determines the aspect of LEE expression and its regulation may be supportive to address the reasons for such low incidence of STEC in clinical diarrhoea and its silent carrier status in animals in this area.
Acknowledgment
Authors acknowledge the financial support received from the Indian Council of Medical Research (ICMR), New Delhi, under extramural research grant (IRIS ID No. 2004-04450), and thank Dr Asis Khan for technical guidance and assistance.
Conflicts of Interest: None.
References
- Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450-79.
- [Google Scholar]
- Non-O157: H7 pathogenic Shiga toxin-producing Escherichia coli: Phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115-23.
- [Google Scholar]
- Enteropathogenic and enterohaemorrhagic Escherichia coli: More subversive elements. Mol Microbiol. 1998;30:911-21.
- [Google Scholar]
- Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol Microbiol. 1999;33:1176-89.
- [Google Scholar]
- Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142-201. Erratum in Clin Microbiol Rev 1998; 11: 403
- [Google Scholar]
- Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113: H21. Infect Immun. 1994;62:3494-505.
- [Google Scholar]
- Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet Microbiol. 1997;54:309-19.
- [Google Scholar]
- Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J Clin Microbiol. 2000;38:1023-31.
- [Google Scholar]
- Prevalence and characteristics of Shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl Environ Microbiol. 2001;67:484-9.
- [Google Scholar]
- Characterization of Shiga toxin producing E. coli and O157 serotype E. coli isolated in France from healthy domestic cattle. Int J Food Microbiol. 2001;63:217-23.
- [Google Scholar]
- Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157: H7. Mol Microbiol. 1992;6:411-7.
- [Google Scholar]
- Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol Microbiol. 1999;32:151-8.
- [Google Scholar]
- Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J Infect Dis. 2000;181:1496-500.
- [Google Scholar]
- Prevalence and genetic profiling of virulence determinants of non-O157 Shiga toxin-producing Escherichia coli isolated from cattle, beef, and humans, Calcutta, India. Emerg Infect Dis. 2002;8:54-62.
- [Google Scholar]
- Dairy farm investigation on Shiga toxin-producing Escherichia coli (STEC) in Kolkata, India with emphasis on molecular characterization. Epidemiol Infect. 2005;133:617-26.
- [Google Scholar]
- Shiga-toxin producing Escherichia coli from healthy cattle in a semi-urban community in Calcutta, India. Indian J Med Res. 1999;110:83-5.
- [Google Scholar]
- Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J Clin Microbiol. 2002;40:4486-92.
- [Google Scholar]
- Low-density macroarray targeting non-locus of enterocyte effacement effectors (nle genes) and major virulence factors of Shiga toxin-producing Escherichia coli (STEC): A new approach for molecular risk assessment of STEC isolates. Appl Environ Microbiol. 2010;76:203-11.
- [Google Scholar]
- Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: Characterization of a new intimin variant. Infect Immun. 2000;68:64-71.
- [Google Scholar]
- Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic Escherichia coli O157: H7. MBio. 2012;3:e00050-12.
- [Google Scholar]
- The interacting Cra and KdpE regulators are involved in the expression of multiple virulence factors in enterohemorrhagic Escherichia coli. J Bacteriol. 2013;195:2499-508.
- [Google Scholar]
- Locus of enterocyte effacement: A pathogenicity island involved in the virulence of enteropathogenic and enterohemorragic Escherichia coli subjected to a complex network of gene regulation. Biomed Res Int. 2015;2015:534738.
- [Google Scholar]
- The LysR-type transcriptional regulator QseD alters type three secretion in enterohemorrhagic Escherichia coli and motility in K-12Escherichia coli. J Bacteriol. 2010;192:3699-712.
- [Google Scholar]
- Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492:113-7.
- [Google Scholar]






