Translate this page into:
Molecular appraisal of intestinal parasitic infection in transplant recipients
Reprint requests: Dr Bijay Ranjan Mirdha, Department of Microbiology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: mirdhabr@hotmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Diarrhoea is the main clinical manifestation caused by intestinal parasitic infections in patients, with special reference to transplant recipients who require careful consideration to reduce morbidity and mortality. Further, molecular characterization of some important parasites is necessary to delineate the different modes of transmission to consider appropriate management strategies. We undertook this study to investigate the intestinal parasitic infections in transplant recipients with or without diarrhoea, and the genotypes of the isolated parasites were also determined.
Methods:
Stool samples from 38 transplant recipients comprising 29 post-renal, two liver and seven bone marrow transplant (BMT) recipients presenting with diarrhoea and 50 transplant recipients (42 post-renal transplant, eight BMT) without diarrhoea were examined for the presence of intestinal parasites by light microscopy using wet mount, modified Ziehl–Neelsen staining for intestinal coccidia and modified trichrome staining for microsporidia. Genotypes of Cryptosporidium species were determined by multilocus genotyping using small subunit ribosomal (SSUrRNA), Cryptosporidium oocyst wall protein (COWP) and dihydrofolate reductase (DHFR) as the target genes. Assemblage study for Giardia lamblia was performed using triose phosphate isomerase (TPI) as the target gene. Samples were also screened for bacterial, fungal and viral pathogens.
Results:
The parasites that were detected included Cryptosporidium species (21%, 8/38), Cystoisospora (Isospora) belli (8%, 3), Cyclospora cayetanensis (5%, 2), G. lamblia (11%, 4), Hymenolepis nana (11%, 4), Strongyloides stercoralis (3%, 1) and Blastocystis hominis (3%, 1). Multilocus genotyping of Cryptosporidium species at SSUrRNA, COWP and DHFR loci could detect four isolates of C. hominis; two of C. parvum, one of mixed genotype and one could not be genotyped. All the C. hominis isolates were detected in adult post-renal transplant (PRT) recipients, whereas the C. parvum isolates included a child with BMT and an adult with PRT. Clostridium difficle, cytomegalovirus and Candida albicans were found in 2, 3 and 2 patients, respectively.
Interpretation & conclusions:
In the present study, C. hominis was observed as an important parasite causing intestinal infections in transplant recipients. Multilocus genotyping of Cryptosporidium species could detect four isolates of C. hominis; two of C. parvum, one of mixed genotype and one could not be genotyped. Genotyping of G. lamblia revealed that assemblage B was most common.
Keywords
Cryptosporidium
genotypes
loci
multilocus
subtypes
transplant
Infections result due to a shift in the equilibrium between host defence mechanisms and invading microorganism, and the associated infective complications are always a major challenge in transplant recipients. Approximately, two-third of patients experience infection-related complications leading to graft failure following transplantation1. The true frequency of intestinal parasitic infections in transplant recipients is often not apparent as many of these patients are asymptomatic2. Only five per cent of human pathogenic parasites have been reported to cause a significant illness in transplant recipients2. The use of cyclosporine as prophylactic immunosuppressive drug has strong parasiticidal effects, and has remarkably reduced the infections in transplant patients3. However, the new immunosuppressive drugs used to prevent graft rejections have resulted in an increase in parasitic infections in these patients. The present study was performed to investigate the frequency of intestinal parasitic infections in transplant recipients with and without diarrhoea. Further, various species and assemblages are known in Cryptosporidium and Giardia lamblia, respectively, that signify different routes of transmission for causing human infections45. Hence, as the secondary aim of the present study, molecular characterization of Cryptosporidium and Giardia isolates was attempted.
Material & Methods
This cross-sectional study was conducted at the department of Microbiology, All India Institute of Medical Sciences, New Delhi, a tertiary care referral and teaching hospital located in north India, from July 2011 to June 2013. The study protocol was approved by the Institutional Ethics Committee. Informed written consent was also obtained from all the patients included in the study. Three consecutive stool specimens were collected for three consecutive days from 38 transplant patients with acute, persistent and chronic diarrhoea and 50 transplant patients without diarrhoea who fulfilled the inclusion and exclusion criteria. Information pertaining to age, gender and history of patient's illness was obtained from each patient using a validated structured questionnaire on receiving the sample. A total of 106 patients were sent from the inpatients (n=68) and outpatient (n=38) departments of the hospital. Diarrhoea was defined as passage of at least three unformed stools in a day and further classified into acute, persistent and chronic diarrhoea, if the diarrhoeal episode was <14 days, between 14 and 29 days and for >30 days duration, respectively6. Patients with food allergy and those who had either used probiotics in the previous three weeks or antiparasitic drugs due to some ailments in the past three months were excluded from the study. Patients who did not comply with the procedures involved in the study were summarily excluded from the study. The stool samples were subjected to microscopic examination using both direct and formal-ether concentration method for the detection of ova, larvae, trophozoites and cysts of intestinal parasites7. In addition, modified Ziehl–Neelsen staining technique7 and modified trichrome staining8 were performed for the detection of intestinal coccidia (Cryptosporidium species, Cyclospora cayetanensis and Cystoisospora belli) and microsporidia, with special reference to Enterocytozoon bieneusi, respectively. The clinical specimens were also screened for bacterial, fungal and viral pathogens using appropriate microbiological diagnostic methods.
For the molecular characterization of intestinal coccidia (Cryptosporidium spp., C. cayetanensis and C. belli), E. bieneusi and G. lamblia, genomic DNA was extracted from all the clinical specimens using QiaAmp mini stool kit (Qiagen, USA) as per manufacturer's protocol. Polymerase chain reaction (PCR) assay was carried out using 18S rRNA as the target gene for the detection of Cryptosporidium species9, C. cayetanensis9, C. belli10 and E. bieneusi11, using previously described primers and PCR conditions. Genus-specific primers were used for Cryptosporidium and species-specific primers for C. cayetanensis, C. belli, E. bieneusi and Giardia species. Genotyping of Cryptosporidium species at multiple loci was performed by PCR-restriction fragment length polymorphism (RFLP) assay. Small subunit ribosomal RNA (SSUrRNA), Cryptosporidium oocyst wall protein (COWP), dihydrofolate reductase (DHFR) using previously described primers and PCR conditions121314 were used for genotyping, and RFLP was performed using restriction enzymes SspI and AseI (New England Biolabs, USA) for SSUrRNA gene (Fig. 1) and RsaI (New England Biolabs, USA) for COWP gene1213 (Fig. 2). Cryptosporidium glycoprotein (Cpgp40/15) locus was amplified using previously described primers and PCR conditions for subgenotyping Cryptosporidium species and RFLP was done using RsaI (New England Biolabs) restriction enzyme15 (Fig. 3). It is known that C. cayetanensis and C. belli are the only known species responsible for human infection, and genotyping was performed by PCR-RFLP assay using 18S rRNA for C. cayetanensis16 and direct sequencing for C. belli to study genetic heterogeneity17, if any. Assemblage study for G. lamblia was performed using PCR-RFLP where triose phosphate isomerase (TPI) was used as a target gene and RsaI as a restriction enzyme4. All the patients with cryptosporidiosis received intravenous fluid replacement and were treated with nitazoxanide for 10-21 days. In conjunction with this regimen, dosage of immunosuppressive drugs was also reduced. The patients with Cyclospora and Cystoisospora (Isospora) infection were treated with albendazole and co-trimoxazole, respectively, for a minimum of 10 days. The patients infected with Giardia and other helminths also received standard therapy.
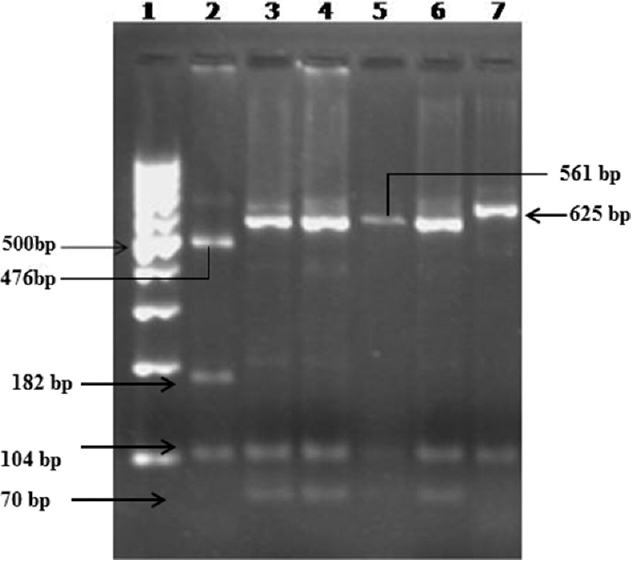
- Restriction fragment length polymorphism using AseI RE for SSUrRNA gene. Lane 1-100 bp DNA ladder, lane 2 - Cryptosporidium felis control (AseI-104, 182, 476 bp), lanes 3, 4 - C. parvum (monkey genotype - AseI-70, 104, 559 bp), lanes 5, 6 - C. hominis, lane 7 - C. parvum (bovine genotype-AseI-104, 625 bp).
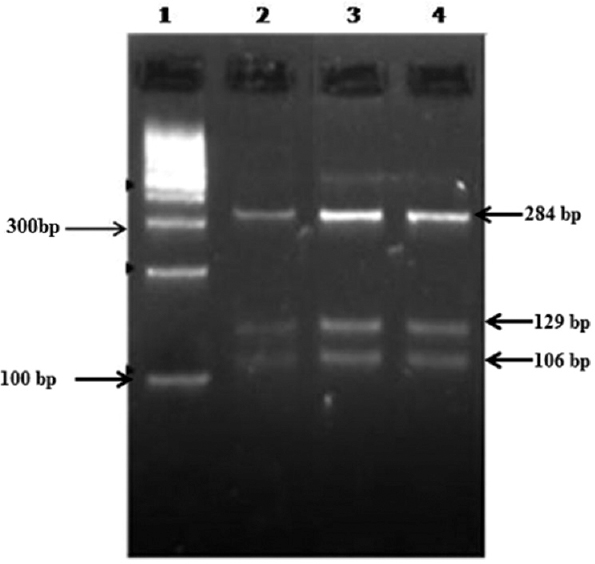
- Restriction fragment length polymorphism assay using RsaI RE for Cryptosporidium oocyst wall protein gene. Lane 1 - 100 bp DNA ladder, lanes 2, 3, 4 - Cryptosporidium hominis isolates (RsaI - 284, 129, 106 bp).
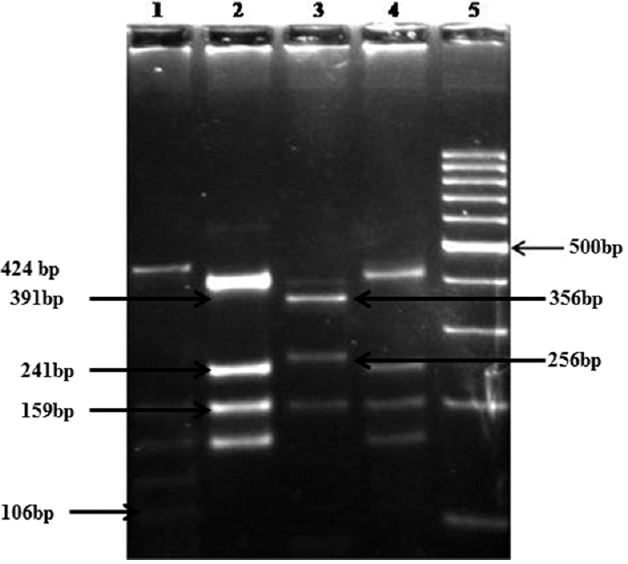
- Restriction fragment length polymorphism assay using RsaI RE for Cpgp40/15 gene. Lane 1 - Ie isolates (424, 159, 134, 129, 106 bp), lanes 2, 4 - Ia (391, 241, 159, 143 bp), lane 3 - IIc (356, 256, 143 bp), lane 5 - 100 bp DNA ladder.
Statistical analysis: The statistical analysis was performed using STATA version 11.2 for Windows (Stata Corp LP, Texas, USA). All values were expressed as mean ± standard deviation for continuous variables and percentages for categorical variables. Categorical variables were compared using Pearson's Chi-square test or Fisher's exact test and continuous variables were assessed by Wilcoxon rank-sum (Mann–Whitney U) test.
Results
A total of 38 transplant recipients comprising 30 adults (21 males) and eight children (6 males) were included in the study with a mean age of 29 ± 14.1 yr. These included 29 post-renal transplant (PRT), two liver and seven bone marrow transplant (BMT) recipients. Of these 38 patients, seven, 11 and 20 patients had episodes of acute, persistent and chronic diarrhoea, respectively. Diarrhoeal episodes in 14 patients could be attributed to the immunosuppressive treatment that included eight patients who received prednisolone and the remaining six received mycophenolic acid. Twenty five diarrhoeal episodes were caused by infectious agents comprising bacterial (Clostridium difficle, n = 2), cytomegalovirus infection (n = 3), Candida albicans in two and parasites (pathogenic) in 19 cases. Parasitic infections were presented as either single parasite or multiple parasites. The parasites (n = 23) that were detected in these patients by light microscopy included Cryptosporidium species (21%, 8/38), C. belli (8%, 3), C. cayetanensis (5%, 2), G. lamblia (11%, 4) Blastocystis hominis (3%, 1), Hymenolepis nana (11%, 4) and Strongyloides stercoralis (3%, 1). No E. bieneusi could be detected in any of the samples. Multiple parasites were observed in only five adult PRT recipients, of whom two were co-infected with Cryptosporidium species with B. hominis and G. lamblia and two patients with H. nana co-infected with C. belli and G. lamblia each. Only one patient had co-infection of G. lamblia and Endolimax nana. All intestinal protozoa (Cryptosporidium spp., C. cayetanensis, C. belli and G. lamblia) positive by light microscopy were also positive by their respective PCR assays.
In comparison, 12 (24%) intestinal parasites including five pathogenic (G. lamblia = 3, H. nana = 2) and seven non-pathogenic protozoa were detected by light microscopy in 12 of the total 50 transplant recipients without diarrhoea (28 adult males). No coccidia or microsporidia could be detected in this group either by light microscopy or PCR assays.
The eight Cryptosporidium isolates were subjected to multilocus genotyping at SSUrRNA, COWP and DHFR gene and Cpgp40/15 loci. Of these seven isolates of Cryptosporidium, four could be genotyped as C. hominis, two as C. parvum, one as mixed genotype and one could not be genotyped. All the C. hominis isolates were detected in adult PRT recipients, whereas the C. parvum isolates were detected in a child with BMT and an adult with PRT. Symptoms included prolonged diarrhoea, fever, abdominal pain and weight loss. The distribution of subtypes was determined at Cpgp40/15 locus. Two Ia and one each of Ie and If subtypes were observed in C. hominis isolates and IId and IIc among C. parvum isolates. The isolate with mixed genotype had IIa subtype. The subtype Ia of C. hominis was associated with abdominal pain and anaemia. Three different fragments of 140, 106 and 48 bp were obtained after restriction digestion of PCR products from both the isolates of C. cayetanensis. All the four G. lamblia parasites were of assemblage B. The stool samples from all the patients with parasitic infections were re-examined after four weeks of receiving treatment. All these patients recovered eventually from the disease and had no recurrence during the six-month follow up.
Discussion
Organ transplant recipients may acquire parasitic infection in three different ways such as (i) transmission with the graft, (ii) de novo infection, or (iii) reactivation of latent infection as a consequence of immunosuppression. Most of the infections due to reactivation are extraintestinal. Relapse of cryptosporidiosis in immunocompromised patients even after treatment can occur, suggesting that the infection may remain in the latent stage; however, no significant evidence is available on the role of reactivation of latent infection of other intestinal protozoan parasites. The reactivation of latent infection of C. felis in cats by administration of prednisolone has been reported18. In the present study, the frequency of intestinal parasitic infections was relatively higher in transplant recipients with diarrhoea (66%, 23/38) as compared to transplant recipients without diarrhoea (24%, 12/50) (P<0.01). The most common parasite identified in the present study was Cryptosporidium species (21%). The global prevalence of cryptosporidial infection in transplant recipients has been reported as 18.8-34.8 per cent19. A study on renal transplant recipients in India identified cryptosporidial diarrhoea in 16.6 per cent of cases20. Similarly, Cryptosporidium spp., has been identified in 1.7-11 per cent of the patients undergoing allogeneic BMT21. Cryptosporidiosis has also been reported in children with liver transplantation22. In contrast, in a study carried out on renal transplant recipients in Brazil, S. stercoralis (11/16) was the most common helminthic infection23.
In developing countries including India, C. hominis is the most common luminal coccidial species responsible for a majority of human infections in immunocompromised patients24. In the present study, C. hominis was the predominant Cryptosporidium spp., and it was also associated with prolonged duration of diarrhoea than infection with C. parvum. C. parvum has been reported in a bone marrow recipient in Iran25. Detection of both C. hominis and C. parvum in our study highlights the possible mode of transmission of this parasite. The zoonotic transmission of Cryptosporidium infection cannot be excluded for transplant recipients undergoing immunosuppressive therapy based on exposure to socio-economic settings. While in the present study, subtype Ia of C. hominis was associated with abdominal pain and anaemia, an association of subtype Ia with older age was observed in the study by Ajjampur et al26.
Infections with C. cayetanensis and C. belli are less common than Cryptosporidium species and have occasionally been reported in renal transplant recipients27. A single case of liver transplant with isosporiasis has also been documented28. Genotyping of G. lamblia was performed to investigate whether different genotypes and/or intraspecies variations within the genotypes had any effect on the clinical symptomatology. Assemblage A, based on PCR-RFLP of tpi gene of G. lamblia, has been reported as the most common genotype associated with giardiasis, whereas assemblage B has been predominantly found in children29. Most of our patients had assemblage B. B. hominis that had long been considered non-pathogenic, has also been incriminated as a diarrhoeagenic agent and has been reported in transplant recipients30.
In conclusion, transplant recipients undergoing immunosuppressive therapy are a major risk group for acquiring intestinal parasitic infections. Improving the level of knowledge about covert parasitic infections and relevant risk factors will have obvious influence on withdrawing the infection rate among this population and would significantly reduce the morbidity. With the accumulated data, it is of further importance to associate genotypes and subgenotypes with special reference to Cryptosporidium spp., with the clinical outcome of the disease.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), Department of Health Research, New Delhi, India, for financial support. Authors thank all the participating patients, faculty and residents of the Departments of Nephrology, Surgery, AIIMS, and Dr. B. R. Ambedkar Institute of Rotary Cancer Hospital.
Conflicts of Interest: None.
References
- Risk factors for rejection and infection in pediatric liver transplantation. Am J Transplant. 2008;8:396-403.
- [Google Scholar]
- Gastrointestinal infections in immunocompromised hosts. Curr Opin Gastroenterol. 2006;22:18-23.
- [Google Scholar]
- Parasitic infections in transplant recipients. Nat Clin Pract Nephrol. 2006;2:490-503.
- [Google Scholar]
- Sensitive PCR-restriction fragment length polymorphism assay for detection and genotyping of Giardia duodenalis in human feces. J Clin Microbiol. 2002;40:446-52.
- [Google Scholar]
- Etiology of diarrhea in patients undergoing allogeneic bone marrow transplantation in South India. Transplantation. 2002;73:1247-51.
- [Google Scholar]
- Fauci AS, Braunwald E, Kasper DL, Longo DL, Jameson JL, Hauser SL, eds. Harrison's Principles of Internal Medicine (17th ed). New York: McGraw-Hills; 2008.
- Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J Clin Microbiol. 1983;18:185-90.
- [Google Scholar]
- A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol. 1993;31:3264-9.
- [Google Scholar]
- Extraction-free, filter-based template preparation for rapid and sensitive PCR detection of pathogenic parasitic protozoa. J Clin Microbiol. 2000;38:2271-7.
- [Google Scholar]
- Detection of Cystoisospora belli by polymerase chain reaction using primers based on small-subunit ribosomal RNA sequences. Eur J Clin Microbiol Infect Dis. 2000;19:631-4.
- [Google Scholar]
- Detection of Enterocytozoon bieneusi (Microsporidia) by polymerase chain reaction (PCR) using species-specific primer in stool samples of HIV patients. Indian J Med Res. 2005;121:215-9.
- [Google Scholar]
- Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol. 1999;65:3386-91.
- [Google Scholar]
- PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150:209-17.
- [Google Scholar]
- A multiplex allele specific polymerase chain reaction (MAS-PCR) on the dihydrofolate reductase gene for the detection of Cryptosporidium parvum genotypes 1 and 2. Parasitology. 2002;125(Pt 1):35-44.
- [Google Scholar]
- Identification of Cpgp40/15 type Ib as the predominant allele in isolates of Cryptosporidium spp. from a waterborne outbreak of gastroenteritis in South Burgundy, France. J Clin Microbiol. 2006;44:589-91.
- [Google Scholar]
- Template preparation for PCR and RFLP of amplification products for the detection and identification of Cyclospora sp. and Eimeria spp. Oocysts directly from raspberries. J Food Prot. 1998;61:1497-503.
- [Google Scholar]
- Polymorphisms in the 18S rDNA gene of Cystoisospora belli and clinical features of cystoisosporiasis in HIV-infected patients. Parasitol Res. 2011;108:679-85.
- [Google Scholar]
- Biological nature of Cryptosporidium sp. isolated from a cat. Parasitol Res. 1991;77:237-40.
- [Google Scholar]
- Prevalence of cryptosporidiosis in renal transplant recipients presenting with acute diarrhea at a single center in Pakistan. J Nephrol. 2014;3:127-131.
- [Google Scholar]
- Intestinal cryptosporidiosis in living related renal transplant recipients. Transplant Proc. 2004;36:2128-9.
- [Google Scholar]
- Infections in children undergoing allogeneic bone marrow transplantation in India. Pediatr Transplant. 2006;10:48-54.
- [Google Scholar]
- Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15:145-54.
- [Google Scholar]
- Parasitic infection in renal transplant recipients. Transplant Proc. 2007;39:460-2.
- [Google Scholar]
- Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124:80-9.
- [Google Scholar]
- Prevalence, molecular characteristics and risk factors for cryptosporidiosis among Iranian immunocompromised patients. Microbiol Immunol. 2012;56:836-42.
- [Google Scholar]
- Multisite study of cryptosporidiosis in children with diarrhea in India. J Clin Microbiol. 2010;48:2075-81.
- [Google Scholar]
- Cyclospora cayetanensis infection in a patient with renal transplant. Türk Hij Deney Biyol Derg. 2009;66:25-7.
- [Google Scholar]
- A rare diarrheic parasite in a liver transplant patient: Cystoisospora belli. Transplant Proc. 2007;39:1693-5.
- [Google Scholar]
- Giardia lamblia groups A and B among young adults in India. Clin Infect Dis. 1998;26:190-1.
- [Google Scholar]
- Blastocystis hominis – An emerging cause of diarrhoea in renal transplant recipients. J Assoc Physicians India. 2003;51:719-21.
- [Google Scholar]






