Translate this page into:
Modified low cost SNP genotyping technique using cycle threshold (Ct) & melting temperature (Tm) values in allele specific real-time PCR
Reprint requests: Dr B. Vishnu Bhat, Department of Paediatrics, Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry 605 006, India e-mail: drvishnubhat@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Genotyping has now become one of the major diagnostic means for almost all diseases. Among the advanced techniques that are used to study single nucleotide polymorphisms (SNPs), only a few are applicable for routine disease diagnosis. Their applicability mainly depends on three factors: cost, time, and accuracy. The primary objective of this study was to propose allele-specific real-time PCR as a rapid, low cost and simple genotyping method for routine diagnostics.
Methods:
Two SNPs, rs3014866 and rs2149356 were analysed using allele-specific real-time PCR. The polymerase chain reaction was carried out using RealQ PCR master mix containing SYBR Green DNA I dye followed by melt curve analysis. The results were validated by agarose gel electrophoresis and DNA sequencing.
Results:
The allelic discrimination and zygosity of the two SNPs were assessed by combined cycle threshold (Ct) and melting temperature (Tm) values. Variations in Ct and Tm values among the two alleles were observed in both rs3014866 (Ct: C allele - 24±1, T allele - 27±1; Tm: C allele - 82.5±0.3, T allele - 86.3±0.2) and rs2149356 (Ct: C allele - 24±1, A allele - 26±1; Tm: C allele - 79.4±0.2, A allele - 80.4±0.3). Based on the variations, homozygous and heterozygous alleles were detected. Agarose gel electrophoresis and DNA sequencing also confirmed the allelic variation and zygosity observed in real-time PCR.
Interpretation & conclusions:
In diagnostic settings where a large number of samples are analysed daily, allele-specific real-time PCR assay may serve as a simple, low cost and efficient method of genotyping.
Keywords
Allele-specific
cycle threshold
economical diagnostic technique
melting temperature
SNP typing
Single nucleotide polymorphisms (SNPs) are predominantly used in various fields of biology starting from evolutionary studies to disease diagnosis/prognosis. Several methods have been used for genotypic analysis, though the search for a cost-effective, potential technique which can be implemented in patient care is still not available. Though TaqMan genotyping is considered the most sensitive method1, but due to its high cost, it is not applicable for routine patient care in developing countries with poor diagnostic settings. Hence, there is a need to have an efficient genotyping technique which can be applied in routine diagnostic settings for handling large number of samples within a few hours and at low cost.
Allele-specific PCR coupled with amplicon melt curve analysis had been used in genotyping for more than a decade. It was found to be the most cost-effective means of genotyping for various diseases2. In this method, genotyping is carried out using two forward primers each specific to one allele and a common reverse primer. After amplification, the amplicons are melted and the alleles are discriminated by the melting temperature (Tm). In 1997, microvolume fluorometer integrated with thermal cycler was used to analyze the amplicon melt curve using SYBR Green-I dye3. Later with the advancement of real-time PCR, the same allele-specific PCR was made rapid with high sensitive amplicon melt curve analysis. In order to make the assay further sensitive, different strategies like usage of unlabelled probes4, multiplex primers5 and energy-transfer-labelled primers6 have been used. However, these methods require significant optimization protocols and technical expertise which make these non-applicable in low resource settings.
In this study, two SNPs (one transition and one transversion) of human origin were analysed to prove the efficiency and cost-effectiveness of allele-specific real-time PCR coupled with amplicon melt curve analysis in genotyping by incorporating GC-tail and 3’ mismatch in the primer sequence. Allelic discrimination and zygosity testing were also made easier with combined assessment of cycle threshold (Ct) and Tm values.
Material & Methods
This study was conducted as part of an ongoing genomic research project in newborns in the Department of Paediatrics, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India. The study was approved by JIPMER Institute Ethics Committee for Human Studies. The data generated during June, 2012 to May, 2013 were utilized for this study. Two intron variants, rs3014866 and rs2149356 of human genes S100A9 and TLR4, respectively, were selected for this study; rs3014866 is a transition mutation and rs2149356 is a transversion mutation. As per HapMap data, the former SNP has minor allele frequency of 35 per cent7 and the later, 36 per cent in Asian population8.
Designing of primers: The designing of allele specific primers is the crucial step involved in allele specific real-time PCR. Two forward primers were designed for each SNP, with varying nucleotide at its 3´ end (shown in bold, Table I) and the remaining sequence being the same. A common reverse primer was designed for both the alleles. Primers were designed using the online software, NetPrimer [(Premier Biosoft) (www.premierbiosoft.com/netprimer/index.html)] and Primer 3 v.0.4.0 (http://bioinfo.ut.ee/primer3_0.4.0/). Primers were synthesized by Vbc bIOTECH, Austria. In order to increase the specificity and feasibility of efficient allelic discrimination, the following modifications were made in the primer sequence9:
Mismatch: Incorporation of mismatch within 5 bases from 3´ end of the primer was shown to increase the specificity of PCR amplification10. We introduced different mismatches for the two forward primers at the second base from 3´end (shown in lower case, Table I) to distinguish between the alleles. Primers with melting temperature between 58 - 63 °C were selected (without GC clamp).
GC clamp: Since the PCR products derived from both primer sets will be of equal length, there will not be sufficient difference in their melting temperatures (Tms). Addition of random GC clamp at the 5´end of primer can modify the Tm of the product by increasing the product length. A short GC clamp was attached to one forward primer and a long GC clamp to the other11. This brings a difference among the Tm of PCR products. A temperature difference of >0.8 °C can easily distinguish between the alleles.
Sample collection and storage: Sample size (n=128) was calculated at 5 per cent level of significance and 90 per cent power based on the minor allele frequency of the two SNPs in Asian population. After getting informed consent from the parents, 0.5 ml of peripheral venous blood was collected in an EDTA vacutainer from the infants admitted to Neonatal intensive care unit (NICU). The samples were stored at 4°C for not more than four days before DNA isolation.
DNA isolation and quantification: Genomic DNA was isolated from the stored blood samples using QIAmp DNA blood Mini kit (Qiagen, Hilden, Germany). The concentration of DNA was measured using Biophotometer Plus (Eppendorf, Germany). The isolated DNA samples were stored at 4°C till further analysis.
Real-time PCR: Although single tube reactions have been reported11, but to avoid PCR inhibition due to addition of GC-clamp and to make pooling of DNA possible, genotyping was carried out in separate tubes12. Pooling of DNA is known to aid in the determination of allele frequency13. PCR amplification was carried out in two separate tubes containing respective forward primer and common reverse primer for each allele. The reaction mixture contained 10 µl 2X RealQ PCR master mix with Green DNA I dye (Ampliqon, Denmark), 1 µl of each forward and reverse primers (20 pmol/µl), 1 µl of template DNA (100 ng/µl) and 7 µl of nuclease free, diethylpyrocarbonate (DEPC) treated water making the final volume 20 µl. Polymerase chain reaction was performed using 48-well, StepOne™ real-time PCR instrument (Applied Biosystems, Foster city, CA, USA) under the following reaction conditions: 95°C for 10 min, followed by cycling for 35 cycles of denaturation at 95°C for 15 sec, and annealing and extension at 60°C for one min. The reactions were carried out in triplicates. No transcript controls (NTC) were used for each PCR run with primer sets for each allele. The samples confirmed by sequencing were used as positive controls.
Melt curve analysis: PCR amplification was immediately followed by melt curve analysis in which the amplicon was melted from 70 to 90°C at an increment of 0.2°C/min. The StepOne software v2.1 automatically gives the negative derivative of fluorescence data, with a peak at the Tm of the amplicon.
Agarose gel electrophoresis: The allelic discrimination was verified using agarose gel electrophoresis. Ten microlitres of the amplicon mixed with two µl of loading dye was loaded in 1.5 per cent agarose gel. Electrophoresis was carried out at 160 V for 24 min.
Sequence validation: The result from real-time PCR and gel electrophoresis was validated by DNA sequencing. As primers used for genotyping were not suitable for sequencing, appropriate primers for sequencing were designed (Table II). DNA samples (100 ng/µl) were amplified in conventional PCR machine (Corbett Research, UK) with 2X Red Dye PCR master mix. The reaction conditions remained the same as that of real-time PCR. After the reaction was over, the amplified samples were purified using QIAquick PCR purification kit (Qiagen, Hilden, Germany). The purified samples were outsourced for sequencing (Eurofins, India).

Results
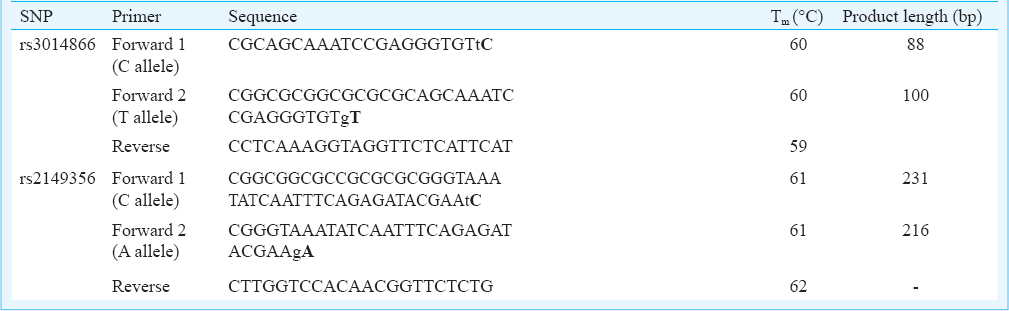
The cycle threshold (Ct) value is the first step in genotyping of SNPs using allele-specific real time PCR. Ct values <30 shows positive amplification of the target. The allelic discrimination of the SNPs, rs3014866 and rs2149356 by means of Ct value is described in Fig. 1. If the Ct value of one primer set is > 30, it indicates the absence of respective allele in the sample, hence homozygous towards the other allele. When both primer sets show Ct value < 30, the sample is heterozygous. Heterozygosity was seen in Fig. 1.a.[1] and Fig. 1.b.[2], with both alleles having Ct value <30. In other Figures homozygosity of the alleles was observed with Ct value < 30. Allelic discrimination was done by the difference in the Ct value between positively amplified alleles. The allele specificity of Ct values <30 was decided based on several trials with all 128 samples and validated with positive bands in agarose gel electrophoresis.
![Amplification plot of SNPs showing allelic variation. (a) rs3014866: [1]+/- homozygous wild type C/C; [2]+/+ heterozygous C/T; [3] -/+ homozygous mutant T/T. (b) rs2149356: [1] +/- homozygous wild type C/C; [2] +/+ heterozygous C/A; [3]-/+ homozygous mutant type A/A.](/content/175/2015/142/5/img/IJMR-142-555-g003.png)
- Amplification plot of SNPs showing allelic variation. (a) rs3014866: [1]+/- homozygous wild type C/C; [2]+/+ heterozygous C/T; [3] -/+ homozygous mutant T/T. (b) rs2149356: [1] +/- homozygous wild type C/C; [2] +/+ heterozygous C/A; [3]-/+ homozygous mutant type A/A.
The melting temperature (Tm) analysis is the second step in assessing allelic variations. After PCR amplification, the PCR product was denatured by heating and this denaturation resulted in the emission of fluorescence. StepOne real-time PCR was capable of preparing the negative derivative of the fluorescence emitted (dissociation curve), giving a peak at the Tm of the amplicon. Based on the amplicon length, Tm varied between the alleles and was used to discriminate the alleles.
In Fig. 2(a), C and T alleles of rs3014866 were distinguished by their Tms, 82.5 ± 0.3°C and 86.3 ± 0.2°C, respectively, with a temperature difference of 4°C (Table II). Similarly, Tms of C and A alleles of rs2149356 were found to be 79.4 ± 0.1°C and 80.4 ± 0.05 °C, respectively, creating a temperature difference of 1°C (Fig. 2b, Table II). Though the Tm difference in rs2149356 was less (1°C), two distinct peaks were observed in the dissociation curve, (Fig.2.b.2), which discriminated the alleles and showed heterozygosity.
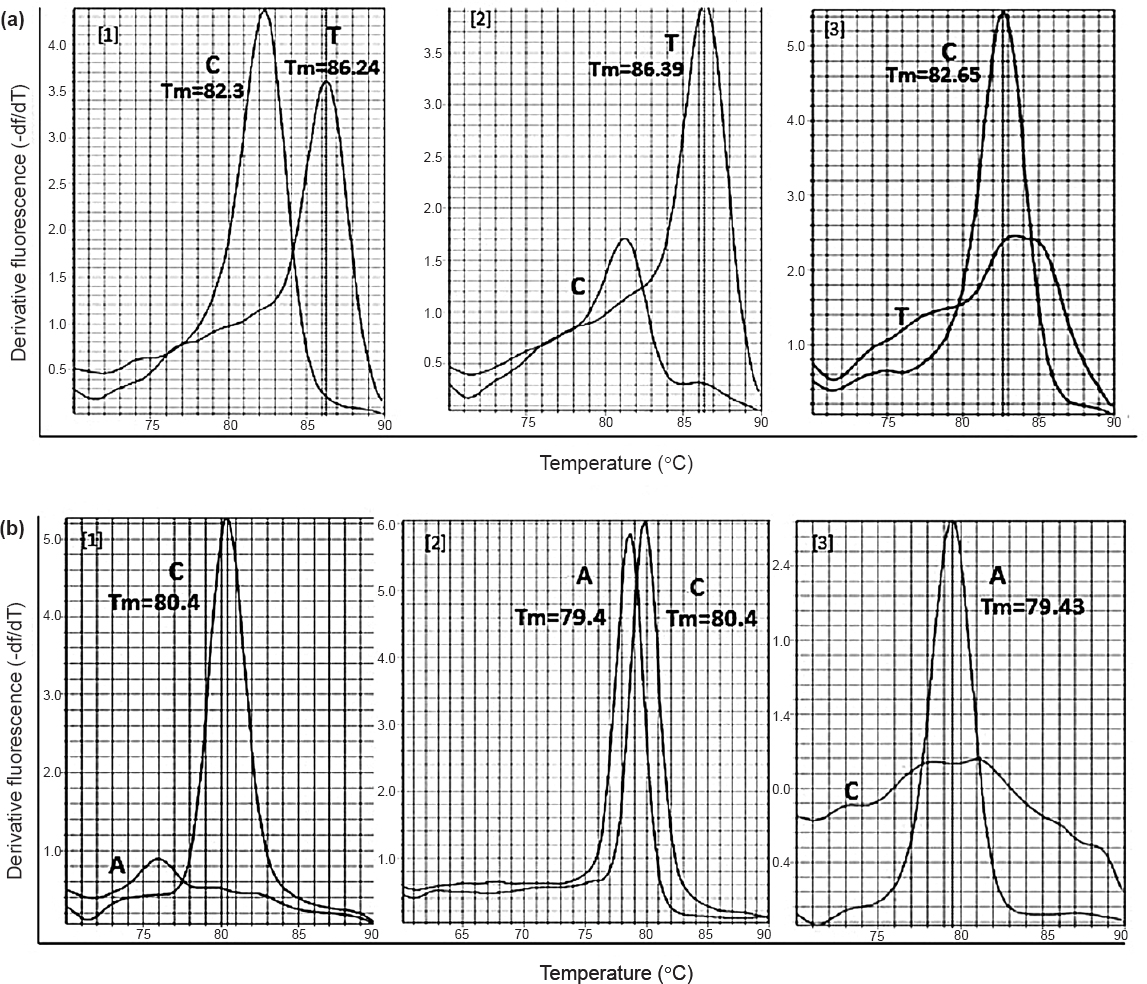
- Dissociation curve of SNPs showing temperature variation between the two alleles. (a) rs3014866 showing a Tm difference of 4 °C (b) rs2149356 showing a Tm difference of 1 °C. *Tm, melting temperature.
The amplicons from real-time PCR were directly observed in agarose gel electrophoresis (Fig. 3). No bands were observed in the samples which showed negative amplification. Both allelic variation and zygosity were verified in gel electrophoresis. Gel detection only serves as a support of results obtained from real-time PCR and is not a necessary step for each sample. DNA sequencing results also substantiated allelic discrimination and zygosity testing. The overall summary of Ct and Tm properties used to discriminate alleles and zygosity in rs3014866 and rs2149356 is given in Table II.
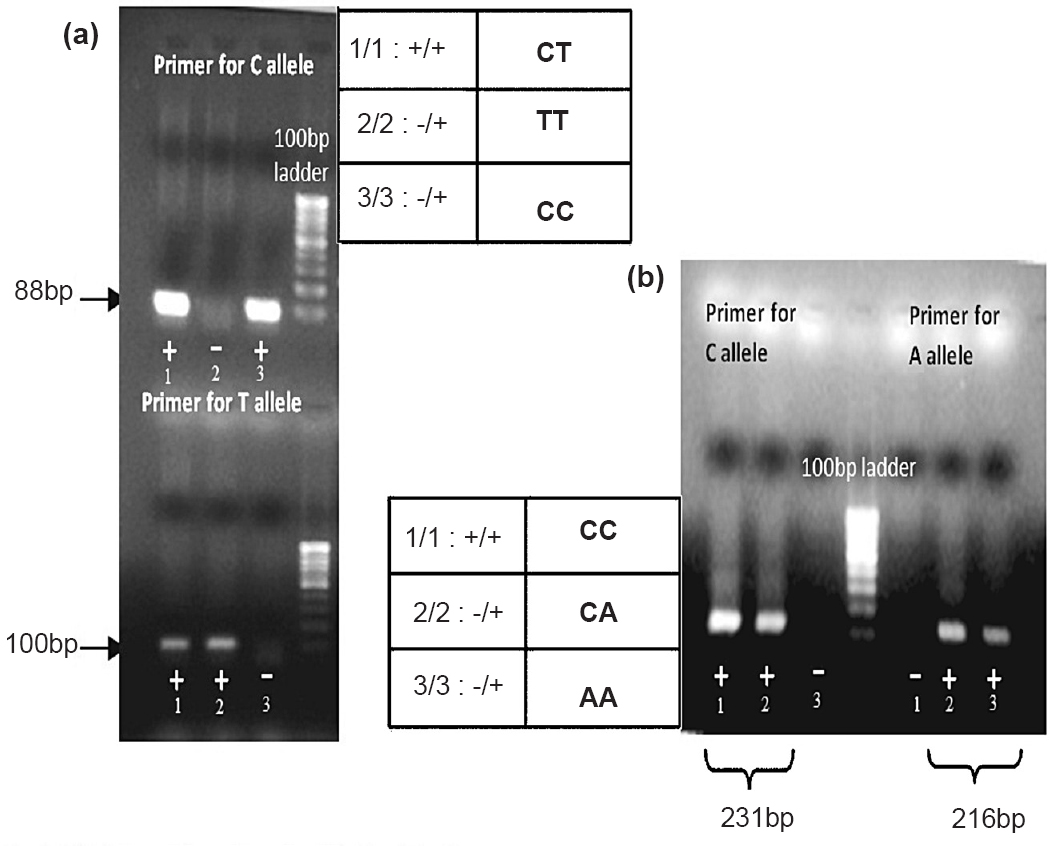
- Gel detection of the amplicons for allelic discrimination. (a) Allelic discrimination of rs3014866 (b) Allelic discrimination of rs2149356.
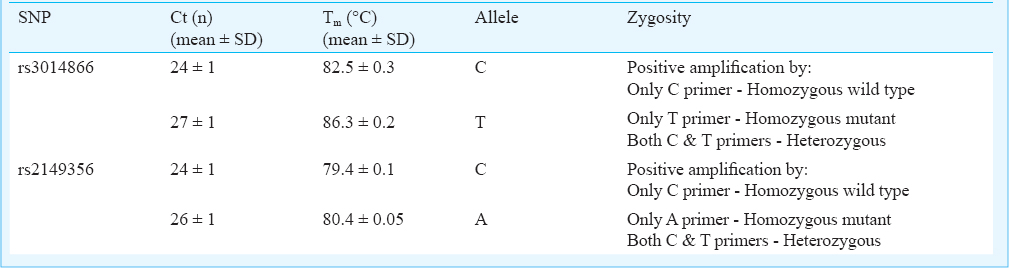
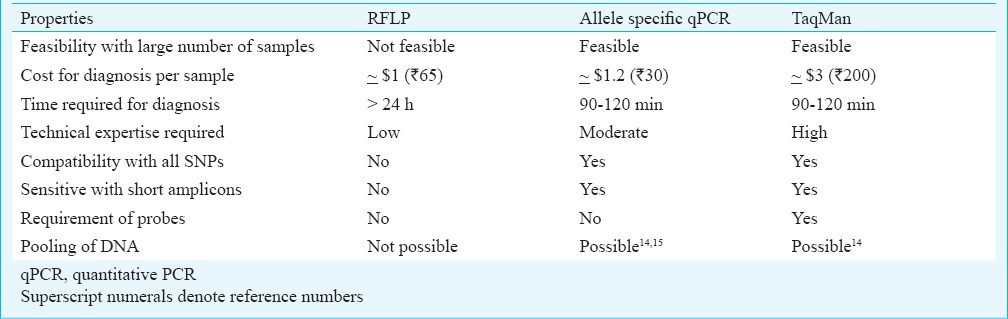
Table IV shows the comparison of allele specific quantitative PCR (qPCR) with other PCR assays being used currently in diagnostics. This modified allele specific qPCR technique was found to be rapid, cheap and sensitive.
Discussion
Allelic discrimination and zygosity testing is one of the most important strategies in genomic diagnostics. In view of applicability, allele-specific real-time PCR remains the efficient and cost-effective method for genotyping. By assessing the Ct and Tm values together, SNP typing by allele-specific PCR becomes more specific in diagnostics.
Allele-specific real-time PCR depends primarily on the proficient designing of primers. Methods like energy-transfer-labelled primers had been used in some studies6. As the forward primers for allele-specific PCR have to be fixed in the exact locus of SNP with no option to move forward or backward, GC content of the primers may be < 40 per cent in some cases6. In such occasions, PCR optimization is the key step to obtain positive amplification of the desired target.
The mismatch introduced in the forward primer not only gives specificity to PCR amplification, but also weakens the non-specific amplifications. Different lengths of GC clamp attached to the 5´´ end of primer sequence increases the specificity of zygosity testing10. The designing of primers used in this study reduced the need for heavy optimization protocols in routine diagnostics. And also when compared to previous techniques like tetra primers4 and energy-transfer-labelled primers5, the primers used in this study were simple enough to be designed with basic skill.
In this study, the fluorescent dye, SYBR Green I was used for detecting DNA amplification and melting temperature of amplicons. LC Green dye has also been used in some earlier studies and found to be effective16. The effect of different dyes on DNA amplification and melting temperature was studied and two dyes, SYTO-82 and SYTO-13 were found to increase sensitivity of real-time PCR assays17. But the cost per reaction of these dyes was 6-folds higher than SYBR Green I17, making these less preferable in diagnostic settings.
The dissociation accuracy depends on the size of the amplicons, short amplicons are greatly differentiated18, which was obvious in this study also by comparing the amplicon size of the two SNPs studied. The rs3014866 had smallest amplicon (≤ 100 bp) and was well differentiated in dissociation curve with temperature difference of 4°C between the two alleles. While the amplicon of rs2149356 was greater than 200 bp and showed only little variation in dissociation curve with temperature difference of 1°C. The PCR reaction for each allele was carried out in separate tubes to reduce the optimization efforts in routine diagnostics. Although it increases the cost per reaction, the total cost for genotyping a SNP is lesser than other real-time PCR assays. Similar separate tube method was followed in previous studies also14.
The important properties of allele-specific PCR were compared with prevailing genomic diagnostic methods like RFLP, TaqMan PCR assays. Allele specific qPCR with SYBR Green I has already been proven to deliver comparable results with TaqMan PCR and other high-density microarrays in gene expression studies19. It was found to be rapid, sensitive, specific, quantifiable and cost-effective20.
The following are the limitations of this method: (i) complexity in primer designing; (ii) Due to the presence of lengthy GC clamps, there was a possibility of non-specific amplifications; and (iii) the primers used for allele discrimination could not be used for validation by DNA sequencing.
In conclusion, the combined analysis of Ct and Tm values in allele-specific real-time PCR provided an accurate means of allelic discrimination and zygosity testing. The total time required for genotyping by this method was approximately two hours which was faster than RFLP technique. The master mix and other reagents used in this method were cheaper compared with TaqMan reagents. The methodology was simple and accurate compared with other high-throughput technologies. Hence, allele-specific real-time PCR may serve as a simple, low cost, efficient genotyping technique in diagnostic settings which handle a large number of samples daily.
Acknowledgment
This study was funded by JIPMER, Puducherry. The first author (BBDD) acknowledges the Indian Council of Medical Research for providing Senior Research Fellowship.
Conflicts of Interest: None.
References
- Cost-effectiveness in the diagnosis of tuberculosis: choices in developing countries. J Infect Dev Ctries. 2014;8:24-38.
- [Google Scholar]
- Allele-specific PCR for a cost-effective & time-efficient diagnostic screening of spinal muscular atrophy. Indian J Med Res. 2012;135:31-5.
- [Google Scholar]
- Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997;245:154-60.
- [Google Scholar]
- Closed-tube genotyping with unlabeled oligonucleotide probes and a saturating DNA dye. Clin Chem. 2004;50:1328-35.
- [Google Scholar]
- SYBR green dye-based probe-free SNP genotyping: introduction of T-Plex real-time PCR assay. Anal Biochem. 2013;441:225-31.
- [Google Scholar]
- High-throughput SNP genotyping by allele-specific PCR with universal energy-transfer-labeled primers. Genome Res. 2001;11:163-9.
- [Google Scholar]
- National Centre for Biotechnology Information. Database for short nucleotide polymorphisms (dnSNP) Available from: http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ ref.cgi?rs=3014866
- [Google Scholar]
- National Centre for Biotechnology Information. Database for short nucleotide polymorphisms (dnSNP) Available from: http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=2149356
- [Google Scholar]
- Allele-specific Holliday junction formation: a new mechanism of allelic discrimination for SNP scoring. Genome Res. 2003;13:1754-64.
- [Google Scholar]
- Single nucleotide polymorphism genotyping using allele-specific PCR and fluorescence melting curves. Biotechniques. 2003;34:1068-72.
- [Google Scholar]
- High throughput SNP genotyping by single-tube PCR with T m -shift primers. Biotechniques. 2005;39:885-93.
- [Google Scholar]
- Allele-specific polymerase chain reaction for the detection of Alzheimer's disease-related single nucleotide polymorphisms. BMC Med Genet. 2013;14:27.
- [Google Scholar]
- High-throughput SNP allele-frequency determination in pooled DNA samples by kinetic PCR. Genome Res. 2000;10:258-66.
- [Google Scholar]
- Determination of allele frequency in pooled DNA: comparison of three PCR-based methods. Biotechniques. 2005;39:853-8.
- [Google Scholar]
- Comparison of real-time PCR with SYBR Green I or 5′-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology. 2003;149:269-77.
- [Google Scholar]
- High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem. 2003;49:853-60.
- [Google Scholar]
- Comparison of multiple DNA dyes for real-time PCR: effects of dye concentration and sequence composition on DNA amplification and melting temperature. Nucleic Acids Res. 2007;35:e127.
- [Google Scholar]
- Closed-tube SNP genotyping without labeled probes/a comparison between unlabeled probe and amplicon melting. Am J Clin Pathol. 2007;127:341-8.
- [Google Scholar]
- Cross-platform comparison of SYBR Green real-time PCR with TaqMan PCR, microarrays and other gene expression measurement technologies evaluated in the MicroArray Quality Control (MAQC) study. BMC Genomics. 2008;9:328.
- [Google Scholar]
- Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol Med Microbiol. 2003;39:81-6.
- [Google Scholar]






