Translate this page into:
Misdiagnosis of leprosy: An underappreciated reason for its continued prevalence
* For correspondence: kabirijdvl@gmail.com
-
Received: ,
Accepted: ,
‘The first step towards diagnosing leprosy is to think of the possibility of leprosy’ D.L. Leiker
Leprosy is diagnosed by certain cardinal clinical features, including pain and/or thickening of peripheral nerves at sites of predilection and around skin lesions and diminution or loss of either sensory (hypoesthesia) or autonomic functions (sweating and axon reflex) in suspicious skin lesions or the skin areas supplied by the peripheral nerves1. Exceptions to this are the absence of nerve involvement in indeterminate form and skin lesions that can precede any evident sign of peripheral nerve damage in lepromatous leprosy (LL). Also, pure neuritic leprosy does not have skin lesions2. The reason for the exclusion of slit skin smear for diagnosis in this definition is due to its low sensitivity except for highly bacillated types.
While the treatment of leprosy has been simplified by adopting uniform multidrug therapy (MDT), the overriding concern is the high incidence of new cases and disabilities3. This could partly be attributed to possible misdiagnosis by non-dermatologists. We have noticed that this trend extends to tertiary hospitals with misdiagnosis by specialists across the spectrum, including physicians, neurologists, and even infertility specialists4. The multisystem involvement of leprosy and reactions can have myriad manifestations, and we aim to highlight these clinical differentials, including neural involvement, so that early diagnosis of leprosy patients is ensured, as dermatologists are rarely the first point of contact for them.
Cutaneous mimickers
Leprosy has a wide spectrum of clinical presentations that often simulate many other dermatological conditions, making it difficult to differentiate, particularly in non-endemic areas. The diverse cutaneous lesions of leprosy include subtle hypopigmented macules, raised plaques of varying sizes, annular plaques, nodules, and diffuse infiltration (Fig. A and B).
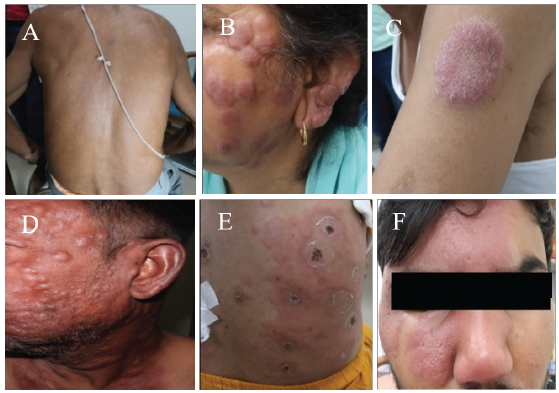
-
(A) Multiple symmetrical hypopigmented macules and plaques in borderline lepromatous leprosy. (B) Multiple plaques and nodules in a patient with borderline lepromatous leprosy. (C) A single annular erythematous plaque with central clearing in borderline tuberculoid leprosy. (D) Multiple nodular lesions in lepromatous leprosy with erythema nodosum leprosum. (E) Necrotic ulcers in erythema nodosum leprosum. (F) Borderline tuberculoid leprosy with type 1 reaction over face misdiagnosed as cellulitis.
The common differentials of hypopigmented macules include pityriasis alba of the face (subtle scaling, aggravating in dry weather) and vitiligo. However, it is important to remember that leprosy lesions are usually never depigmented, unlike vitiligo5. Post-kala-azar dermal leishmaniasis (PKDL) is an important differential owing to a polymorphic clinical picture wherein skin lesions mimic leprosy, with both diseases sharing common endemic zones6. The absence of neural involvement and hypoesthesia distinguishes PKDL from leprosy, apart from positive skin smears. Erythematous plaques of leprosy (Fig. C) may require differentiation from granuloma annulare7, sarcoidosis, diffuse cutaneous leishmaniasis, and follicular mucinosis8. The prominent scaling of psoriasis, scarring in the centre of lupus vulgaris, and prominent photosensitivity of subacute lupus erythematosus lesions distinguish these conditions from leprosy.
Nodular lesions of LL (Fig. D) require differentiation from sarcoidosis, cutaneous lymphomas, PKDL, and mycosis fungoides. However, neural involvement is consistently present in LL, with patchy or complete glove and stocking hypoesthesia, and skin smears are invariably positive for acid-fast bacilli (AFBs).
Leprosy has occasionally been mislabelled as systemic sclerosis in patients presenting with skin thickening and digital resorption9. Borderline tuberculoid (BT) leprosy presenting as chronic macrocheilia can be mistaken for granulomatous cheilitis, and the distinction between the two conditions is often challenging because of the paucibacillary nature of this leprosy spectrum10.
Leprosy reactions and their mimickers
Type I (T1R) and Type II (T2R) are leprosy reactions presenting with inflammatory-looking edematous plaques (T1R) or evanescent painful nodules (T2R). Leprosy reactions have been misdiagnosed as systemic lupus erythematosus11,12, erythema multiforme13, sweet syndrome14,15, vasculitis (Fig. E), arthritis, or collagenosis16,17. T2Rs frequently have joint symptoms and may be misdiagnosed as rheumatoid arthritis18. An edematous and intensely erythematous leprosy plaque undergoing T1R can be mistaken for cellulitis, especially over the face (Fig. F). Erythematous papules and nodules, along with systemic features like fever, polyarthritis, and eye involvement in T2R, can be mistaken as sarcoidosis19,20. Leprosy reaction misdiagnosed as urticaria is also not uncommon21. T2R can mimic cutaneous tuberculosis and differentiating the two conditions is paramount, especially in areas endemic for both these infections22. There are reports of T2R without typical ENL lesions masquerading as lymphomas23. We have encountered instances of necrotic (ulcerated) ENL patients being admitted in surgical wards diagnosed as necrotizing fasciitis and cases of ENL necroticans being misdiagnosed as vasculitis (Fig. E). However, signs of underlying leprosy are evident with facial and ear lobe infiltration (thickened skin) apart from the clinical features of leprosy (vide supra).
Neural leprosy, a missed cause of mononeuritis multiplex
Mononeuropathy multiplex, defined as an affliction of two or more nerves that cannot be explained by a single root or plexus injury, with asymmetric, non–length-dependent subacute damage, is often not suspected to be consequent to leprosy. While Mononeuritis multiplex (MM) is the most common neuropathy in leprosy, there are other patterns like polyneuropathy (distal, symmetric small fiber sensory polyneuropathy), autonomic neuropathy, cranial nerve affliction, ganglionitis, and neuritis (seen in reactions)24. In addition, leprosy can cause neuropathic pain.
While a clinician may suspect leprosy due to skin lesions, this is not always the case with pure neuritic leprosy (PNL). The diagnosis of PNL needs expertise, as subtly thickened nerve(s) can be missed even by specialists. A single thickened nerve is the hallmark of PNL but may also be noted in neurofibroma, schwannoma, malignant nerve sheath tumor, or localized perineurial hypertrophic neuropathy5,24. The considerations in the case of multifocal thickened nerves are neurofibromatosis, Charcot-Marie-Tooth (CMT) disease types 1 and 3, acromegaly, Refsum disease, and chronic inflammatory demyelinating polyneuropathy, in addition to leprosy5,24.
Notably, leprosy is characterised by negative symptoms of loss of pain and touch sensation, and positive sensory symptoms such as paresthesia, dysesthesia, or pain are less common. Proprioception and motor function are largely unaffected in the early stages, which allows patients to use their anaesthetic limbs and consequently results in painless trauma, ulcerations, and trophic changes. Unlike the tuberculoid pole, the latter is especially seen in the lepromatous pole. A heightened immune response in the latter can lead to marked motor palsy, thus making it difficult to use the anaesthetic hands/feet. Preserved tendon reflexes are an important differential diagnostic sign in leprosy, in contrast to the loss of reflexes seen in most other neuropathies5,24,25.
PNL, described by Wade26, is suspected by enlarged nerves and diagnosed by nerve biopsy, nerve conduction studies (NCS), and molecular tools. In the case of nerve biopsies (sural nerve or radial cutaneous nerve), the presence of endoneurial infiltrates, endoneurial fibrosis, perineurial thickening and reduced number of myelinated nerve fibres are diagnostic for leprosy even in the absence of AFB27. Due to limitations associated with nerve biopsy, including the risk of nerve damage, poor sampling and low sensitivity, a biopsy of hypoesthetic regions can be performed. It shows diagnostic changes in 58.6 per cent of patients28. In the case of non-representative biopsy findings, multi-targeting nested PCR and ELISPOT and can be a useful ancillary diagnostic tool for PNL29. NCS shows reduced amplitudes of Compound Muscle Action Potentials (CMAPs) and Sensory Nerve Action Potentials (SNAPs), together with focal slowing of conduction at sites of nerve enlargement24. These findings are not diagnostic of leprosy, but can be used to identify the nature and extent of neuropathy and also to monitor therapeutic responses. Imaging, including high-resolution ultrasonography (HRUS) and Color Doppler (CD) are auxiliary tools that help to detect nerve enlargement, though CD has been shown to be useful in reactions30.
Conclusions
The goals of the World Health Organization (WHO) now focus on interruption of transmission and zero new ‘autochthonous’ leprosy cases in a given area or country for at least three consecutive years31. Without addressing misdiagnosis and mistreatment, the aims enshrined in the WHO document of a 70 per cent reduction in the annual number of new cases detected is a deceptive target as this does not account for the large number of missed cases by non-dermatologists which would contribute to continued transmission. There is an urgent need to inculcate clinical specialists from endemic countries where leprosy is an issue in advisory groups apart from ‘eminence-based’ experts as the ground reality in terms of misdiagnosis rarely finds its way in guidelines31. To achieve these goals in an endemic country, physicians and neurologists should also be made aware of leprosy and its varied presentations5.
Declaration
It is to declare that a written informed consent was obtained by the authors from patients for using clinical photographs for publication in a journal for academic purposes.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
This editorial is published on occasion of the World Leprosy Day - January 30, 2025
References
- Differential Diagnosis. In: Hastings RC, ed. Leprosy (2nd ed). Edinburgh: Churchill Livingstone; 1994. p. :291-320.
- [Google Scholar]
- Leprosy. Available from: https://www.who.int/news-room/fact-sheets/detail/leprosy, accessed on January 1, 2025.
- Current leprosy multi-drug treatment duration for MB patients (12 months) produces good clinical outcomes over many years. Lepr Rev. 2021;92:97-101.
- [CrossRef] [Google Scholar]
- Case Report: Successful outcome of primary infertility with multidrug therapy in a long-standing case of hypogonadism resulting from lepromatous leprosy: Shattering the dogma of irreversible infertility in leprosy. Am J Trop Med Hyg. 2021;106:47-50.
- [CrossRef] [PubMed] [Google Scholar]
- Differential diagnosis. Jopling’s Handbook of Leprosy (7th ed). New Delhi: CBS Publishers & Distributors; 2023.
- Post kala-azar dermal leishmaniasis coexisting with borderline tuberculoid leprosy. Br J Dermatol. 2007;157:811-3.
- [CrossRef] [PubMed] [Google Scholar]
- Borderline tuberculoid leprosy masquerading as granuloma annulare: A clinical and histological pitfall. Am J Dermatopathol. 2017;39:296-9.
- [CrossRef] [PubMed] [Google Scholar]
- A hypopigmented plaque on the face in a leprosy endemic area and the consequent histological confirmation of follicular mucinosis: An uncommon differential of leprosy in children. Int J Dermatol. 2020;59:e471-3.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy simulating systemic sclerosis: A case report. Rev Bras Reumatol Engl Ed. 2017;57:630-2.
- [CrossRef] [PubMed] [Google Scholar]
- Borderline tuberculoid leprosy misdiagnosed as cheilitis granulomatosamiescher: A case report. Clin Case Rep. 2022;10:e6240.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multibacillary leprosy with positive serology misdiagnosed as systemic lupus erythematosus. BMJ Case Rep. 2022;15:e251497.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multibacillary leprosy mimicking systemic lupus erythematosus: Case report and literature review. Lupus. 2015;24:1095-102.
- [CrossRef] [PubMed] [Google Scholar]
- Next-generation sequencing-assisted diagnosis of a case of leprosy misdiagnosed as erythema multiforme. Ann Clin Microbiol Antimicrob. 2022;21:40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Case report: Type 2 leprosy reaction mimicking sweet syndrome: A case report and literature review. Am J Trop Med Hyg. 2024;110:487-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Type 2 leprosy reaction resembling Sweet syndrome: Review of new and published cases. Australas J Dermatol. 2020;61:e234-7.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy mimicking autoimmune diseases: A case series. Clin Exp Rheumatol. 2024;42:746-51.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy misdiagnosed as rheumatoid arthritis. Intern Med J. 2023;53:445-6.
- [CrossRef] [PubMed] [Google Scholar]
- Lepromatous leprosy simulating rheumatoid arthritis - Report of a neglected disease. An Bras Dermatol. 2017;92:389-91.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy initially misdiagnosed as sarcoidosis, adult-onset still disease, or autoinflammatory disease. J Clin Rheumatol. 2011;17:432-5.
- [CrossRef] [PubMed] [Google Scholar]
- Lepromatous leprosy masquerading as acute sarcoidosis: A case report and literature review. Minn Med. 2008;91:30-3.
- [PubMed] [Google Scholar]
- Leprosy misdiagnosed as chronic urticaria in a migrant. J Travel Med. 2024;31:taad099.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy with subsequent type 2 reaction masquerading as cutaneous tuberculosis: A case report and diagnostic pitfalls in a non-endemic area. Infez Med. 2024;32:538-43.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Type II reaction without erythema nodosum leprosum masquerading as lymphoma. Lepr Rev. 2012;83:378-83.
- [CrossRef] [PubMed] [Google Scholar]
- Disorders of peripheral nerves. In: Newman NJ, ed. Bradley and Daroff’s neurology in clinical practice (8th ed). Amsterdam, Netherlands: Elsevier; 2022. p. :1853-1929.e14.
- [Google Scholar]
- Clinical, electroneuromyographic and morphological studies of pure neural leprosy in a Brazilian referral centre. Lepr Rev. 2004;75:242-53.
- [PubMed] [Google Scholar]
- Pure neuritic leprosy: Resolving diagnostic issues in acid fast bacilli (AFB)-negative nerve biopsies: A single centre experience from South India. Ann Indian Acad Neurol. 2015;18:292-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Histological changes in the nerve, skin and nasal mucosa of patients with primary neuritic leprosy. Acta Leprol. 2000;12:11-8.
- [PubMed] [Google Scholar]
- Utility of multi-target nested PCR and ELISPOT assays for the detection of paucibacillary leprosy: A possible conclusion of clinical laboratory misdiagnosis. Front Cell Infect Microbiol. 2022;12:814413.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- High-resolution sonography: A new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis. 2009;3:e498.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Interruption of transmission and elimination of leprosy disease. Available from: https://www.who.int/publications/i/item/9789290210467, accessed on December 19, 2024.





