Translate this page into:
Microbicides for HIV prevention
Reprint requests: Prof. Gita Ramjee, Director, HIV Prevention Research Unit, Medical Research Council, Durban, South Africa & Professor, Department of Epidemiology & Population Health, London School of Hygiene & Tropical Medicine, London, UK Gita.Ramjee@mrc.ac.za
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Although the HIV incidence rate has slowed in some countries, HIV remains a serious health challenge, particularly in the developing world. The epidemic is increasingly feminised, with young women at high risk of acquiring the virus. There is thus a clear requirement for acceptable woman-initiated methods of HIV prevention. Foremost among these are vaginally-applied substances known as microbicides; early research into potential microbicides focussed on non-HIV-specific compounds such as surfactants and polyanionic entry inhibitors. However, proof of the microbicide concept as a viable prevention strategy was not provided until the CAPRISA 004 trial of a microbicide containing the HIV-specific antiretroviral tenofovir was completed in mid-2010. Confirmation of the proof of concept provided by CAPRISA 004 by at least two major trials will hopefully lead to licensure of the product by 2018. Parallel studies are planned to ascertain the feasibility of implementation of these products in the public sector with subsequent research focussed on appropriate and acceptable methods of delivery of the active ingredient, and to increase adherence through other delivery systems such as vaginal rings.
Keywords
Adherence
developing countries
HIV
microbicide
prevention
vaginal
women
Introduction
Although UNAIDS reports that the peak of the global HIV epidemic appears to have passed, several countries continue to experience rising HIV incidence, most notably in eastern Europe and central Asia1. The largest epidemics, in terms of absolute numbers of people living with HIV remain in sub-Saharan Africa (22.5 million people) and south and south-east Asia (4.1 million people), although both these regions have experienced a decline in adult HIV prevalence in the last decade1. The global epidemic is still feminised, with women accounting for 52 per cent of all people living with HIV1. In sub-Saharan Africa, women account for some 60 per cent of infections, while in Asia this proportion is approximately 35 per cent1. The mode of transmission in sub-Saharan Africa is largely heterosexual, with generalised country epidemics; while in Asia, the epidemic remains concentrated among identifiable groups engaging in high-risk behaviour such as injection drug users, men who have sex with men, sex workers, and their clients. Despite the continued concentrated nature of the Asian epidemic, HIV is beginning to spread more widely, particularly to the female partners of men who inject drugs or pay for sex1. UNAIDS estimates that in India, 90 per cent of people newly infected acquired the virus through sexual activity1.
Women are particularly vulnerable to HIV infection for a variety of intersecting biological, social and cultural reasons. Principle among these is the large mucosal surface area of the female genital tract which is exposed to infected inocula during unprotected sex2. In men, the foreskin is the major site of HIV infection2, which has a comparatively smaller surface area, thus reducing vulnerability to infection. Women are also made vulnerable to HIV infection by cultural proscriptions limiting their control over when and how they have sex, their knowledge about safe sex practices, their access to condoms, and their ability to negotiate their use3–6. There is thus a clear need for a focus on HIV prevention methods for women, which include educational, legal and cultural empowerment, coupled with the development and provision of woman-initiated biomedical means for reducing vulnerability to infection.
Currently available evidence-based biomedical HIV prevention options are limited to condoms (both male and female)78, and medical male circumcision9–11. Many examples of potential biomedical HIV prevention modalities trialled in the past three decades (such as vaccines12–15, microbicides16 and the vaginal diaphragm17) have not shown significant evidence of efficacy. However, promising data on new vaccines18 and several antiretroviral (ARV)-based methods have recently shown that prevention of transmission or acquisition of HIV may be possible through non-barrier or non-surgical modalities19–23. The majority of these antiretroviral-based formulations are oral tablets containing the active ingredients tenofovir and/or emtricitabine, while one is a 1 per cent tenofovir topical gel intended to be applied to the vagina. In the case of oral tablet-based formulations, the pathway to dissemination to the populations in need may be shorter than for the topical gel, since the pills are already licensed for HIV treatment, and only the indication will need to be changed. In the case of 1 per cent tenofovir gel, additional clinical trials are planned or underway to provide the evidence necessary for licensure as a preventive method.
The concept of a topical vaginally applied “virucide”, the application of which would be controlled by women themselves, was mooted in a 1990 by Zena Stein3. Several types of such topical vaginally applied gels (later referred to as microbicides) have been tested in the past. These have included surfactants, polyanionic entry inhibitors, and acid buffers16 (Table). None of the previous generations were able to prevent the acquisition of HIV infection by women, and two (N9 and cellulose sulphate) may have somewhat unexpectedly increased susceptibility to infection through damaging effects on the vaginal epithelium2432 which may have been caused by very frequent use of the product. In addition to the completed trials (Table), several other candidate substances have reached phase I and II testing, including another acid buffer (ACIDFORM – phase I), VivaGel (phase I), dapivirine gel or ring (antiretroviral - phase I/II), tenofovir gel (phase I/II/IIb), and Praneem polyherbal vaginal tablets (phase I and II)16.
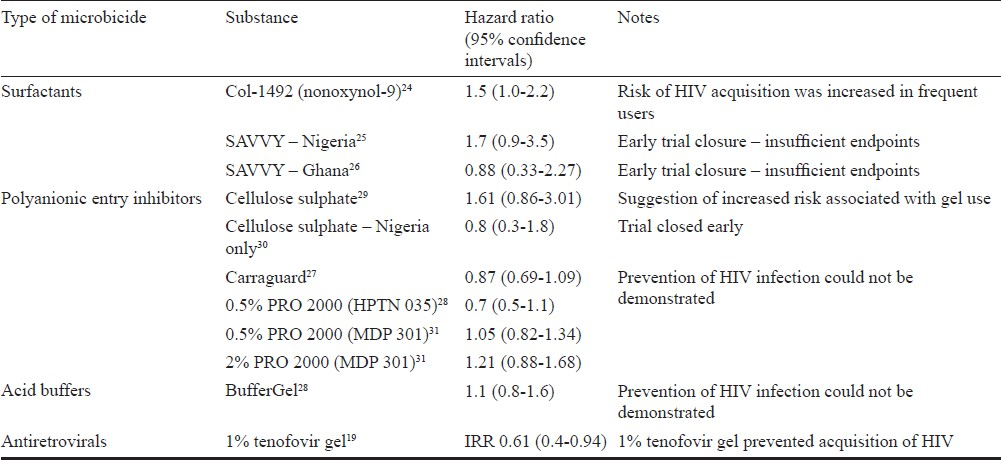
Surfactants
The surfactant products were the first to be tested, and were hypothesised to act through the non-specific disruption of the membranes of bacteria and viruses. N9, a compound previously marketed as a spermicide, was extensively studied243334, and in the most definitive testing conducted among a population of 892 sex workers in South Africa, Benin, Côte d’Ivoire, and Thailand, was not found to prevent the acquisition of HIV, with some suggestion of enhanced risk of acquisition among users of the active product24. Results of later trials conducted using another surfactant SAVVY (C31G) were compromised by lower than expected HIV incidence in the trial populations, and no firm conclusions on the ability of the product to affect the acquisition of HIV could be reached2526.
Polyanions
Among the polyanionic entry inhibitors tested, two of the three (Carraguard and PRO 2000) were found to be safe for use2728, but did not have any effect on HIV acquisition. A third member of the group - cellulose sulphate - was tested in large scale efficacy trials in Uganda, South Africa, Benin and India24, and in Nigeria30 among women considered to be at high risk for acquisition of HIV. Unfortunately, review of the safety data from the trials revealed that the investigational product may have increased the risk of acquisition of HIV35, resulting in early trial stoppage.
Carraguard is a gel product derived from carrageenan, a seaweed extract, which was tested in a phase III trial in South Africa among 6000 women. Although Carraguard was safe to use, it was not found to affect the acquisition of HIV27. However, promising data have indicated that Carraguard could have an impact on the acquisition of human papillomavirus36, the virus which causes cervical cancer3738, and that it could be used as a delivery vehicle for antiretroviral molecules such as MIV-15039.
PRO 2000 was tested in two major advanced phase clinical trials. The HIV Prevention Trials Network (HPTN) 035 trial recruited 3099 women from sites in South Africa, Zambia, Zimbabwe, Malawi and Philadelphia. Tantalisingly, the trial showed that women using 0.5 per cent PRO 2000 were 30 per cent less likely to acquire HIV than women using the placebo gel28. However, this result was not confirmed by the much larger Microbicide Development Program (MDP) 301 trial which recruited 9385 women at sites in South Africa, Zambia, Tanzania and Uganda. This trial tested two concentrations of PRO 2000 (0.5 and 2%) against placebo; the 2 per cent arm was stopped early on the advice of the data safety monitoring committee for reasons of futility. The effect of 0.5 per cent PRO 2000 on acquisition of HIV was not significantly different from that of the placebo gel in the MDP trial31.
Acid buffers
Evidence shows that microorganisms and sperm are detrimentally affected by the acidification of the surrounding medium4041, which suggests a possible mechanism for a microbicide with dual contraceptive and anti-HIV activity. Unfortunately, this hypothesis has yet to be borne out by definitive evidence (although clinical trials on acidifying microbicide substances continue), as the only such product to reach efficacy testing - BufferGel - could not be shown to affect the acquisition of HIV by women in the HPTN 035 trial28.
ARV-based microbicides
Evidence that microbicides could effectively prevent the acquisition of HIV by heterosexual women was provided by the CAPRISA (Centre for the AIDS Program of Research in South Africa) 004 trial, which tested 1 per cent tenofovir gel against placebo and found that acquisition of HIV was reduced by 39 per cent overall in women using the active product, and by 54 per cent in highly adherent users19. This exciting result heralded a new wave of research on the use of antiretrovirals for HIV prevention rather than treatment. As expected, this first proof of concept trial was not designed to be a licensure trial and will require confirmation. Two trials will contribute the additional data required for licensure. The Microbicide Trials Network (MTN) VOICE (Vaginal and Oral intervention to control the Epidemic) trial has completed enrolment of over 5000 women from sites in South Africa, Zimbabwe and Uganda and is scheduled to report results in early 2013. The trial is testing both oral tablets (tenofovir disoproxil fumarate and Truvada) and vaginal gel (1% tenofovir gel) against respective oral and gel placebos. It is hoped that should VOICE show a high level of efficacy for 1 per cent tenofovir gel, with additional safety data, then the gel can be expected to obtain US FDA approval irrespective of the dosing schedule; CAP 004 dosing was use of gel 12 h before sex and 12 h after sex with no more than two doses in 24 h (known as the BAT 24 dosing schedule). In contrast, the VOICE trial is testing a daily dosing regimen. Another trial named FACTS 001 (Follow on Consortium of Tenofovir Studies) spearheaded by South African scientists, will provide additional safety data for licensure. This study is expected to start in September 2011 at several clinical research sites in South Africa. This study will aim to confirm the BAT 24 dosing regimen. It is estimated that completion of trials and regulatory submission and approval is likely to take several years with the gel expected in the market around 2018.
Given that the field is now highly energized with the outcome of CAP 004, the focus of attention has also turned to parallel studies undertaken to understand the best mechanisms of introducing the gel to the public sector. The CAPRISA group will undertake two studies; one to ascertain the feasibility of introducing 1 per cent tenofovir gel through family planning clinics in Durban, South Africa (CAP 008) and a second (CAP 009) to determine future ARV therapeutic options for people exposed to short term ARVs in CAP 004. Similarly the MTN group is planning the MTN 018 study to assess the effectiveness of either monthly or quarterly clinic follow up among former VOICE participants receiving either oral tablets or vaginal gel. However, all these trials will not generate the safety data required for adolescent and postmenopausal use. Additional studies are planned to gather this information.
Rectal microbicides
Along with risk of vaginal HIV transmission, the prevalence of both heterosexual and homosexual anal sex is high in sub-Saharan Africa and elsewhere. HIV prevalence among men who have sex with men (MSM) in Africa ranges from 6.2 per cent in Egypt to 30.9 per cent in Cape Town42. Cross-sectional studies from Malawi, Namibia and Botswana reported that 53.7 per cent (N=537) of MSM had both male and female partners. This figure was higher in Egypt (73.3%) and 39 per cent in Uganda. Other studies have shown that prevalence of anal intercourse is high among heterosexual men and women. An anonymous survey by Kalichman et al43 of 2593 men and 1818 women in Cape Town showed that 14 per cent of men and 10 per cent of women engaged in anal intercourse. Other studies showed a prevalence of anal sex of 42 per cent among sex workers in Kwazulu-Natal44, 40.8 per cent among sex workers in Kenya45 and 12 per cent among secondary school students in Nigeria46. It is evident from these studies that the need exists for both vaginal and rectal application of microbicides.
The recognition of the importance of investigating safety of rectal application of microbicides has prompted recent research outlining appropriate pre-clinical assessments to be made in macaques47. This research has highlighted that different formulations for rectal and vaginal use may be needed, since the epithelium of the rectal compartment is somewhat more fragile and vulnerable to damage than that of the vaginal compartment. Although several pre-clinical studies have been conducted concerning the rectal application of microbicides48–52, the MTN 006 trial is the first to report on potential anti-HIV efficacy of human rectal use of a formulation of 1 per cent tenofovir gel53. In accordance with the conclusions and cautions reported by Patton et al47, the MTN 006 study concluded that rectal use of the 1 per cent tenofovir gel was neither completely safe nor acceptable, and that reformulation for rectal use will be necessary. The study also indicated that daily use of the gel inhibited ex vivo HIV infection of tissue samples. Although human rectal safety and acceptability studies were conducted with UC781 gel54–56, with indications that the gel was safe and acceptable, further development of the product ceased in 2010 following formulation difficulties57, leaving tenofovir gel as the foremost potential rectal microbicide in clinical trials58.
Acceptability of microbicides
The impetus for the development of a woman-controlled HIV preventive method has primarily been the lack of viable options which women could apply and control themselves, without co-operation of a male partner. However, this concept may be difficult to incorporate into the daily lives of many of the populations of women at high risk of infection. Notably, acceptability of microbicides among women varies widely between countries – African women have indicated that a microbicide would be a highly desirable product, while acceptability among Indian women was considerably lower, perhaps due to perceptions of lower or non-existent risk59. Studies have indicated a range of degrees of disclosure of microbicide use to male partners, from full disclosure to covert use5960. The degree of disclosure is potentially linked to culturally-specific norms regulating women's sexual behaviour. For example, one study found that married Indian women were fearful of using a microbicide without their husband's consent61, and another reported that domestic violence and community gender norms have a significant negative impact on women's ability to adopt HIV protective measures62–64. African women have reported that the discovery of undisclosed use by their partners or husbands could put them at risk of violence or other forms of retribution, and that they therefore, would prefer to disclose use, but may frame the microbicide as a “hygiene” product without relating it to prevention of HIV59. Microbicides may both challenge existing sexual norms and thereby empower women to control their own health, or could be incorporated into existing patriarchal power structures without changing the status quo65 (if men demand control over microbicide use by their female partners, or if covert use becomes the dominant usage context). Key informant narratives reveal that women are frequently expected to engage in various vaginal practices for the benefit of their male partners, but not themselves, emphasising the lack of bodily autonomy that many women experience66. Montgomery et al65 suggested that the introduction of microbicides would result in “routinisation” of the products and their eventual incorporation into pre-existing practices and systems65. Acceptability of microbicide use will also be influenced by many intersecting factors such as country-setting, ethnic group, educational level, gender, age and socio-economic group6667. Much of the past research on microbicide acceptability has focussed on the properties of the gels alone, but Tanner68 suggested a renewed focus on researching acceptability as it relates to relational and contextual factors, which may provide more accurate estimates of likelihood of use.
Oral pre-exposure prophylaxis (PrEP)
In November 2010, the results of the iPrEx trial were released, which indicated that oral dosage with the antiretroviral combination tablet emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) was effective in preventing the acquisition of HIV in men and transgender women who have sex with men – an overall reduction in infection of 44 per cent was recorded among participants in the active arm21. This was followed in mid-2011 by the release of the results of three more trials of oral antiretroviral tablets for HIV prevention – the Partners PrEP study, the TDF2 study, and the HPTN 052 study.
In the Partners PrEP study 4758 serodiscordant couples were recruited at sites in Kenya and Uganda; the HIV-negative partners were randomised to receive TDF, or the FTC/TDF combination tablet. Those who received TDF had 62 per cent fewer incidents of HIV infection compared to those receiving placebo tablets, while those receiving FTC/TDF had 73 per cent fewer infections22.
The TDF2 study randomised 1219 HIV-negative Botswanan men and women to receive either FTC/TDF tablets or placebo tablets. Overall, the risk of infection among participants randomised to the active drug was reduced by 63 per cent, while in participants who were known to have a supply of study drugs, the risk of infection was reduced by 78 per cent23.
The HPTN 052 study differed somewhat from those described above, in that it focussed on prevention of transmission, rather than acquisition of HIV. One thousand seven hundred and sixty three HIV-serodiscordant couples were enrolled in HPTN 052 at 13 sites across Africa, Asia and the Americas. The HIV-positive partner was required to have a CD4 count of between 350 and 550 cells/μl at enrolment and, therefore, did not immediately require antiretroviral treatment. The participants were divided into two groups – one of which initiated antiretroviral therapy immediately, and in another therapy was delayed until the infected partner's CD4 count dropped below 250 cells/μl or they developed an AIDS-related illness. Immediate initiation of therapy by the HIV-positive partner reduced transmission of HIV to the negative partner by an astonishing 96 per cent20.
Combination prevention
The tool box for HIV prevention seems more robust now than ever with the addition of numerous partially effective methods such as male circumcision, oral ARV-based prevention, ARV-based vaginal microbicides, clean needle exchange programmes, voluntary counselling and testing, a positive signal for an HIV vaccine, male and female condoms, and STI prevention. It is evident that a combination of interventions will have to be packaged according to an individual's risk profile. Individuals who engage in high risk behaviour such as multiple partnerships, anal intercourse or who have an HIV discordant partner for example, will require a package of highly effective interventions including ARVs. It should be possible to tailor packages for young single women and men, married couples in which one of the partners is a migrant worker, MSM, intravenous drug users and men and women engaging in heterosexual anal sex.
Challenges and future directions
In addition to decisions relevant to methods of supply to the population at large, the implications of cost of PrEP for the countries most affected by the HIV epidemic is an important factor. The current leading oral PrEP option – FTC/TDF – could cost as much as $1000 per person per month69, a burden that would prove untenable in countries struggling to supply drugs to millions of people for HIV treatment alone. New licensing and manufacturing agreements may be necessary to improve the availability and supply of drugs for PrEP. In addition, the mode of delivery of microbicide gels remains to be refined, since the gel applicator forms a substantial component of the cost of the microbicide mode of PrEP70.
ARV resistance
A frequent concern relating to ARV-based prevention methods is the possible development and transmission of resistance in persons who use such methods, and are unlucky enough to contract HIV while on treatment. Current data from completed microbicide clinical trials are possibly insufficient for conclusions to be drawn on this phenomenon in microbicides; neither tenofovir-related, nor thymidine-related mutations were detected in women who seroconverted during CAPRISA 00419. Data from the MTN 001 study showed that vaginal dosing with tenofovir gel resulted in high concentrations of the active ingredient in vaginal tissue, but that oral dosing with TDF tablets produced far lower vaginal tissue drug concentrations. Drug concentrations in blood were far higher with oral dosing than vaginal dosing71. These data indicate that the risk of development of viral resistance could potentially be reduced by vaginal gel dosing rather than oral tablet dosing since translocation of the active ingredient is limited. Further data informing the debate concerning viral resistance will be contributed by the VOICE trial. The FACTS 001 phase III trial19 testing the safety and effectiveness of 1 per cent tenofovir gel will also provide additional information on the potential development of resistance and support for the licensure of this promising product. Utilisation of microbicides with two or more active ingredients which target different aspects of the virus life-cycle could also help to prevent the development of resistance72.
Product adherence
Adherence to product use could be a critical factor influencing both the real-world effectiveness of any microbicide product and the development of viral resistance in infected users. In the clinical trial environment participants receive intensive safe-sex counselling, supplies of products and condoms and other support, which may artificially increase both the actual and reported use of the investigational product (trial participants may feel compelled to inflate reports of product use due to social desirability bias). Reported adherence to microbicide use during clinical trials has been found to vary quite widely, with a range from 45 to 95 per cent of unprotected sex acts covered by microbicides16. Both intermittent (coitally dependent; e.g. BAT24) and daily microbicide dosing approaches are or have been investigated. While it is still too early to determine the comparative success of the two gel dosing strategies, other microbicide formulations being developed may reduce the dosing burden upon users. Foremost among these are the intravaginal rings (IVR)– flexible silicone or thermoplastic elastomer devices which are inserted into the vagina and remain in situ for a period of time while slowly releasing the active drug ingredient73. Similar devices have been in use for some time as delivery vehicles for hormone replacement therapy and contraceptives. The principle IVR currently undergoing testing contains dapivirine7475, a non-nucleoside antiretroviral, while IVRs containing tenofovir are also under development76. Dapivirine trials are expected to commence in 2012.
Many women in need of HIV prevention methods are aged between 18 and 30 yr77. These women are of reproductive age and may want to have children. ARV-based microbicides specifically target HIV, are not effective in preventing other sexually transmitted infections and are not spermicidal. Ideally one would like to have products available that have both contraceptive and non-contraceptive properties. Providing women with a range of choices is likely to have a substantial impact on acceptability and adherence.
Although some research has been ongoing into the possible delivery of PrEP via injectables, no trials have yet reported results on this possibility. The TMC278LA trial of the long acting injectable HIV drug rilpivirine conducted by St Stephen's AIDS Trust in the United Kingdom was terminated prior to completion on the basis of safety information78.
Ethical issues
Many ethical concerns will arise when ARV-based drugs currently used in treatment for HIV are provided to HIV negative individuals at high risk of contracting the virus. Many countries where these drugs will be most useful for HIV prevention are the same countries where the demand for treatment for HIV positive people is greatest. Widespread use of ARV-based prevention options will require regular HIV testing and safety monitoring (liver and renal parameters)79. Health systems strengthening together with trained human resources will be key to the success of these options.
Conclusion
Recent advances in the use of antiretrovirals for prevention of HIV have renewed the hope that effective methods will soon be available to the wider population. Although several recent successes have been achieved with the use of oral formulations, topical microbicides and other vaginally applied formulations will hopefully also become a component of the HIV prevention tool-kit soon. The development of a wider range of delivery methods and formulations with multiple activity (dual contraceptive and anti-HIV activity for example) will provide women with an expanded range of mechanisms for ensuring their own health and that of their families.
References
- UNAIDS. In: Report on the global AIDS epidemic 2010. Geneva: Joint United Nations Programme on HIV/AIDS; 2010.
- [Google Scholar]
- HIV prevention: The need for methods women can use. Am J Public Health. 1990;80:460-2.
- [Google Scholar]
- The value of site preparedness studies for future implementation of phase 2/IIb/III HIV prevention trials - Experience from the HPTN 055 study. J Acquir Immune Defic Syndr. 2008;47:93-100.
- [Google Scholar]
- Violence, rape, and sexual coercion: everyday love in a South African township. Gend Dev. 1997;5:41-6.
- [Google Scholar]
- Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev (1):CD003255.
- [Google Scholar]
- The effectiveness of condoms in reducing heterosexual transmission of HIV. Fam Plann Perspect. 1999;31:272-9.
- [Google Scholar]
- Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 trial. PLoS Med. 2005;2(11):e298.
- [Google Scholar]
- Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657-66.
- [Google Scholar]
- Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643-56.
- [Google Scholar]
- Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654-65.
- [Google Scholar]
- Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881-93.
- [Google Scholar]
- Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11:507-15.
- [Google Scholar]
- Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661-71.
- [Google Scholar]
- The last decade of microbicide clinical trials in Africa: from hypothesis to facts. AIDS. 2010;24(Suppl 4):S40-S9.
- [Google Scholar]
- MIRA Tecem. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: A randomised controlled trial. Lancet. 2007;370:251-61.
- [Google Scholar]
- MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209-20.
- [Google Scholar]
- Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168-74.
- [Google Scholar]
- Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- [Google Scholar]
- Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-99.
- [Google Scholar]
- University of Washington. Pivotal study finds that HIV medications are highly effective as prophylaxis against HIV infection in men and women in Africa. University of Washington, International Clinical Research Center. 2011. Available from: http://depts.washington.edu/uwicrc/research/studies/files/PrEP_PressRelease-UW_13Jul2011.pdf
- [Google Scholar]
- CDC. CDC trial and another major study find PrEP can reduce risk of HIV infection among heterosexuals 2011. 2011. Available from: http://www.cdc.gov/nchhstp/newsroom/PrEPHeterosexuals.html
- [Google Scholar]
- Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971-7.
- [Google Scholar]
- SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One. 2008;3(1):e1471.
- [Google Scholar]
- SAVVY (C31G) gel for prevention of HIV infection in women: a Phase 3, double-blind, randomized, placebo-controlled trial in Ghana. PLoS One. 2007;2(12):e1312.
- [Google Scholar]
- Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977-87.
- [Google Scholar]
- Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25:957-66.
- [Google Scholar]
- Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463-72.
- [Google Scholar]
- Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: Results of a phase III trial in Nigeria. PLoS One. 2008;3(11):e3784.
- [Google Scholar]
- PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): A phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376:1329-37.
- [Google Scholar]
- Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J Infect Dis. 2009;200:599-608.
- [Google Scholar]
- Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477-82.
- [Google Scholar]
- Evaluation of a low-dose nonoxynol-9 gel for the prevention of sexually transmitted diseases: A randomized clinical trial. Sex Transm Dis. 2001;28:394-400.
- [Google Scholar]
- CONRAD. 2007. Phase III trials of cellulose sulfate microbicide for HIV prevention closed. Press release [online] issued 2007 January 31. Available from: http://www.conrad.org/news-pressreleases-10.html/
- [Google Scholar]
- Carraguard, a vaginal microbicide, protects women against HPV infection. In: Proceedings of the 26th International Papillomavirus Conference & Clinical and Public Health Workshops. 2010.
- [Google Scholar]
- Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365-71.
- [Google Scholar]
- Relation of human papilloma virus status to cervical lesions and consequences for cervical-cancer screening: A prospective study. Lancet. 1999;354:20-5.
- [Google Scholar]
- Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex Transm Dis. 2007;34:9-14.
- [Google Scholar]
- Acid sensitivity of cell-free and cell-associated HIV-1: Clinical implications. AIDS Res Hum Retroviruses. 1990;6:1433-6.
- [Google Scholar]
- The rate at which human sperm are immobilized and killed by mild acidity. Fertil Steril. 2000;73:687-93.
- [Google Scholar]
- The global epidemic of HIV infection among men who have sex with men. Curr Opin HIV AIDS. 2009;4:300-7.
- [Google Scholar]
- Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex Transm Infect. 2009;85:411-5.
- [Google Scholar]
- Prevalence of HIV among truck drivers visiting sex workers in KwaZulu-Natal, South Africa. Sex Transm Dis. 2002;29:44-9.
- [Google Scholar]
- Anal and dry sex in commercial sex work, and relation to risk for sexually transmitted infections and HIV in Meru, Kenya. Sex Transm Infect. 2006;82:392-6.
- [Google Scholar]
- Sexual behavior and risk of HIV/AIDS among adolescents in public secondary schools in Osogbo, Osun State, Nigeria. Int J Adolesc Med Health. 2009;21:387-94.
- [Google Scholar]
- A summary of preclinical topical microbicide rectal safety and efficacy evaluations in a pigtailed macaque model. Sex Transm Dis. 2009;36:350-6.
- [Google Scholar]
- Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob Agents Chemother. 2009;53:1797-807.
- [Google Scholar]
- The pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Proceedings of the 26th Microbicides 2010 Conference 2010 May 22-25
- [Google Scholar]
- Testing rectal safety of tenofovir in the macaque model. In: Proceedings of the Microbicides Conference 2010. 2010.
- [Google Scholar]
- Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008;5:e157.
- [Google Scholar]
- Vaginal and rectal topical microbicide development: Safety and efficacy of 1.0% Savvy (C31G) in the pigtailed macaque. Sex Transm Dis. 2006;33:691-5.
- [Google Scholar]
- RMP-02/MTN-006: A phase 1 placebo-controlled trial of rectally applied 1% vaginal TFV gel with comparison to oral TDF. In: Proceedings of the 18th Conference on Retroviruses and Opportunistic Infections. 2011. 27 February-2 March
- [Google Scholar]
- Acceptability of UC781 gel as a rectal microbicide among HIV-uninfected women and men. AIDS Behav. 2010;14:618-28.
- [Google Scholar]
- A Phase 1 rectal safety and acceptability study of UC781 microbicide gel Proceedings of the 16th Conference on Retroviruses and Opportunistic Infections. 2009 February 8-11
- [Google Scholar]
- Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob Agents Chemother. 2007;51:1608-15.
- [Google Scholar]
- CONRAD. Clinical Trials. 2011. Available from: http://www.conrad.org/microbicides-trials.html
- [Google Scholar]
- AVAC. Microbicide and PrEP candidates in ongoing clinical trials. Available from: http://www.avac.org/ht/a/GetDocumentAction/i/3109
- [Google Scholar]
- Acceptability of a microbicide among women and their partners in a 4-country phase I trial. Am J Public Health. 2004;94:1159-64.
- [Google Scholar]
- Covert use, vaginal lubrication, and sexual pleasure: A qualitative study of urban U.S. women in a vaginal microbicide clinical trial. Arch Sex Behav. 2010;39:748-60.
- [Google Scholar]
- Acceptability of vaginal and rectal products in India: Implications for the development of a microbicide. In: Proceedings of Second International AIDS Society Conference on HIV Pathogenesis and Treatment. 2003.
- [Google Scholar]
- When HIV-prevention messages and gender norms clash: the impact of domestic violence on women's HIV risk in slums of Chennai, India. AIDS Behav. 2003;7:263-72.
- [Google Scholar]
- Acceptability and adherence of a candidate microbicide gel among high-risk women in Africa and India. Cult Health Sex. 2010;12:739-54.
- [Google Scholar]
- Re-framing microbicide acceptability: Findings from the MDP301 trial. Cult Health Sex. 2010;12:649-62.
- [Google Scholar]
- Preferences and practices related to vaginal lubrication: Implications for microbicide acceptability and clinical testing. J Womens Health (Larchmt). 2005;14:424-33.
- [Google Scholar]
- Factors affecting acceptability and adherence of a candidate microbicide gel among high-risk women in Africa and India. In: Proceedings of the Microbicides 2010. 2010.
- [Google Scholar]
- Perceptions of acceptability and utility of microbicides in Ghana, West Africa: A qualitative, exploratory study. Sahara J. 2008;5:11-8.
- [Google Scholar]
- Gilead HIV breakthrough of the year stymied by $12,000 cost, side effects. 2011. Available from: http://www.bloomberg.com/news/2011-02-28/gileads-12-000-a-year-hiv-prevention-pill-fails-to-win-physiciansupport.html
- [Google Scholar]
- Microbicide applicator: Overview and regulatory issues: WHO/ICMR/CONRAD/IPM Regulatory Issues in Microbicide Research. 2007.
- [Google Scholar]
- MTN. Study comparing tenofovir gel and oral tablet finds gel provides more drug to tissue: Preferences for HIV prevention products differ among U.S. and African women. 2011. Available from: http://www.mtnstopshiv.org/node/2929
- [Google Scholar]
- Is HIV drug resistance a limiting factor in the development of anti-HIV NNRTI and NRTI-based vaginal microbicide strategies? Antiviral Res. 2006;71:343-50.
- [Google Scholar]
- Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25:483-8.
- [Google Scholar]
- Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416-23.
- [Google Scholar]
- CONRAD. Dual-Protection Technologies. 2011. Available from: http://www.conrad.org/research-technologies.html
- [Google Scholar]
- South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenagers?. Cape Town: HSRC Press; 2009.
- ClinicalTrials.gov. Pre-exposure prophylaxis using TMC278LA. 2011. Available from: http://clinicaltrials.gov/show/NCT01049932?order=2121
- [Google Scholar]
- Pre-exposure prophylaxis for HIV infection: What if it works? Lancet. 2007;370:89-93.
- [Google Scholar]






