Translate this page into:
Mechanism of intestinal folate transport during folate deficiency in rodent model
Reprint requests: Dr Jyotdeep Kaur, Department of Biochemistry, Postgraduate Institute of Medical Education & Research, Chandigarh 160 012, India e-mail: jyotdeep2001@yahoo.co.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Folate deficiency is a public health problem and is the most notable for its association with neural tube defect in developing embryo, megaloblastic anaemia, cancers and cardiovascular diseases. The mechanisms of the intestinal folate uptake process have been earlier characterized. However, much less is known about regulation. In this study we evaluated the mechanistic insights of folate absorption in an in vivo model of folate deficiency.
Methods:
Male Wistar rats were fed folate-containing diet (2 mg/kg folic acid) or a folic acid-free diet over 3 months and folate transport was studied in intestinal brush border membrane vesicles (BBMV).
Results:
The characterization of the folate transport system in intestinal brush border membrane (BBM) suggested it to be a carrier mediated, acidic pH stimulated, and Na+ independent. Folate deficiency increased the folate transport by altering the Vmax without changing the Km of folate transport process. The increased transport efficiency of the BBM was associated with upregulation of folate transporters at both mRNA and protein level.
Interpretation & conclusions:
Folate deficiency resulted in significant upregulation of intestinal folate uptake, by increasing number of transporters without any change in specificity of transporters towards its substrate. The observed upregulation was associated with significant increase in reduced folate carrier (RFC) and proton coupled folate transporter (PCFT) expressions, suggesting the transcriptional and translational regulation of folate uptake during folate deficiency.
Keywords
Folate deficiency
folate transport
methylation
PCFT
RFC
Folic acid is required for the purine and pyrimidine nucleotides synthesis, for the metabolism of several amino acids including homocysteine and for methylation of biological molecules1–3. Folate deficiency is a public health problem that is the most no in its association with neural tube defect in developing embryo4, megaloblastic anaemia, cancers and cardiovascular disease1. Since mammals cannot synthesize folate de novo, they must obtain these derivatives from the outside environment, necessitating an efficient intestinal absorptive mechanism. The intestine is exposed to folate from two sources: dietary source, where absorption of vitamin occurs in small intestine, and a large intestinal bacterial source, where the vitamin is synthesized by the normal microflora and absorbed by the large intestine56. The folate uptake shows the characteristics of a carrier mediated process with low pH optimum that operates efficiently within the acidic microclimate of intestinal surfaces, duodenum and upper jejunum7–9. Several transport systems for the folate uptake have been described. Besides folate uptake, these transporters act as important determinants for the chemotherapeutic potential of various antifolates. Among different transporters, the most well characterized folate transporters are proton coupled folate transporter (PCFT) and the reduced folate carrier (RFC) which mediate the uptake of folate at acidic and alkaline/neutral pH optima respectively and are ubiquitously expressed579–11. Folate deficiency might affect gene expression by disrupting DNA methylation, thereby involving in pathogenesis of various diseases. Thus studying the mechanistic aspects of folate regulation under folate deficient conditions is of nutritional, phsiological and clinical importance. Previous studies suggested that moderate folate deficiency altered folate uptake and induced changes in adenomatous polypopses coli (WNT/APC) pathway, a possible link between folate status and cancer12 and it also decreased the affinity of folate recepter (FR) or folate binding protein (FBP) in tumour tissues of mice13. Studies done on rodent model of folate deficiency suggested upregulated mRNA expression of RFC and PCFT in intestine and that the increase in mRNA expression of RFC under these conditions involves reduced folate carrier promoter B (RFCpB)14–16. However, little is knowm regarding the activity of folate transporters at membrane surfaces and regulation of folate transporters at protein level under folate deficient conditions and whether there is any change in expression profile of folate transporters with the maturation of intestinal crypt cells to villus tip. Moreover, the association of decreased folate levels with S-adenosyl methionine (SAM): S-adenosyl homocysteine (SAH) ratio and genomic DNA hypomethylation may lead to a possible link of folate deficiency with cancer. The present study was therefore, carried out to delineate the mechanism of intestinal folate uptake in vivo model of folate deficiency and its association with SAM levels and genomic DNA methylation.
Material & Methods
Chemicals: Radiolabelled [3’, 5’, 7, 9-3H]-folic acid, potassium salt with specific activity 24.0 Ci/mmol and S-adenosyl-[methyl- 3H]-methionine with specific activity70.0 Ci/mmol were purchased from Amersham Pharmacia Biotech (Hong Kong). Prokaryotic CpG DNA methyl transferase was procured from the New England Biolabs, USA. Moloney Murine Leukemia Virus reverse transcriptase (RevertAid™ M-MuLV RT) kit was purchased from the MBI Fermentas, Life Sciences, USA. RNAlater (RNA stabilization solution) and diethylpyrocarbonate were obtained from Ambion, Inc. Austin, USA. Color Burst electrophoresis marker, methotrexate, bovine serum albumin and DL-dithiothreitol (DTT) or Cleland's reagent were purchased from Sigma Aldrich Co, USA. Cellulose nitrate membrane filters (0.45 μm) were obtained from Millipore Corporation (Bedford, MA, USA). Peptides were obtained from USV Limited, Mumbai India).
Animals: The study was performed in the department of Biochemistry, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India. Weaning male albino rats (Wistar strain) weighing 40-50 g were obtained from central animal house of the institute. These were housed in polypropylene cages in the departmental animal house under hygienic conditions at controlled temperature (23 ± 1°C) and humidity (44-55%). The rats were randomized into two groups of eight animals each. Folate deficiency was produced in a group of rats by feeding them a folate-deficient diet and 10 g succinylsulphathiazole per kg diet for three months. Inclusion of succinylsulphathiazole facilitates induction of severe folate deficiency by eradicating intestinal microflora. Animals in control group were pair fed and received a control diet with folic acid (2 mg/kg diet) and did not get succinylsulphathiazole as described earlier17. Blood (1 ml) was drawn from tail vein of the rats every 4 wk. After 3 months of dietary treatment, the animals were sacrificed under pentothal anaesthesia. Starting from the ligament of Trietz, the entire intestine was removed and thoroughly washed with ice-cold saline. The protocol was approved by the institute's ethics committee on animal experiments.
Determination of serum folate, and SAM and SAH levels in intestine: Folate concentration was assayed microbiologically using Lactobacillus casei as described earlier8. The intestinal SAM and SAH levels were determined by the method of Wagner et al18.
Isolation of intestinal epithelial cells: The intestinal epithelial cells were isolated following the method of Weiser et al19.
Preparation of brush border membrane vesicles (BBMV) from isolated intestinal epithelial cells: BBMV were prepared from the isolated total intestinal cells from control and folate-deficient diet fed rats at 4°C20 with some modifications21. The final pellet containing cells was homogenized by adding 2 mM Tris-50 mM mannitol buffer and 10mM MgCl2 was added to the homogenate followed by intermittent shaking for 10 min. The contents were centrifuged at 3,000xg for 15 min and the supernatant was then run at 27,000xg for 30 min. The pellet thus obtained, was suspended in small amount of loading buffer containing 280 mM mannitol, 20 mM HEPES-Tris, pH 7.4 and centrifuged at 27,000xg for 30 min. The final pellet obtained was suspended in loading buffer so as to obtain protein concentration of approximately 5 mg/ml. These BBMV were used to study [3H]-folic acid uptake.
BBMV were also isolated from the cells representing villus tip, mid villus and crypt base isolated from the rat intestine. The respective cell fractions from two animals were pooled for this purpose to get sufficient BBMV. These BBMV were used to determine [3H]-folic acid uptake across the crypt villus axis and to analyse the RFC and PCFT protein levels in different cell types.
Transport of [3H]-folic acid: Uptake studies were performed at 37°C using the incubation buffer of 100 mM NaCl, 80 mM mannitol, 10 mM HEPES, 10 mM 2-morpholinoethanesulphonic acid (MES), pH 5.5 and 0.5 μM of [3H]-folic acid unless otherwise mentioned. Ten μl of isolated BBMV (50 μg protein) from control and folate-deficient diet fed rats for different specific assays were added to incubation buffer containing [3H]-folic acid of known concentration. Reaction was stopped by adding ice-cold stop solution containing 280 mM mannitol, 20 mM HEPES-Tris, pH 7.4 followed by rapid vacuum filtration. Non-specific binding to the filters was determined by residual filter counts after filtration of the incubation buffer and labelled substrate without vesicles22. The radioactivity retained by the filters was determined by liquid scintillation counting (Beckman Coulter LS 6500). For the determination of pH optima for folate uptake, transport of 0.5 μM [3H]-folic acid was measured in the incubation buffer [100mM NaCl, 80 mM mannitol, 10 mM HEPES, 10 mM 2-morpholinoethanesulfonic acid (MES/Tris)] pH 4.5 to 8.0, prepared by varying concentration of MES or Tris and for determination of kinetic constants Km and Vmax, transport of [3H]-folic acid was measured by varying the concentration of [3H]-folic acid from 0.125 to 4.0 μM in the incubation buffer of pH 5.5.
Reverse transcriptase-PCR analysis: Total RNA from all animals was isolated from the upper 1cm of jejunal tissues or cells representing villus-tip, mid villus and crypt base cells by using RNA isolation Kit (Taurus Scientific, USA). cDNA synthesis was carried out from the purified and intact total RNA according to manufacturer's instructions. Expression of reduced folate carrier (RFC), proton coupled folate receptor (PCFT), glycereldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin was evaluated using sequence specific primers corresponding to the sequence in the open reading frame. Twenty-μL PCR mixture was prepared in 1x PCR buffer consisting of 0.6U of Taq polymerase, 1 μM of primer for β-actin, RFC and PCFT along with 200 μM of each dNTP. In optimized PCR, the initial denaturation step was carried out for 2 min at 95°C. The denaturation, annealing, and elongation steps were carried out respectively for 1 min at 94°C, 45 sec at 64°C (PCFT) or 56°C (GAPDH) or 60°C (β-actin) and 1 min at 72°C for 35 cycles. In case of RFC denaturation, annealing and elongation steps were carried out respectively for 30 sec at 94°C, 30 sec at 52.1°C, 30 sec at72°C for 35 cycles. The final extension step was carried out for 10 min at 72°C. The primers were designed using Primer3 Input (version 0.4.0) (http://frodo.wi.mit.edu/,USA). The sequences of the primers used were as follows: 5’-CATGCTAAGCGAACTGGTGA-3’(sense) and 5’-TTTCCACAGGACATGGACA-3’ (antisense) for RFC, AAGCCAGTTATGGGCAACAC (sense) and GGATAGGCTGTGGTCAAGGA (antisense) for PCFT, 5’CACTGTGCCCATCTATGAGGG3’(sense) and 5’TCCACATCTGCTGGAAGGTGG3’ (antisense) for β-actin and CCTTCATTGACCTCAACTACAT (sense) and CCAAAGTTGTCATGGATGACC (antisense) for GAPDH. The expected PCR products of size 120, 300, 588, and 400 bp were obtained for rRFC, rPCFT, rβ-actin, and rGAPDH respectively when electrophoresed on 1.2% agarose gel. The densitometric analyses of products were determined by using ‘Scion image’ software (www.scioncorp.com, USA).
Western blot analysis: For protein expression studies, BBMV (100-150 μg) isolated from epithelial cell were resolved on 10 per cent SDS-PAGE and transferred to nitrocellulose membrane for 20 min at 25V. Western blotting was performed using the procedure described by Towbin et al24 using polyclonal primary antibodies as rabbit anti-rat RFC (1:500 dilutions) raised against specific region of rat RFC synthetic peptide corresponding to amino acids 495-512. The polyclonal antibodies against PCFT were raised against a peptide ADPHLEFQQFPQSP corresponding to amino acids 446-449 of this protein, conjugated to KLH. This synthetic peptide was used for immunization in two rabbits. For β-actin (loading control) the blots were probed with polyclonal antibodies (Santa Cruz biotechnology, Santa Cruz, CA). Immunodetection was performed with goat anti-rabbit and rabit-antimouse immunoglobin G conjugated to horseradish peroxidase (HRP, 1:2,000 dilutions) with the use of metal enhanced DAB system (Thermo Scientific, USA). The specific bands were quantified by using ‘Scion image’ software.
Genomic DNA methylation studies: DNA isolation was performed by the conventional method25 using a lysis buffer containing proteinase K. The methylation status of CpG sites in genomic DNA was determined by the in vitro methyl acceptance capacity of DNA using [3H-methyl]-AdoMet as a methyl donor and a prokaryotic CpG DNA methyltransferase26.
Statistical analysis: Each uptake assay was performed three times with eight independent preparations from each group. Group means were compared by using the Student's t-test, and analysis of variance was used to measure significance levels on data obtained from experiments involving folate uptake as a function time, pH and substrate concentration.
Results
The membrane vesicle preparations were evaluated for their purity by biochemical and functional criteria. The specific activity of alkaline phosphatase and sodium-potassium-adenosine triphosphatase (Na+, K+-ATPase) were studied for checking the purity of BBMV. A 12-15 fold increase in alkaline phosphatase activity was observed in the isolated BBMV with minimum activity of Na+, K+-ATPase in comparison to the respective homogenates.
The body weight of rats fed folate deficient diet were found to be significantly (P<.05) lower than the body weight of pair fed controls, which were 228 ± 15 and 187.8 ± 19 (g) in control and folate different diet fed rats respectively. There was a significant decrease (P<0.01) in serum folate levels (Fig. 1), which decreased progressively during the treatment in the folate deficient diet fed rats compared to controls. The serum folate levels at the time of sacrifice were 102 ± 12 and 8.5 ± 3 (μg/l) in control and folate different diet fed rats respectively, indicating a severe folate deficient status of rats fed folate deficient diet.
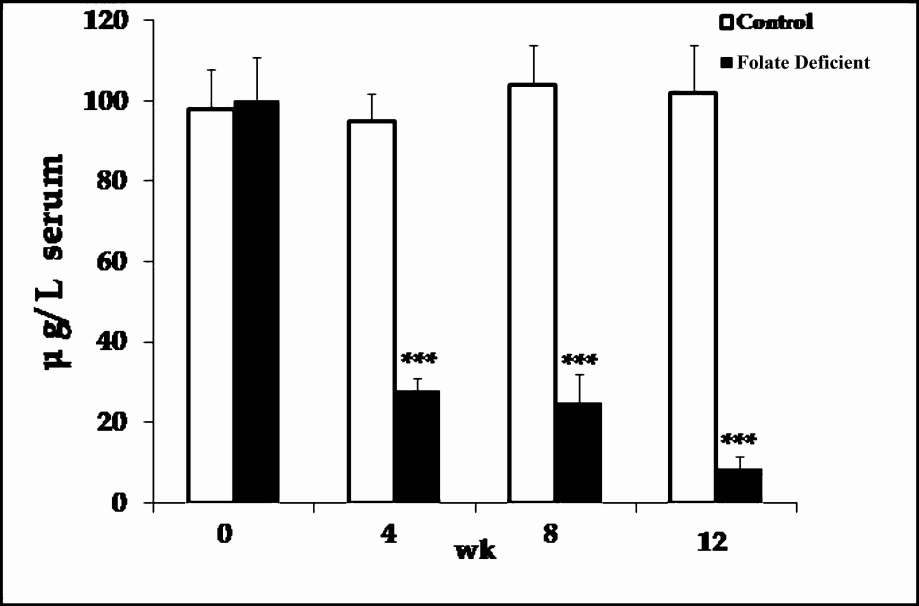
- Serum folate levels of control and folate deficient diet fed rats as a function of time in weeks. Results are presented as mean ± SD of three experiments. ***P<0.01 vs. control.
Effect on folic acid deficiency on folate uptake across intestinal BBM: The time course of folate uptake at various time intervals in an acidic medium (pH 5.5) revealed that uptake in intestinal brush border membrane was maximum at 30 sec time interval in both the groups of rats, with higher uptake at all time intervals in rats fed folate deficient diet (Fig. 2). The pH gradient created by varying extracellular pH (4.5- 8) showed that transmembrane pH served driving force for folate transport. With the change in extracellular pH from 8 to 4.5, keeping intracellular pH constant at 7.4, an increase in folic acid uptake was observed, with maximum uptake at pH 5.5 in both the groups of rats and the uptake was (13-49%) higher in BBMV prepared from folate deficient diet fed rats as compared to controls (Fig. 3). To delineate the mechanisms involved in upregulation of folic acid uptake during folate deficiency, the kinetic constant Km and Vmax of folate uptake were measured by changing extracellular substrate concentration from 0.125 to 4 μM. The initial velocity determined at 30 sec and at pH 5.5 showed that in both groups the saturation phenomenon with a plateau at 1.5 μM of substrate concentration was indicative of Michaelis-Menten kinetics (Fig. 4a), indicating that carrier-mediated transport was responsible for the folic acid transport. At different concentrations of folic acid, there was 23- 28 per cent (P<0.001) more folic acid uptake in intestine of rats fed folate deficient diet as compared to control. The increase in transport was associated with increase in Vmax (132 ± 1.6 in control vs. 222 ± 0.9 pmol/30 sec/mg protein in folate deficient rats) (P<0.001) of folate uptake process, without any significant change in Km (2.5±0.08 in 2.6±0.1 μM in folate deficient rats, respectively), suggesting that there was not any change in affinity of transporters towards its substrate. Moreover, when studied the uptake across crypt villus axis, the uptake was found to be maximum at villus tip followed by mid villus and then crypt base (Fig. 4b).
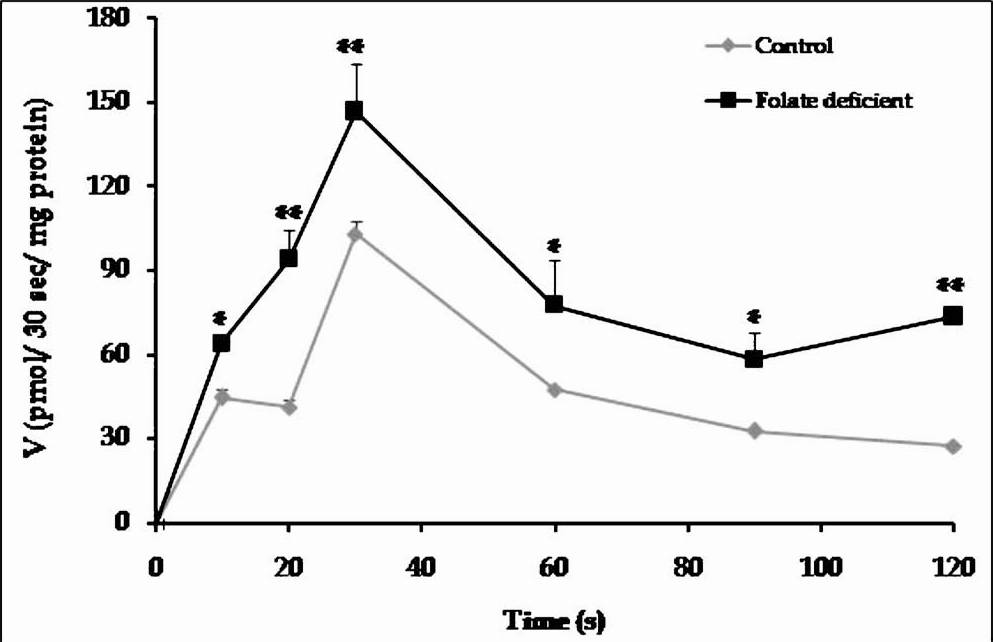
- Time course of folate uptake in the intestinal brush border membrane vesicles (BBMV). Each point represents the mean ± SD of four determinations. P*<0.05, **<0.01 vs. control.
![Uptake of [3H]-folic acid in the intestinal brush border membrane (BBM) as a function of pH optimum. Each data point is mean ± SD of 4 separate uptake determinations. P*<0.05, **<0.01, ***<0.001 vs. control.](/content/175/2012/136/5/img/IJMR-136-758-g003.png)
- Uptake of [3H]-folic acid in the intestinal brush border membrane (BBM) as a function of pH optimum. Each data point is mean ± SD of 4 separate uptake determinations. P*<0.05, **<0.01, ***<0.001 vs. control.
![(a) Uptake of [3H]-folic acid in the intestinal BBM as a function of substrate concentration. (b) Uptake of [3H]-folic acid in the intestinal BBM along crypt-villus axis. Each data point is mean± SD of 4 separate uptake determinations. CB-crypt base; MV-midvillus and VT- villus tip. P*<0.05, **<0.0l, ***<0.001 vs. control and each value is mean ± SD of four separate uptake determinations. *, ***P<0.001 vs. crypt base, ###P<... vs. mid-villus.](/content/175/2012/136/5/img/IJMR-136-758-g004.png)
-
(a) Uptake of [3H]-folic acid in the intestinal BBM as a function of substrate concentration. (b) Uptake of [3H]-folic acid in the intestinal BBM along crypt-villus axis. Each data point is mean± SD of 4 separate uptake determinations. CB-crypt base; MV-midvillus and VT- villus tip. P*<0.05, **<0.0l, ***<0.001 vs. control and each value is mean ± SD of four separate uptake determinations. *, ***P<0.001 vs. crypt base, ###P<... vs. mid-villus.
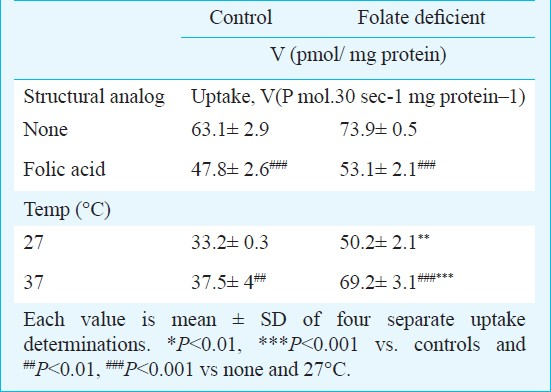
To determine the structural specificities of folate transport system (Table I), the inhibitory effects of unlabelled folic acid, at a concentration of 1 μM was determined on uptake of 0.5 μM folic acid into brush border membrane vesicles of both the groups of rats. The results showed that unlabelled folic acid significantly decreased (P <0.001 vs. P<0.001 for control and folate deficient diet fed rats, respectively) folic acid uptake, indicating competition for folate transport. Also decreasing temperature from 37 to 27°C, decreased uptake by 31 per cent (P<0.01) and 32 per cent (P<0.001), respectively in control and folic acid deficient diet fed groups respectively, with a temperature co-efficient of 1.43 and 1.48 in the control and folate deficient diet-fed groups.
Effect of dietary folate on tissue mRNA levels of RFC and PCFT: The finding that folic acid uptake was markedly higher in intestinal BBM of folate deficient rats as compared to controls led us to study the regulation of folate transporters at the molecular level. For this RT-PCR analysis was performed with the use of specific primers for rRFC, rPCFT and β–actin or GAPDH to obtain fragments of sizes 120, 300 and 588 or 400 bp. The results showed that the levels of rRFC and rPCFT mRNA were significantly higher (P<0.001) in intestinal BBM of rats fed folate deficient diet. When studied along the crypt-villus axis, the expression of both rRFC and rPCFT increased along crypt-villus axis, with maximum expression at villus tip (VT) followed by mid-villus (MV) and then crypt base (CB).
Effect of dietary folate on protein levels of RFC and PCFT in intestine: The finding that the folate deficiency resulted in a significant increase in mRNA levels of both the PCFT and the RFC, led us to study whether this increase in mRNA levels was associated with protein levels. To investigate the effect of folate deficiency on the level of expression of the PCFT and the RFC protein, Western blotting was performed on the intestinal brush border membrane vesicles prepared from the intestine of both the groups of rats. Western analysis was performed with the use of specific polyclonal anti-rRFC, anti-rPCFT, and anti-β-actin antibodies. The results showed a significant increase in rRFC (P<0.01) and rPCFT (P<0.05) protein levels in BBM isolated from folate deficient rats as compared to controls.
Genomic DNA methylation analysis: The effect of folate deficiency was studied on the genomic DNA methylation in the intestine. DNA was isolated from the intestine and genomic DNA methylation was studied using the labelled S-adenosylmethionine (Fig. 5). The amount of SAM incorcorporated into DNA was inversely proportional to degree of methylation. It was observed that DNA from folate deficient diet fed rats incorporated more SAM relative to that from control group. Results indicated the hypo-methylation of DNA under the conditions of folate deficiency. Moreover, there was an increase in SAH/SAM ratio in rats fed deficient diet, which is an indicator of folate deficiency.
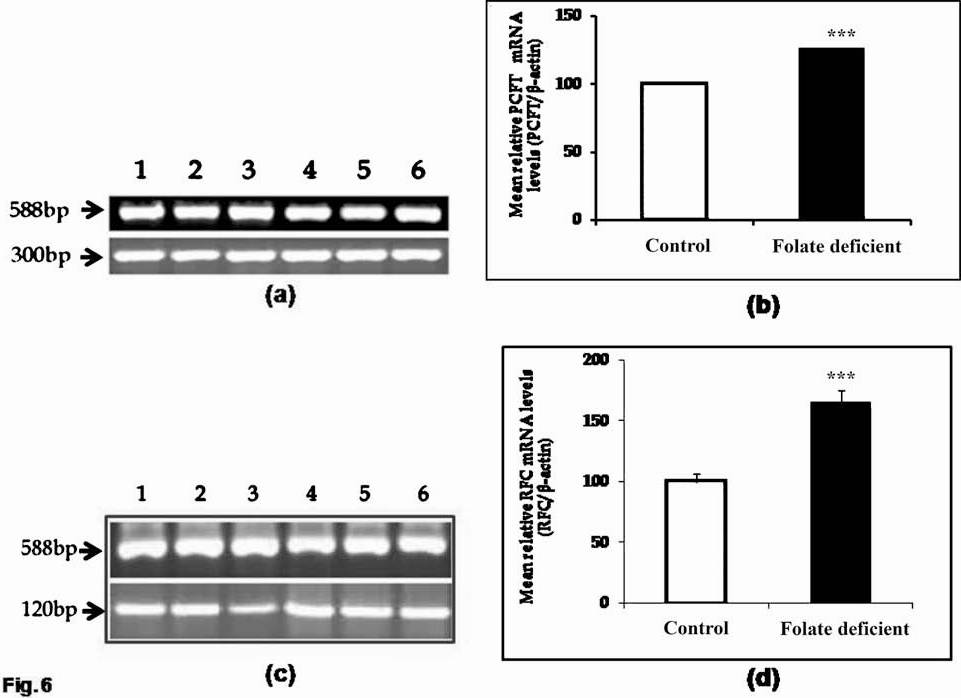
- RT-PCR analysis of RFC (120 bp) and PCFT (300 bp) with β-actin(588 bp) as an internal control in intestine. (a and c) Resolved on 1.2 per cent agarose gel electrophoresis and (b and d) densitometric analysis representing relative change in PCFT and RFC mRNA expression. Data shown are representative of 5 separate sets of experiments. Lanes 1-3: Control; 4-6: Folate deficient. ***P<0.001 vs. control.
Discussion
Our results with the intestinal BBM vesicles isolated from folate deficient diet fed rats showed more uptake in acidic micro environment at pH 5.5 reinforcing earlier observations that acidic pH to be a potential driving force of folate transport across intestinal BBM57 and highlighting the role of PCFT in the uptake of folate across intestinal BBM, as PCFT shows maximum transport activity at acidic pH5727. Folate deficiency leads to significant upregulation in the intestinal folate uptake, this observed increase in folate uptake was associated with increase in Vmax without any significant change in Km of folate uptake process, in accordance with previous studies2328 indicating that folate deficiency induced increased folate uptake results from the increased number or activity of folate transporters rather than the altered affinity of transporters towards its substrate. The Km values observed here might represent the affinity of PCFT for folic acid. For the assessment of the specificity of the folate transport system, unlabelled folic acid used in incubation medium significantly reduced the uptake in both the groups, with more decrease in rats fed folate deficient diet.
The temperature coefficient of the folate uptake showed that transport in intestinal BBM surfaces follows first order rate equation as uptake decreased when there was decrease in temperature from 37 to 27°C, suggesting effect of temperature on folate uptake29.
To evaluate the mechanism of upregulation of folate uptake, the expression profile of folate transporters PCFT and RFC was of prime importance, as both are believed to be the transporters responsible for folate uptake78. The upregulation of the folate uptake across the intestinal absorptive surface was associated with an increase in mRNA levels both PCFT (transporter responsible for folate uptake in acidic microenvironment) and RFC (that is also believed to be responsible for intestinal folate uptake).
Further, this increase in intestinal folate uptake along with mRNA expression of folate transporters was associated with parallel increase in protein levels of both RFC and PCFT transporters, suggesting the possible involment of transcriptional and translational regulatory mechanisms in regulating intestinal folate uptake during folate deficiency, these results are supported by earlier studies where the mRNA expression of RFC and PCFT increased under folate deficient conditions12–1623. However, our results on mRNA expression were contradictory to the earlier study, which showed increased folate uptake in association with decreased expression of folate transporters when Caco-2 cells were grown in folate deficient conditions28. This might be due to difference in the model for study i.e. cell line vs. animal model of folate deficiency, and the concentration of folate the cells were exposed to. The role of folate transport regulation across crypt-villus axis was evaluated in rats. Higher distribution of RFC and PCFT mRNA levels in villus tip suggests that large number of folate transporters is expressed at the villus tip and this distribution of RFC and PCFT occurs within maturation of intestinal stem cells.
The results observed in the present model of folate deficiency depicted that the folate deficiency in rats was associated with upregulation in the folate uptake by increasing the Vmax without changing the affinity of folate transporters and this increased uptake was associated with upregulation in folate transporter expression. Howover, in our earlier studies carried out in animal model of chronic alcoholism, there was a decreased uptake of folate associated with decreased affinity as well as number of transporter molecules82930. The decreased uptake under these conditions was associated with reduced expression of folate transporters831, thus, suggesting different regulatory mechanisms involved in the uptake of folate in two different models of folate deficiency. In the present study, there was a state of severe folate deficiency as compared to folate deficiency observed in chronic alcoholism82930. Hence, it can be postulated that circulating folate levels might be the determinants of the adaptive response of the increased uptake of folate and increased expression of folate transporters.
In summary, the results showed that the folate deficiency resulted in a significant upregulation of intestinal folate uptake, by increasing number of transporters without any change in specificity of transporters towards its substrate. The observed up-regulation was associated with significant increase in RFC and PCFT expressions, suggesting the transcriptional and translational regulation of folate uptake during folate deficiency. Moreover, the decrease in serum folate levels was associated with a decrease in genomic DNA methylation and an increase in homocysteine levels as observed by increase in SAH: SAM ratio.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research, New Delhi, for financial support.
References
- Folate status in various pathophysiological conditions. IUBMB Life. 2008;60:834-42.
- [Google Scholar]
- New perspectives on folate transport in relation to alcoholism-induced folate malabsorption--association with epigenome stability and cancer development. FEBS J. 2009;276:2175-91.
- [Google Scholar]
- Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med. 2009;11:e4.
- [Google Scholar]
- Is low or high body weight associated with an increased risk of neural tube defects? Praxis (Bern. 1994;2006(95):2019-26.
- [Google Scholar]
- Reduced levels of folate transporters (PCFT and RFC) in membrane lipid rafts result in colonic folate malabsorption in chronic alcoholism. J Cell Physiol. 2011;226:579-87.
- [Google Scholar]
- Evidence for the existence of a carrier-mediated folate uptake mechanism in human colonic luminal membranes. Am J Physiol. 1997;272:G1408-15.
- [Google Scholar]
- Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917-28.
- [Google Scholar]
- Down-regulation of reduced folate carrier may result in folate malabsorption across intestinal brush border membrane during experimental alcoholism. FEBS J. 2007;274:6317-28.
- [Google Scholar]
- Folate transport by human intestinal brush-border membrane vesicles. Am J Physiol. 1987;252:G229-36.
- [Google Scholar]
- Ontogenic regulation of folate transport across rat jejunal brush-border membrane. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1068-73.
- [Google Scholar]
- Folate malabsorption is associated with down-regulation of folate transporter expression and function at colon basolateral membrane in rats. Br J Nutr. 2011;107:1-9.
- [Google Scholar]
- Moderate folate depletion modulates the expression of selected genes involved in cell cycle, intracellular signaling and folate uptake in human colonic epithelial cell lines. J Nutr Biochem. 2008;19:328-35.
- [Google Scholar]
- Characterization of folate receptor from normal and neoplastic murine tissue: influence of dietary folate on folate receptor expression. Clin Cancer Res. 1996;2:1135-41.
- [Google Scholar]
- Folate uptake in the human intestine: promoter activity and effect of folate deficiency. J Cell Physiol. 2003;196:403-8.
- [Google Scholar]
- Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol. 2007;293:C1669-78.
- [Google Scholar]
- Structure and regulation of the murine reduced folate carrier gene: identification of four noncoding exons and promoters and regulation by dietary folates. J Biol Chem. 2005;280:5588-97.
- [Google Scholar]
- Folate deficiency results in alteration in intestinal brush border membrane composition and enzyme activities in weanling rats. J Nutr Sci Vitaminol (Tokyo). 2006;52:163-7.
- [Google Scholar]
- A rapid high-performance liquid chromatographic procedure for the simultaneous determination of methionine, ethionine, S-adenosylmethionine, S-adenosylethionine, and the natural polyamines in rat tissues. Anal Biochem. 1984;140:108-16.
- [Google Scholar]
- Synthesis of plasmalemmal glycoproteins in intestinal epithelial cells.Separation of Golgi membranes from villus and crypt cell surface membranes; glycosyltransferase activity of surface membrane. J Cell Biol. 1978;77:722-34.
- [Google Scholar]
- Divalent metal is required for both phosphate transport and phosphate binding to phosphorin, a proteolipid isolated from brush-border membrane vesicles. J Biol Chem. 1984;259:9059-63.
- [Google Scholar]
- Long-term alcohol ingestion alters the folate-binding kinetics in intestinal brush border membrane in experimental alcoholism. Alcohol. 2007;41:441-6.
- [Google Scholar]
- Decreased expression of transporters reduces folate uptake across renal absorptive surfaces in experimental alcoholism. J Membr Biol. 2007;220:69-77.
- [Google Scholar]
- Adaptive regulation of intestinal folate uptake: effect of dietary folate deficiency. Am J Physiol Cell Physiol. 2000;279:C1889-95.
- [Google Scholar]
- Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350-4.
- [Google Scholar]
- Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J Nutr. 1999;129:1945-50.
- [Google Scholar]
- Moderate folate deficiency does not cause global hypomethylation of hepatic and colonic DNA or c-myc-specific hypomethylation of colonic DNA in rats. Am J Clin Nutr. 1995;61:1083-90.
- [Google Scholar]
- The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol. 2008;74:854-62.
- [Google Scholar]
- The effect of folate status on the uptake of physiologically relevant compounds by Caco-2 cells. Eur J Pharmacol. 2010;640:29-37.
- [Google Scholar]
- Evaluation of the kinetic properties of the folate transport system in intestinal absorptive epithelium during experimental ethanol ingestion. Mol Cell Biochem. 2007;304:265-71.
- [Google Scholar]
- Role of signaling pathways in the regulation of folate transport in ethanol-fed rats. J Nutr Biochem. 2009;20:291-7.
- [Google Scholar]
- Low folate transport across intestinal basolateral surface is associated with down-regulation of reduced folate carrier in in vivo model of folate malabsorption. IUBMB Life. 2009;61:236-43.
- [Google Scholar]






